Leptin Levels of the Perinatal Period Shape Offspring’s Weight Trajectories through the First Year of Age
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patients
2.2. Procedures
2.3. Statistical Analysis
2.4. Study Endpoints
3. Results
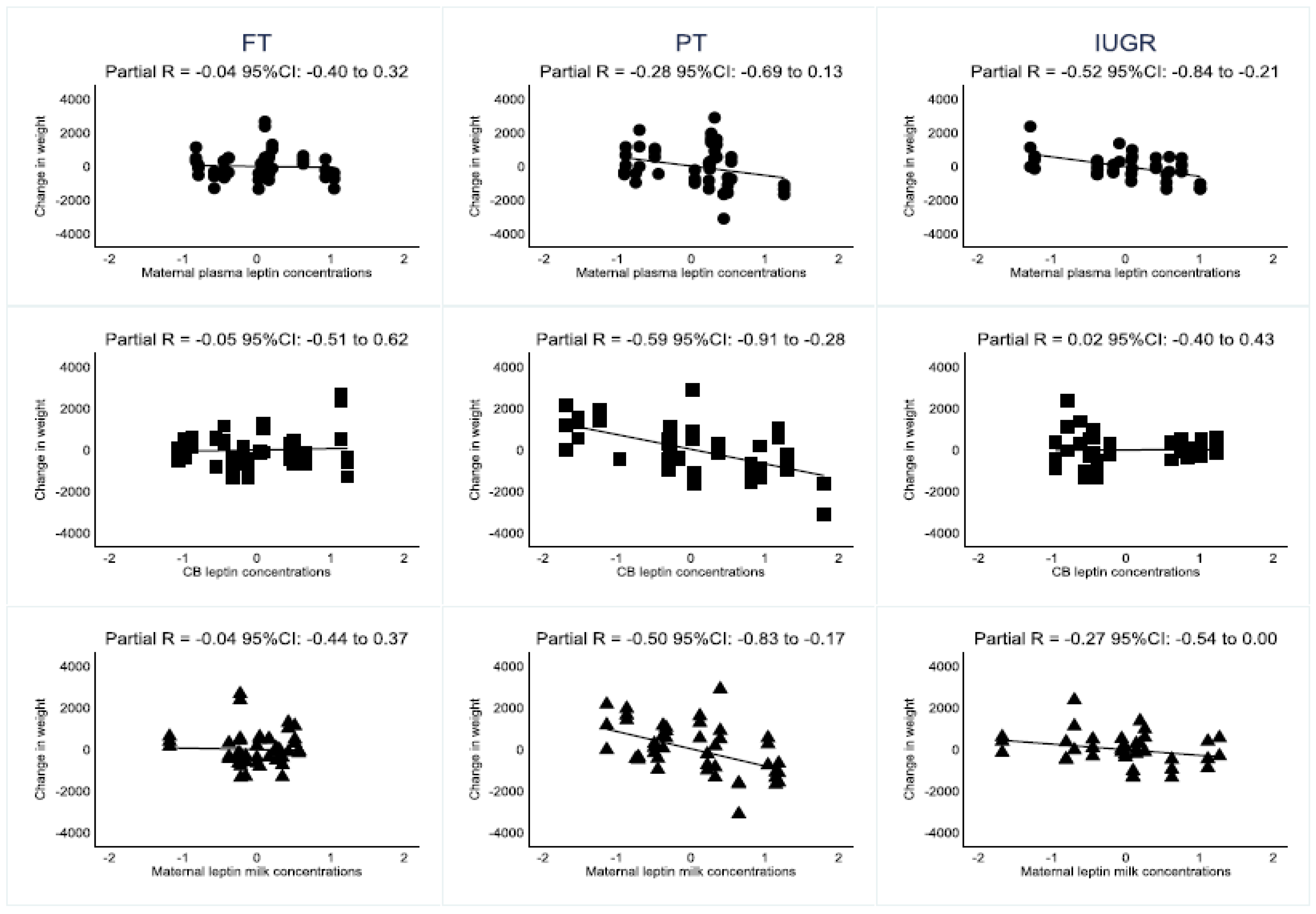
3.1. Association of Leptin Concentrations and Neonate’s Weight Trajectories
3.2. Association of Leptin Concentrations and Neonate’s Subgroups Weight Trajectories
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Picó, C.; Palou, A. Leptin as a breast milk component for the prevention of obesity. Nutr. Rev. 2018, 76, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, A.A.; Khaire, A.A. Leptin as a predictive marker for metabolic syndrome. Cytokine 2019, 121, 154735. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.F.; McAinch, A.J.; Romano, T.; Wlodek, M.E.; Hryciw, D.H. Leptin in pregnancy and development: A contributor to adulthood disease? Am. J. Physiol. Endocrinol. Metab. 2015, 308, E335–E350. [Google Scholar] [CrossRef]
- Weyermann, M.; Beermann, C.; Brenner, H.; Rothenbacher, D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin. Chem. 2006, 52, 2095–2102. [Google Scholar] [CrossRef]
- Stefaniak, M.; Dmoch-Gajzlerska, E.; Mazurkiewicz, B.; Gajzlerska-Majewska, W. Maternal serum and cord blood leptin concentrations at delivery. PLoS ONE 2019, 7, e0224863. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Rifas-Shiman, S.L.; Williams, C.J.; Fargnoli, J.L.; Kelesidis, T.; Gillman, M.W. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: A prospective cohort study. Pediatrics 2009, 123, 682–689. [Google Scholar] [CrossRef]
- Chaoimh, C.N.; Murray, D.M.; Kenny, L.C.; Irvine, A.D.; Hourihane, J.O.; Kiely, M. Cord blood leptin and gains in body weight and fat mass during infancy. Eur. J. Endocrinol. 2016, 175, 403–410. [Google Scholar] [CrossRef][Green Version]
- Karakosta, P.; Chatzi, L.; Plana, E.; Margioris, A.; Castanas, E.; Kogevinas, M. Leptin levels in cord blood and anthropometric measures at birth: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2011, 25, 150–163. [Google Scholar] [CrossRef]
- Bagias, C.; Sukumar, N.; Weldeselassie, Y.; Oyebode, O.; Saravanan, P. Cord Blood Adipocytokines and Body Composition in Early Childhood: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health. 2021, 18, 1897. [Google Scholar] [CrossRef]
