Silymarin from Milk Thistle Fruits Counteracts Selected Pathological Changes in the Lenses of Type 1 Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Diabetes Induction, and In Vivo Study Design
2.2. Assessment of Basic Serological Parameters
2.3. Assessment of Protein Level in the Lenses
2.4. Assessment of Antioxidative Enzyme Activity in the Lenses and the Serum
2.5. Assessment of Thiol and Glutathione Levels in the Lenses
2.6. Assessment of Oxidative Damage Markers, Vitamin C, and Advanced Glycation End Product Level in the Lenses and the Serum
2.7. Assessment of the Total Oxidative Stress Markers in the Lenses and the Serum
2.8. Assessment of Polyol Pathway Markers in the Lenses
2.9. Apparatus and Statistical Analysis
3. Results
3.1. Effect of Silymarin on Body Weight and Serological Parameters in Rats with Type 1 Diabetes Mellitus
3.2. Effect of Silymarin on Protein Levels in the Lenses of Rats with Type 1 Diabetes Mellitus
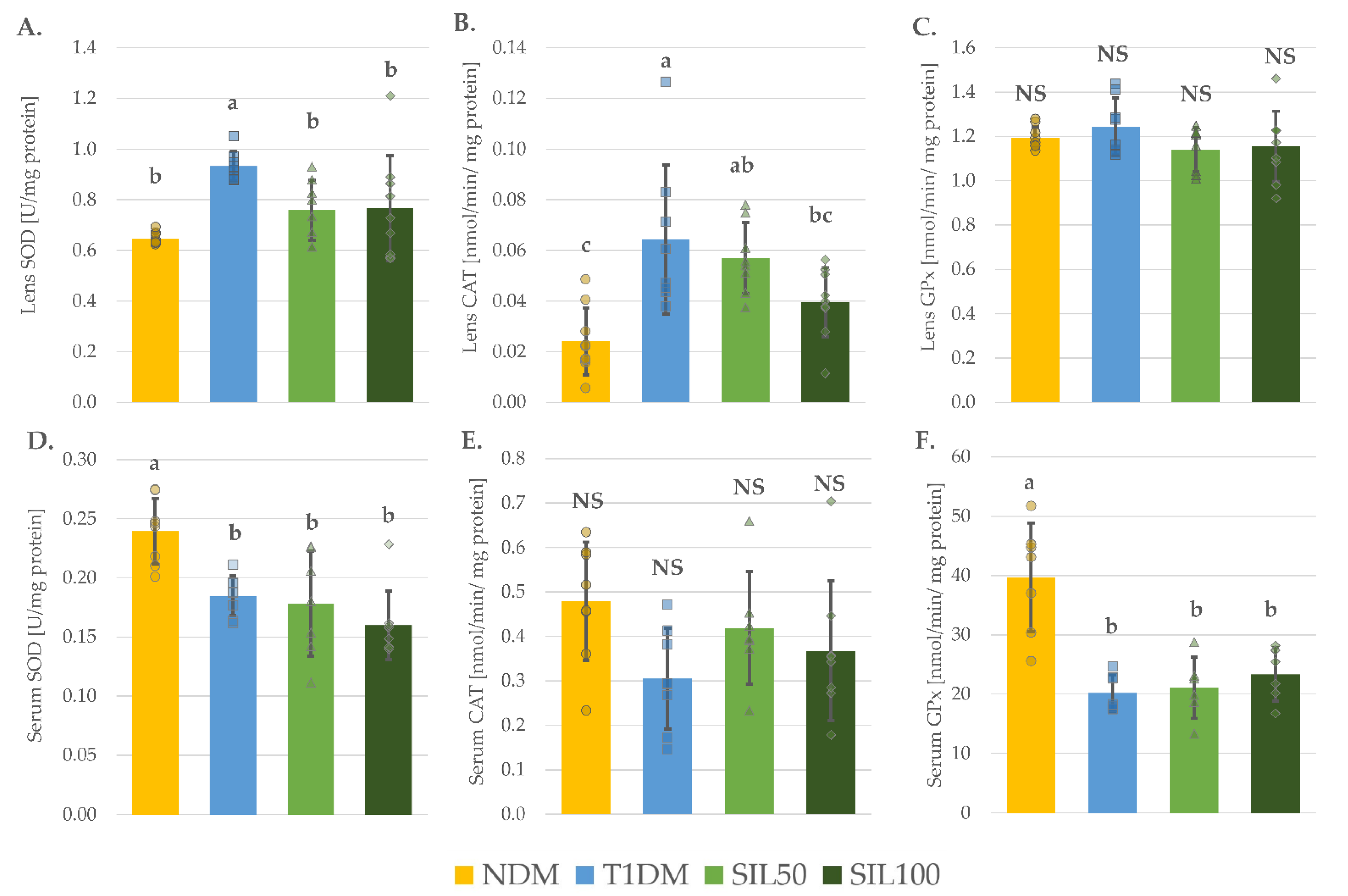
3.3. Effect of Silymarin on Antioxidative Enzymes in the Serum and the Lenses of Rats with Type 1 Diabetes Mellitus
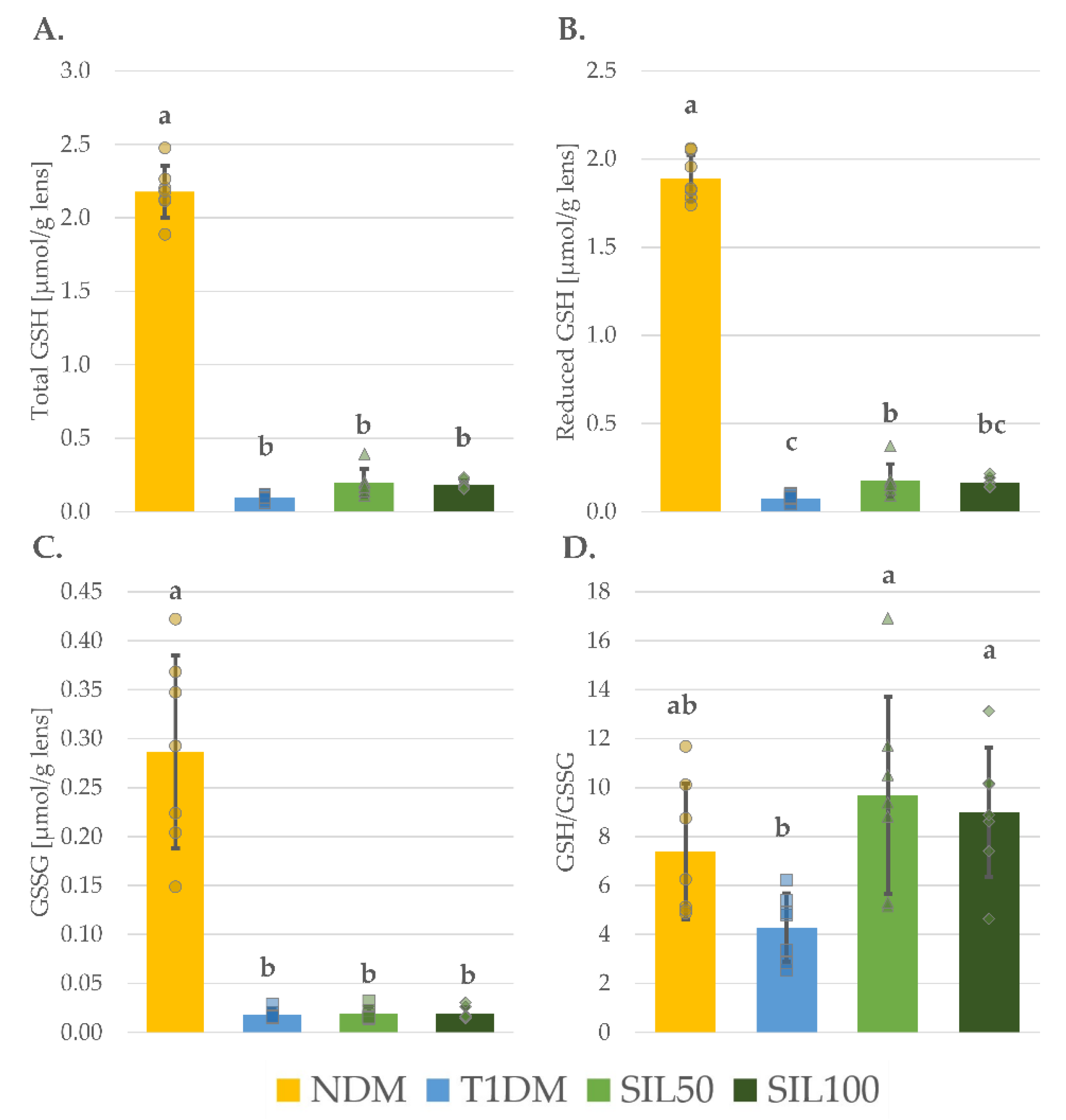
3.4. Effect of Silymarin on Thiols in the Lenses of Rats with Type 1 Diabetes Mellitus
3.5. Effect of Silymarin on Oxidative Damage and Glycation in the Lenses and the Serum of Rats with Type 1 Diabetes Mellitus
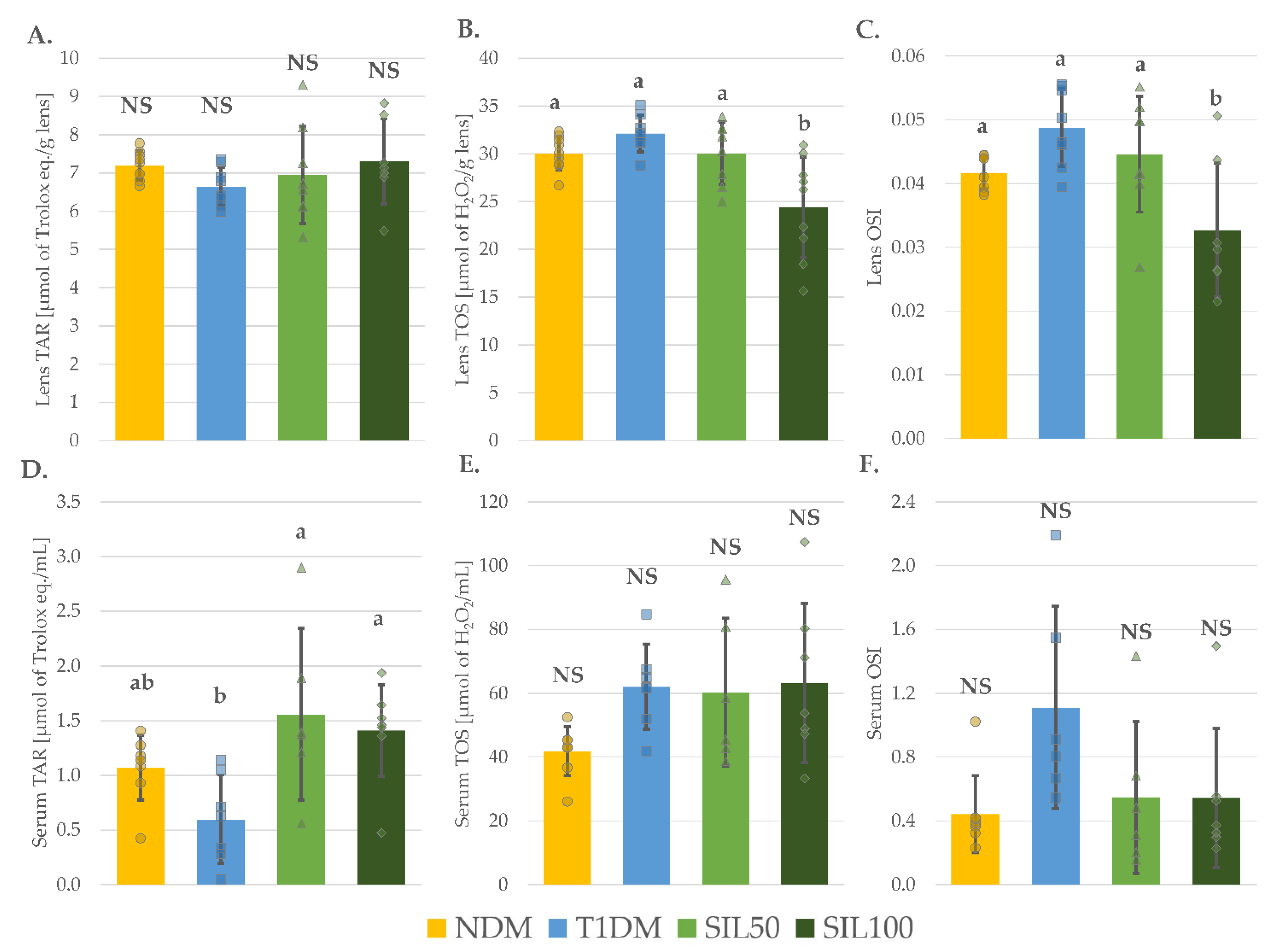
3.6. Effect of Silymarin on Total Oxidative Stress Markers in the Lenses and the Serum of Rats with Type 1 Diabetes Mellitus
3.7. Effect of Silymarin on Polyol Pathway in the Lenses of Rats with Type 1 Diabetes Mellitus
3.8. General Effect of Silymarin on the Serum and the Lenses of Rats with Type 1 Diabetes Mellitus
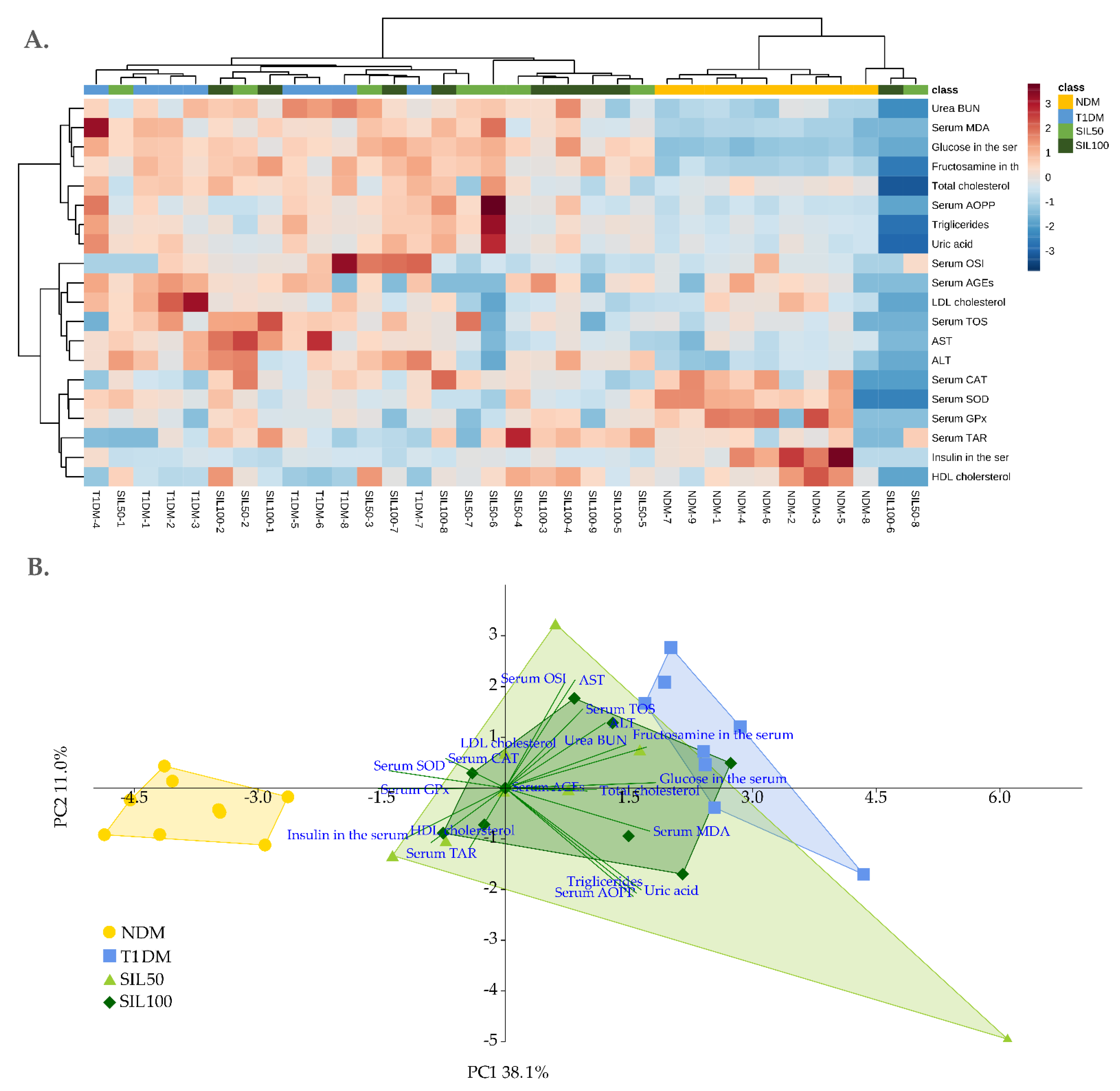

| Parameter/Group | NDM | T1DM | SIL50 | SIL100 |
|---|---|---|---|---|
| Serum | ||||
| PC 1 | −3.81 ± 0.74 c | 2.52 ± 0.82 a | 0.87 ± 2.30 b | 0.79 ± 1.22 b |
| PC 2 | −0.41 ± 0.52 NS | 0.85 ± 1.42 NS | −0.34 ± 2.33 NS | −0.05 ± 1.13 NS |
| Lenses | ||||
| PC 1 | 4.97 ± 0.58 a | −2.19 ± 0.77 c | −1.76 ± 0.50 bc | −1.46 ± 0.27 b |
| PC 2 | 0.16 ± 1.01 b | 2.33 ± 0.68 a | −0.68 ± 1.63 bc | −1.63 ± 2.10 c |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Assessment Report on Silybum marianum (L.) Gaertn., Fructus; European Medicines Agency: London, UK, 2018. [Google Scholar]
- Janeczko, M.; Kochanowicz, E. Silymarin, a popular dietary supplement shows anti-Candida activity. Antibiotics 2019, 8, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evren, E.; Yurtcu, E. In vitro effects on biofilm viability and antibacterial and antiadherent activities of silymarin. Folia Microbiol. 2015, 60, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition… and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H.J. Silymarin as supportive treatment in liver diseases: A narrative review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef] [Green Version]