- Ren, R.X.; Shen, Y. A meta-analysis of relationship between birth weight and cord blood leptin levels in newborns. World J. Pediatr. 2010, 15, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Hechler, C.; Gebauer, C.; Kiess, W.; Kratzsch, J. Leptin in maternal serum and breast milk: Association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr. Res. 2011, 70, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Worthington, C.R.; Bahorski, J.S.; Fields, D.A.; Gower, B.A.; Fernández, J.R.; Chandler-Laney, P.C. Associations Among Maternal Adiposity, Insulin, and Adipokines in Circulation and Human Milk. J. Hum. Lact. 2021, 37, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Grieger, J.A.; Hutchesson, M.J.; Cooray, S.D.; Bahri Khomami, M.; Zaman, S.; Segan, L.; Teede, H.; Moran, L.J. A review of maternal overweight and obesity and its impact and cardiometabolic outcomes during pregnancy and postpartum. Ther. Adv. Reprod. Health 2021, 15, 2633494120986544. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lamb, K.E.; Grimes, C.; Laws, R.; Bolton, K.; Ong, K.K.; Campbellk, K. Rapid weight gain during infancy and subsequent adiposity: A systematic review and meta-analysis of evidence. Obesity Rev. 2018, 19, 321–332. [Google Scholar] [CrossRef]
- Yajnik, C.S. Transmission of obesity-adiposity and related disorders from the mother to the baby. Ann. Nutr. Metab. 2014, 64, 8–17. [Google Scholar] [CrossRef]
- Kapur, A. Links between maternal health and NCDs. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 2–42. [Google Scholar] [CrossRef]
- Kratzsch, J.; Bae, Y.J.; Kiess, W. Adipokines in human breast milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 27–38. [Google Scholar] [CrossRef]
- Savino, F.; Sardo, A.; Rossi, L.; Benetti, S.; Savino, A.; Silvestro, L. Mother and Infant Body Mass Index, Breast Milk Leptin and Their Serum Leptin Values. Nutrients 2016, 8, 383. [Google Scholar] [CrossRef]
- Garofoli, F.; Mazzucchelli, I.; Angelini, M.; Klersy, C.; Tinelli, C.; Carletti, G.V.; Calcaterra, V.; Gardella, B.; Tzialla, C. The dynamical interplay of perinatal leptin with birthweight and 3-month weight, in full-term, preterm, IUGR mother-infant dyads. J. Matern. Fetal Neonatal Med. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- De Knegt, V.E.; Hedley, P.L.; Kanters, J.K.; Thagaard, I.N.; Krebs, L.; Christiansen, M.; Lausten-Thomsen, U. The Role of Leptin in Fetal Growth during Pre-Eclampsia. Int. J. Mol. Sci. 2021, 22, 4569. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; Byrne, J.; Mahony, R.M.; Foley, M.E.; McAuliffe, F.M. Leptin, fetal growth and insulin resistance in non-diabetic pregnancies. Early Hum. Dev. 2014, 90, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Hamilton, J.K. Effect of maternal weight, adipokines, glucose intolerance and lipids on infant birth weight among women without gestational diabetes mellitus. Can. Med. Assoc. J. 2012, 184, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.M.; Sichieri, R.; Moreira, M.E.; Moura, A.S. Early postnatal growth in preterm infants and cord blood leptin. J. Perinatol. 2004, 24, 751–756. [Google Scholar] [CrossRef]