- Tighe, S.P.; Akhtar, D.; Iqbal, U.; Ahmed, A. Chronic liver disease and silymarin: A biochemical and clinical review. J. Clin. Transl. Hepatol. 2020, 8, 454–458. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/silybin and chronic liver disease: A marriage of many years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phyther. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, H. Anti-Parkinson potential of silymarin: Mechanistic insight and therapeutic standing. Front. Pharmacol. 2018, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Haddadi, R.; Shahidi, Z.; Eyvari-Brooshghalan, S. Phytomedicine silymarin and neurodegenerative diseases: Therapeutic potential and basic molecular mechanisms. Phytomedicine 2020, 79, 153320. [Google Scholar] [CrossRef]
- Camini, F.C.; Costa, D.C. Silymarin: Not just another antioxidant. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190206. [Google Scholar] [CrossRef]
- Vahabzadeh, M.; Amiri, N.; Karimi, G. Effects of silymarin on metabolic syndrome: A review. J. Sci. Food Agric. 2018, 98, 4816–4823. [Google Scholar] [CrossRef]
- Soleymani, S.; Ayati, M.H.; Mansourzadeh, M.J.; Namazi, N.; Zargaran, A. The effects of silymarin on the features of cardiometabolic syndrome in adults: A systematic review and meta-analysis. Phyther. Res. 2022, 36, 842–856. [Google Scholar] [CrossRef]
- Stolf, A.M.; Cardoso, C.C.; Acco, A. Effects of silymarin on diabetes mellitus complications: A review. Phyther. Res. 2017, 31, 366–374. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 diabetes mellitus. Nat. Rev. Dis. Prim. 2017, 3, 17016. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Vieira-Potter, V.J.; Karamichos, D.; Lee, D.J. Ocular complications of diabetes and therapeutic approaches. BioMed Res. Int. 2016, 2016, 3801570. [Google Scholar] [CrossRef] [Green Version]
- Nowroozzadeh, M.H.; Thornton, S.; Watson, A.; Syed, Z.A.; Razeghinejad, R. Ocular manifestations of endocrine disorders. Clin. Exp. Optom. 2022, 105, 105–116. [Google Scholar] [CrossRef]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in diabetes mellitus. World J. Diabetes 2019, 10, 140–153. [Google Scholar] [CrossRef]
- Peterson, S.R.; Silva, P.A.; Murtha, T.J.; Sun, J.K. Cataract surgery in patients with diabetes: Management strategies. Semin. Ophthalmol. 2018, 33, 75–82. [Google Scholar] [CrossRef]
- Stefek, M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscip. Toxicol. 2011, 4, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Tewari, D.; Samoila, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal plants and natural products used in cataract management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef] [Green Version]
- Lim, V.; Schneider, E.; Wu, H.; Pang, I. Cataract preventive role of isolated phytoconstituents: Findings from a decade of research. Nutrients 2018, 10, 1580. [Google Scholar] [CrossRef] [Green Version]
- Patil, K.K.; Gacche, R.N. Inhibition of glycation and aldose reductase activity using dietary flavonoids: A lens organ culture studies. Int. J. Biol. Macromol. 2017, 98, 730–738. [Google Scholar] [CrossRef]
- Huang, X.; Qi, M.; Ye, H.; Lin, W.; Zheng, L.; Lin, J. Experimental study on natural anti-oxidants protecting lens against oxidative injuries. Chin. J. Pathophysiol. 2001, 17, 120–123. [Google Scholar]
- Fallah Huseini, H.; Zaree Mahmodabady, A.; Heshmat, R.; Raza, M. The effect of Silybum marianum (L.) Gaertn. seed extract (silymarin) on galactose induced cataract formation in rats. J. Med. Plants 2009, 8, 7–12. [Google Scholar]
- Tohidi, V.; Azimpour, S. Effect of silymarin in treatment of experimentally induced cataract in rabbit. J. Vet. Clin. Res. 2018, 9, 15–23. [Google Scholar]
- Malekinejad, H.; Rezabakhsh, A.; Rahmani, F.; Hobbenaghi, R. Silymarin regulates the cytochrome P450 3A2 and glutathione peroxides in the liver of streptozotocin-induced diabetic rats. Phytomedicine 2012, 19, 583–590. [Google Scholar] [CrossRef]
- Vessal, G.; Akmali, M.; Najafi, P.; Moein, M.R.; Sagheb, M.M. Silymarin and milk thistle extract may prevent the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Ren. Fail. 2010, 32, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Roghani, M.; Khalili, M.; Baluchnejadmojarad, T.; Ahmani, M. Protective effect of silymarin on learning and memory deficiency in streptozotocin-diabetic rats. J. Gorgan Univ. Med. Sci. 2013, 15, 35–42. [Google Scholar]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosenbrough, N.J.; Lewis Farr, A.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Kosecik, M.; Erel, O.; Sevinc, E.; Selek, S. Increased oxidative stress in children exposed to passive smoking. Int. J. Cardiol. 2005, 100, 61–64. [Google Scholar] [CrossRef]
- Patel, D.K.; Kumar, R.; Kumar, M.; Sairam, K.; Hemalatha, S. Evaluation of in vitro aldose reductase inhibitory potential of different fraction of Hybanthus enneaspermus Linn F. Muell. Asian Pac. J. Trop. Biomed. 2012, 2, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Wojnar, W.; Kaczmarczyk-Sedlak, I.; Zych, M. Diosmin ameliorates the effects of oxidative stress in lenses of streptozotocin-induced type 1 diabetic rats. Pharmacol. Rep. 2017, 69, 995–1000. [Google Scholar] [CrossRef]
- Wojnar, W.; Zych, M.; Kaczmarczyk-Sedlak, I. Antioxidative effect of flavonoid naringenin in the lenses of type 1 diabetic rats. Biomed. Pharmacother. 2018, 108, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Wojnar, W.; Zych, M.; Kaczmarczyk-Sedlak, I. Wpływ naryngeniny na odpowiedź antyoksydacyjną oraz status oksydacyjny w soczewkach szczurów z cukrzycą. Herbalism 2018, 1, 17–30. [Google Scholar] [CrossRef]
- Sedlak, L.; Wojnar, W.; Zych, M.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E.; Kaczmarczyk-Sedlak, I. Effect of resveratrol, a dietary-derived polyphenol, on the oxidative stress and polyol pathway in the lens of rats with streptozotocin-induced diabetes. Nutrients 2018, 10, 1423. [Google Scholar] [CrossRef] [Green Version]