- Karakosta, P.; Roumeliotaki, T.; Chalkiadaki, G.; Sarri, K.; Vassilaki, M.; Venihaki, M.; Malliaraki, N.; Kampa, M.; Castanas, E.; Kogevinas, M.; et al. Cord blood leptin levels in relation to child growth trajectories. Metabolism 2016, 65, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Steinbrekera, B.; Colaizy, T.T.; Vasilakos, L.K.; Johnson, K.J.; Santillan, D.A.; Haskell, S.E.; Roghair, R.D. Origins of neonatal leptin deficiency in preterm infants. Pediatr. Res. 2019, 85, 1016–1023. [Google Scholar] [CrossRef]
- Aydin, S.; Ozkan, Y.; Erman, F.; Gurates, B.; Kilic, N.; Colak, R.; Gundogan, T.; Catak, Z.; Bozkurt, M.; Akin, O.; et al. Presence of obestatin in breast milk: Relationship among obestatin, ghrelin, and leptin in lactating women. Nutrition 2008, 24, 689–693. [Google Scholar] [CrossRef]
- Dundar, N.O.; Anal, O.; Dundar, B.; Ozkan, H.; Caliskan, S.; Büyükgebiz, A. Longitudinal investigation of the relationship between breast milk leptin levels and growth in breast-fed infants. J. Pediatr. Endocrinol. Metab. 2005, 2, 181–187. [Google Scholar] [CrossRef]
- Andreas, N.J.; Hyde, M.J.; Herbert, B.R.; Jeffries, S.; Santhakumaran, S.; Mandalia, S.; Holmes, E.; Modi, N. Impact of maternal BMI and sampling strategy on the concentration of leptin, insulin, ghrelin and resistin in breast milk across a single feed: A longitudinal cohort study. BMJ Open 2016, 6, e010778. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Giannì, M.L.; Morniroli, D.; Leone, L.; Roggero, P.; Agostoni, C.; De Cosmi, V.; Mosca, F. Hormones in Breast Milk and Effect on Infants’ Growth: A Systematic Review. Nutrients 2019, 11, 1845. [Google Scholar] [CrossRef]
- Joung, K.E.; Martin, C.R.; Cherkerzian, S.; Kellogg, M.; Belfort, M.B. Human Milk Hormone Intake in the First Month of Life and Physical Growth Outcomes in Preterm Infants. J. Clin. Endocrinol. Metab. 2021, 106, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Arisak, O.; Ichikawa, G.; Koyama, S.; Sairenchi, T. Childhood obesity: Rapid weight gain in early childhood and subsequent cardiometabolic risk. Clin. Pediatr. Endocrinol. 2020, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Raspini, B.; Porri, D.; De Giuseppe, R.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; Monti, M.C.; Vacca, M.; et al. Prenatal and postnatal determinants in shaping offspring’s microbiome in the first 1000 days: Study protocol and preliminary results at one month of life. Ital. J. Pediatr. 2020, 6, 45. [Google Scholar] [CrossRef]
- Van den Elsen, L.W.J.; Verhasselt, V. Human Milk Drives the Intimate Interplay Between Gut Immunity and Adipose Tissue for Healthy Growth. Front. Immunol. 2021, 2, 645415. [Google Scholar] [CrossRef]
| FT (n = 16) | PT (n = 16) | IUGR (n = 13) | p-Value | Post Hoc Comparison p-Value—Bonferroni | |