- Wojnar, W.; Zych, M.; Borymski, S.; Kaczmarczyk-Sedlak, I. Chrysin reduces oxidative stress but does not affect polyol pathway in the lenses of type 1 diabetic rats. Antioxidants 2020, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Mahendiran, K.; Elie, C.; Nebel, J.C.; Ryan, A.; Pierscionek, B.K. Primary sequence contribution to the optical function of the eye lens. Sci. Rep. 2014, 4, 5195. [Google Scholar] [CrossRef] [Green Version]
- Lam, D.; Rao, S.K.; Ratra, V.; Liu, Y.; Mitchell, P.; King, J.; Tassignon, M.-J.; Jonas, J.; Pang, C.P.; Chang, D.F. Cataract. Nat. Rev. Dis. Prim. 2015, 1, 15014. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Vasavada, A. Cataract and surgery for cataract. BMJ 2006, 333, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.J.; Afshari, N.A. Cataract and systemic disease: A review. Clin. Exp. Ophthalmol. 2021, 49, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Recoba, R.; Barrón, H.; Alvarez, C.; Favari, L. Silymarin increases antioxidant enzymes in alloxan-induced diabetes in rat pancreas. Comp. Biochem. Physiol. Part C 2003, 136, 205–212. [Google Scholar] [CrossRef]
- Soto, C.; Pérez, J.; García, V.; Uría, E.; Vadillo, M.; Raya, L. Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus. Phytomedicine 2010, 17, 1090–1094. [Google Scholar] [CrossRef]
- Fallah Huseini, H.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phyther. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef]
- Meng, S.; Yang, F.; Wang, Y.; Qin, Y.; Xian, H.; Che, H.; Wang, L. Silymarin ameliorates diabetic cardiomyopathy via inhibiting TGF-β1/Smad signaling. Cell Biol. Int. 2019, 43, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Sheela, N.; Jose, M.A.; Sathyamurthy, D.; Kumar, B.N. Effect of silymarin on streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in rats. Iran. J. Kidney Dis. 2013, 7, 117–123. [Google Scholar]
- Yön, B.; Belviranl, M.; Okudan, N. The effect of silymarin supplementation on cognitive impairment induced by diabetes in rats. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 20180109. [Google Scholar] [CrossRef]
- Oliveira, M.L.M.; da Cunha, A.L.; de Geraldo, L.W.; Caetano, C.F.; Caldeira, C.D. Silymarin attenuates hepatic and pancreatic redox imbalance independent of glycemic regulation in the alloxan-induced diabetic rat model. Biomed. Environ. Sci. 2020, 33, 690–700. [Google Scholar]
- Nansseu, J.R.N.; Fokom-Domgue, J.; Noubiap, J.J.N.; Balti, E.V.; Sobngwi, E.; Kengne, A.P. Fructosamine measurement for diabetes mellitus diagnosis and monitoring: A systematic review and meta-analysis protocol. BMJ Open 2015, 5, e007689. [Google Scholar] [CrossRef] [Green Version]
- Vergès, B. Dyslipidemia in type 1 diabetes: A masked danger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Almalki, D.A.; Alghamdi, S.A.; Al-Attar, A.M. Comparative study on the influence of some medicinal plants on diabetes induced by streptozotocin in male rats. BioMed Res. Int. 2019, 2019, 3596287. [Google Scholar] [CrossRef] [Green Version]
- Al-Attar, A.M.; Alsalmi, F.A. Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J. Biol. Sci. 2019, 26, 118–128. [Google Scholar] [CrossRef]
- Sajedianfard, J.; Behroozi, Z.; Nazifi, S. The effects of a hydroalcoholic extract of silymarin on serum lipids profiles in streptozotocin induced diabetic rats. Comp. Clin. Path. 2014, 23, 779–784. [Google Scholar] [CrossRef]
- El-Far, M.; Negm, A.; Wahdan, M. Promising biopharmaceutical use of silymarin and silibinin as antidiabetic natural agents in streptozotocin-induced diabetic rats: First comparative assessment. World J. Pharm. Parmaceutical Sci. 2014, 4, 7–28. [Google Scholar]
- Sharma, M.; Pillai, K.K.; Anwer, T.; Najmi, A.K.; Haque, S.E.; Sultana, Y. Protective effect of silymarin in streptozotocin-induced diabetic dyslipidaemia in rats. Orient. Pharm. Exp. Med. 2010, 10, 165–172. [Google Scholar] [CrossRef]
- Rahimi, R.; Karimi, J.; Khodadadi, I.; Tayebinia, H.; Kheiripour, N.; Hashemnia, M.; Goli, F. Silymarin ameliorates expression of urotensin II (U-II) and its receptor (UTR) and attenuates toxic oxidative stress in the heart of rats with type 2 diabetes. Biomed. Pharmacother. 2018, 101, 244–250. [Google Scholar] [CrossRef]
- Numan, A.T.; Hadi, N.A.; Mohammed, S.N.; Hussain, S.A. Use of silymarine as adjuvant in type 1 diabetes mellitus patients poorly controlled with insulin. J. Fac. Med. 2010, 52, 75–79. [Google Scholar]
- Voroneanu, L.; Nistor, I.; Dumea, R.; Apetrii, M.; Covic, A. Silymarin in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Res. 2016, 2016, 5147468. [Google Scholar] [CrossRef] [Green Version]
- Hadi, A.; Pourmasoumi, M.; Mohammadi, H.; Symonds, M.; Miraghajani, M. The effects of silymarin supplementation on metabolic status and oxidative stress in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2018, 41, 311–319. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Chung, S.S.M.; Kador, P.F. Diabetic cataract: Mechanisms and management. Diabetes. Metab. Res. Rev. 2010, 26, 172–180. [Google Scholar] [CrossRef]
- Pintor, J. Sugars, the crystalline lens and the development of cataracts. Biochem. Pharmacol. Open Access 2012, 1, 1000e119. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.; Martin, K.A.; Hwa, J. Aldose reductase, oxidative stress, and diabetic mellitus. Front. Pharmacol. 2012, 3, 87. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.A.; Di, L.; Kerns, E.H. The effect of plasma protein binding on in vivo efficacy: Misconceptions in drug discovery. Nat. Rev. Drug Discov. 2010, 9, 929–939. [Google Scholar] [CrossRef]
- In Vivo Pharmacology Training Group. The fall and rise of in vivo pharmacology. Trends Pharmacol. Sci. 2002, 23, 13–18. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, D.; Zhang, J.; Yuan, J. Metabolism, transport and drug-drug interactions of silymarin. Molecules 2019, 24, 3693. [Google Scholar] [CrossRef] [Green Version]
- Sayin, N.; Kara, N.; Pekel, G. Ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 92–108. [Google Scholar] [CrossRef]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Zheng, H.X.; Chen, D.J.; Zu, Y.X.; Wang, E.Z.; Qi, S.S. Chondroitin sulfate prevents STZ induced diabetic osteoporosis through decreasing blood glucose, antioxidative stress, anti-inflammation and OPG/RANKL expression regulation. Int. J. Mol. Sci. 2020, 21, 5303. [Google Scholar] [CrossRef]
- Alghazeer, R.; Alghazir, N.; Awayn, N.; Ahtiwesh, O.; Elgahmasi, S. Biomarkers of oxidative stress and antioxidant defense in patients with type 1 diabetes mellitus. Ibnosina J. Med. Biomed. Sci. 2018, 10, 198. [Google Scholar] [CrossRef]
- Pradeep, S.R.; Srinivasan, K. Ameliorative influence of dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) on eye lens abnormalities via modulation of crystallin proteins and polyol pathway in experimental diabetes. Curr. Eye Res. 2018, 43, 1108–1118. [Google Scholar] [CrossRef]
- Barman, S.; Srinivasan, K. Zinc supplementation ameliorates diabetic cataract through modulation of crystallin proteins and polyol pathway in experimental rats. Biol. Trace Elem. Res. 2019, 187, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Ahsan, H. Biomarkers of inflammation and oxidative stress in ophthalmic disorders. J. Immunoass. Immunochem. 2020, 41, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Higashi, K.; Mori, A.; Sakamoto, K.; Ishii, K.; Nakahara, T. Anti-cataract effect of resveratrol in high-glucose-treated streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2018, 41, 1586–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Chen, Y.; Zhao, T.; Wang, Z. Effect of astaxanthin on metabolic cataract in rats with type 1 diabetes mellitus. Exp. Mol. Pathol. 2020, 113, 104372. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Dai, Y.; Xie, Q.; Wu, P.; Chen, M.; Liu, C. Anti-cataract effects of coconut water in vivo and in vitro. Biomed. Pharmacother. 2021, 143, 112032. [Google Scholar] [CrossRef]
- Kilari, E.K.; Putta, S. Delayed progression of diabetic cataractogenesis and retinopathy by Litchi chinensis in STZ-induced diabetic rats. Cutan. Ocul. Toxicol. 2017, 36, 52–59. [Google Scholar] [CrossRef]
- Fan, X.; Monnier, V.M.; Whitson, J. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 2017, 156, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Dalle-Donne, I.; Milzani, A.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid. Redox Signal. 2008, 10, 445–473. [Google Scholar] [CrossRef]
- Christy, X.; Liu, X.; Liu, Y.; Wu, H. The important functions of GSH-dependent enzyme glutaredoxin. In Glutathione in Health and Disease; Erkekoglu, P., Kocer-Gumusel, B., Eds.; IntechOpen: London, UK, 2018; pp. 27–43. [Google Scholar]
- Lou, M.F. Redox regulation in the lens. Prog. Retin. Eye Res. 2003, 22, 657–682. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative stress indexes for diagnosis of health or disease in humans. Oxid. Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Mathebula, S.D. Polyol pathway: A possible mechanism of diabetes complications in the eye. Afr. Vis. Eye Health 2015, 74, 13. [Google Scholar] [CrossRef]
- Jahan, H.; Choudhary, M.I. Glycation, carbonyl stress and AGEs inhibitors: A patent review. Expert Opin. Ther. Pat. 2015, 25, 1267–1284. [Google Scholar]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’Nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Wu, C.-H.; Huang, S.-M.; Yen, G.-C. Silymarin: A novel antioxidant with antiglycation and antiinflammatory properties in vitro and in vivo. Antioxid. Redox Signal. 2011, 14, 353–366. [Google Scholar] [CrossRef]
- Engle, S.; Whalen, S.; Joshi, A.; Pollard, K.S. Unboxing cluster heatmaps. BMC Bioinform. 2017, 18, 63. [Google Scholar] [CrossRef] [Green Version]
- Zych, M.; Wojnar, W.; Borymski, S.; Szałabska, K.; Bramora, P.; Kaczmarczyk-Sedlak, I. Effect of rosmarinic acid and sinapic acid on oxidative stress parameters in the cardiac tissue and serum of type 2 diabetic female rats. Antioxidants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janas, A.; Kruczek, E.; Londzin, P.; Borymski, S.; Czuba, Z.P.; Folwarczna, J. Negligible effect of estrogen deficiency on development of skeletal changes induced by type 1 diabetes in experimental rat models. Mediat. Inflamm. 2020, 2020, 2793804. [Google Scholar] [CrossRef] [PubMed]
- Ringnér, M. What is principal components analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Torres-Piedra, M.; Ortiz-Andrade, R.; Villalobos-Molina, R.; Singh, N.; Medina-Franco, J.L.; Webster, S.P.; Binnie, M.; Navarrete-Vázquez, G.; Estrada-Soto, S. A comparative study of flavonoid analogues on streptozotocin-nicotinamide induced diabetic rats: Quercetin as a potential antidiabetic agent acting via 11β-Hydroxysteroid dehydrogenase type 1 inhibition. Eur. J. Med. Chem. 2010, 45, 2606–2612. [Google Scholar] [CrossRef]
- Bolli, A.; Marino, M.; Rimbach, G.; Fanali, G.; Fasano, M.; Ascenzi, P. Flavonoid binding to human serum albumin. Biochem. Biophys. Res. Commun. 2010, 398, 444–449. [Google Scholar] [CrossRef]
- Gecibesler, I.H.; Aydin, M. Plasma protein binding of herbal-flavonoids to human serum albumin and their anti-proliferative activities. An. Acad. Bras. Cienc. 2020, 92, 1–16. [Google Scholar] [CrossRef]
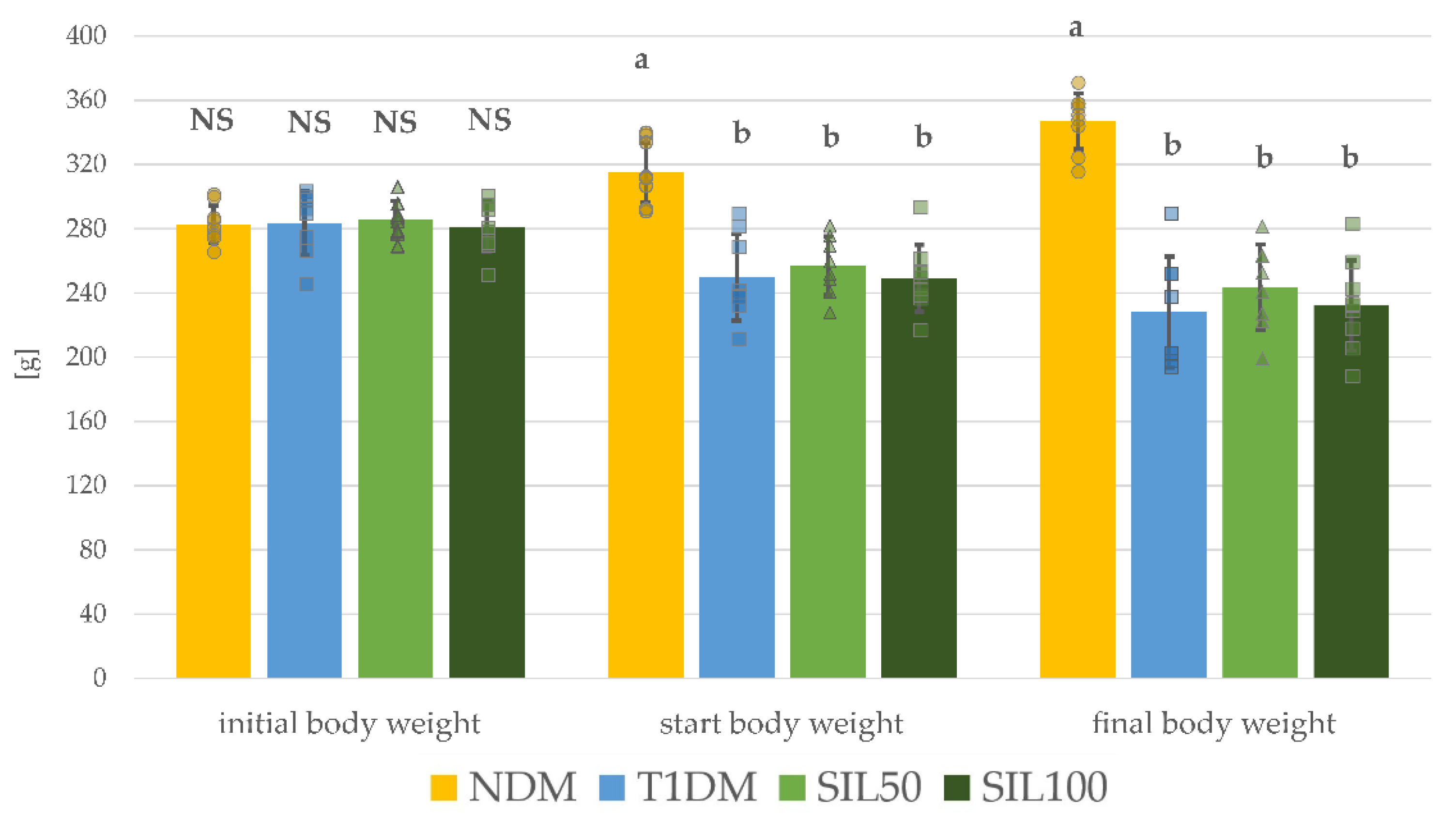

| Parameter/Group | NDM | T1DM | SIL50 | SIL100 |
|---|---|---|---|---|
| Glucose [mg/dL] | 141.4 ± 33.0 b | 641.8 ± 80.9 a | 661.8 ± 75.2 a | 631.3 ± 149.6 a |
| Insulin [µg/L] | 0.434 ± 0.272 a | 0.089 ± 0.053 b | 0.069 ± 0.041 b | 0.083 ± 0.035 b |
| Fructosamine [µmol/L] | 281.9 ± 29.5 b | 495.6 ± 65.2 a | 475.6 ± 58.4 a | 496.2 ± 65.0 a |
| AST [U/L] | 16.50 ± 4.55 NS | 30.22 ± 12.61 NS | 25.68 ± 16.59 NS | 26.03 ± 11.74 NS |
| ALT [U/L] | 4.33 ± 1.67 b | 9.86 ± 2.78 a | 10.50 ± 3.49 a | 9.76 ± 3.05 a |
| Total cholesterol [mg/dL] | 84.3 ± 5.8 b | 101.5 ± 6.3 a | 82.9 ± 20.4 b | 89.7 ± 13.8 ab |
| HDL-C [mg/dL] | 48.7 ± 18.5 a | 19.1 ± 5.3 b | 47.9 ± 11.5 a | 45.5 ± 14.6 a |
| LDL-C [mg/dL] | 9.15 ± 2.76 b | 13.67 ± 5.48 a | 8.15 ± 2.66 b | 8.31 ± 1.97 b |
| Triglycerides [mg/dL] | 74.7 ± 3.5 NS | 96.9 ± 13.2 NS | 96.2 ± 33.6 NS | 91.1 ± 10.0 NS |
| Uric acid [mg/dL] | 5.30 ± 0.35 b | 6.72 ± 0.90 a | 6.67 ± 1.76 b | 6.33 ± 0.98 ab |
| Urea BUN [mg/dL] | 36.9 ± 7.8 b | 69.6 ± 15.6 a | 62.2 ± 13.0 a | 58.1 ± 16.1 a |
| Parameter/Group | NDM | T1DM | SIL50 | SIL100 |
|---|---|---|---|---|
| Total protein [mg/g] | 563.2 ± 99.7 NS | 536.4 ± 123.5 NS | 487.3 ± 112.1 NS | 521.6 ± 69.9 NS |
| Soluble protein [mg/g] | 443.8 ± 25.2 NS | 408.5 ± 39.2 NS | 438.7 ± 11.8 NS | 446.0 ± 43.3 NS |
| Total/soluble protein ratio | 1.28 ± 0.27 NS | 1.32 ± 0.34 NS | 1.11 ± 0.27 NS | 1.18 ± 0.20 NS |
| Parameter/Group | NDM | T1DM | SIL50 | SIL100 |
|---|---|---|---|---|
| Serum | ||||
| MDA [nmol/mL] | 11.06 ± 1.39 b | 33.94 ± 15.98 a | 30.11 ± 10.84 a | 28.70 ± 6.56 a |
| AOPP [μmol/L] | 23.46 ± 2.49 NS | 38.39 ± 9.59 NS | 38.58 ± 22.73 NS | 33.16 ± 11.20 NS |
| AGEs [μg/mL] | 6.59 ± 1.55 b | 9.12 ± 0.75 a | 6.89 ± 0.49 b | 7.49 ± 1.71 b |
| Lenses | ||||
| MDA [nmol/g] | 5.89 ± 0.27 b | 7.48 ± 0.94 a | 6.29 ± 0.66 b | 6.17 ± 0.95 b |
| AOPP [nmol/mg of protein] | 3.72 ± 0.41 b | 4.69 ± 0.94 a | 2.43 ± 0.50 c | 2.62 ± 0.51 c |
| PCG [nmol/mg of protein] | 0.020 ± 0.003 b | 0.027 ± 0.005 a | 0.027 ± 0.006 a | 0.020 ± 0.004 b |
| G6PD [U/mg of protein] | 0.040 ± 0.006 NS | 0.029 ± 0.011 NS | 0.035 ± 0.015 NS | 0.041 ± 0.015 NS |
| GR [nmol/min/mg of protein] | 0.51 ± 0.19 NS | 0.33 ± 0.10 NS | 0.43 ± 0.22 NS | 0.34 ± 0.10 NS |
| Vitamin C [μg/g] | 7.69 ± 0.14 a | 7.17 ± 0.06 b | 7.23 ± 0.09 b | 7.14 ± 0.11 b |
| AGEs [μg/g] | 7.47 ± 1.88 b | 10.83 ± 0.49 a | 11.01 ± 1.31 a | 9.91 ± 0.82 a |
| Parameter/Group | NDM | T1DM | SIL50 | SIL100 |
|---|---|---|---|---|
| Glucose [μmol/g] | 0.90 ± 0.60 b | 4.63 ± 1.11 a | 4.98 ± 1.18 a | 5.09 ± 0.73 a |
| AR [nmol/min/mg of protein] | 0.086 ± 0.011 b | 0.107 ± 0.021 a | 0.109 ± 0.013 a | 0.123 ± 0.029 a |
| Sorbitol [μmol/g] | 1.23 ± 0.15 c | 30.62 ± 1.85 ab | 29.67 ± 2.85 b | 31.52 ± 1.66 a |
| SDH [μU/mg of protein] | 1.97 ± 0.46 c | 2.93 ± 0.87 b | 2.41 ± 0.56 bc | 3.81 ± 1.13 a |
| Fructose [μmol/g] | 0.076 ± 0.002 c | 0.135 ± 0.007 ab | 0.142 ± 0.008 a | 0.132 ± 0.015 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borymska, W.; Zych, M.; Dudek, S.; Kaczmarczyk-Sedlak, I. Silymarin from Milk Thistle Fruits Counteracts Selected Pathological Changes in the Lenses of Type 1 Diabetic Rats. Nutrients 2022, 14, 1450. https://doi.org/10.3390/nu14071450
Borymska W, Zych M, Dudek S, Kaczmarczyk-Sedlak I. Silymarin from Milk Thistle Fruits Counteracts Selected Pathological Changes in the Lenses of Type 1 Diabetic Rats. Nutrients. 2022; 14(7):1450. https://doi.org/10.3390/nu14071450
Chicago/Turabian StyleBorymska, Weronika, Maria Zych, Sławomir Dudek, and Ilona Kaczmarczyk-Sedlak. 2022. "Silymarin from Milk Thistle Fruits Counteracts Selected Pathological Changes in the Lenses of Type 1 Diabetic Rats" Nutrients 14, no. 7: 1450. https://doi.org/10.3390/nu14071450
APA StyleBorymska, W., Zych, M., Dudek, S., & Kaczmarczyk-Sedlak, I. (2022). Silymarin from Milk Thistle Fruits Counteracts Selected Pathological Changes in the Lenses of Type 1 Diabetic Rats. Nutrients, 14(7), 1450. https://doi.org/10.3390/nu14071450