|---|---|---|---|---|---|
| Maternal age, years # | 31 (30–32) | 32 (29–36) | 32 (30–37) | 0.377 | - |
| Cesarean section delivery § | 1 (6.3%) | 11 (68.8%) | 10 (76.9%) | <0.001 | PT vs. FT: 0.002 |
| IUGR vs. FT: <0.001 | |||||
| IUGR vs. PT: >0.90 | |||||
| Maternal BMI before pregnancy * | 23.5 (3.65) (range 16.5–29.8) | 23.5 (3.98) (range 18.7–30.48) | 23 (3.68) (range 19.0–31.2) | 0.814 | - |
| Maternal BMI at delivery * | 28 (3.63) (range 20.8–33.2) | 27.5 (4.0) (range 20.8–35.0) | 26.0 (3.35) (range 220–33.6) | 0.228 | - |
| Male neonate § | 8 (50%) | 7 (43.8%) | 5 (38.5%) | 0.929 | - |
| Gestational Age, weeks # | 40.5 (39.4–41.0) | 33.7 (29.7–34.6) | 36.6 (32.1–37.3) | <0.001 | PT vs. FT: <0.001 |
| IUGR vs. FT: <0.001 | |||||
| IUGR vs. PT: 0.217 | |||||
| APGAR 1′ | 10 (9–10) | 7.5 (5.5–9) | 9 (6–9) | <0.001 | PT vs. FT: <0.001 |
| IUGR vs. FT: 0.027 | |||||
| IUGR vs. PT: 0.304 | |||||
| APGAR 5′ | 10 (10–10) | 9 (8–10) | 10 (7–10) | 0.022 | PT vs. FT: 0.003 |
| IUGR vs. FT: 0.100 | |||||
| IUGR vs. PT: 0.402 | |||||
| Maternal plasmatic leptin concentration pg/mL # | 44,473.5 (22,214.5–50,302.4) | 75,575.2 (27,219.9–89,243.3) | 71,768.5 (41,890.1–12,1378.1) | 0.213 | - |
| Maternal milk leptin concentration pg/mL # | 620.6 (462.3–881.6) | 622.0 (329.7–1235.0) | 844.3 (444.9–1008.9) | 0.782 | - |
| CB leptin concentration pg/mL # | 19,280.5 (11,800.8–32,894.8) | 3958.1 (1982.9–11,545.6) | 1588.8 (1223.1–6912.0) | <0.001 | PT vs. FT: <0.001 |
| IUGR vs. FT: <0.001 | |||||
| IUGR vs. PT: 0.632 | |||||
| Birth weight g # | 3242.5 (3022.5–3585.0) | 1802.5 (1367.5–2203.5) | 1770.0 (1265.0–2265.0) | <0.001 | PT vs. FT: <0.001 |
| IUGR vs. FT: <0.001 | |||||
| IUGR vs. PT: >0.90 | |||||
| Change in weight g # (increase at 12 months) | 6135 (5893–7110) | 7062 (6275–8565) | 6295 (5970–6955) | 0.272 | |
| % Change in weight (increase at 12 months) | 191.7 (183.4–224.8) | 374.9 (288.9–564.6) | 341.6 (288.5–549.8) | <0.001 | PT vs. FT: <0.001 |
| IUGR vs. FT: <0.001 | |||||
| IUGR vs. PT: >0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garofoli, F.; Mazzucchelli, I.; Angelini, M.; Klersy, C.; Ferretti, V.V.; Gardella, B.; Carletti, G.V.; Spinillo, A.; Tzialla, C.; Ghirardello, S. Leptin Levels of the Perinatal Period Shape Offspring’s Weight Trajectories through the First Year of Age. Nutrients 2022, 14, 1451. https://doi.org/10.3390/nu14071451
Garofoli F, Mazzucchelli I, Angelini M, Klersy C, Ferretti VV, Gardella B, Carletti GV, Spinillo A, Tzialla C, Ghirardello S. Leptin Levels of the Perinatal Period Shape Offspring’s Weight Trajectories through the First Year of Age. Nutrients. 2022; 14(7):1451. https://doi.org/10.3390/nu14071451
Chicago/Turabian StyleGarofoli, Francesca, Iolanda Mazzucchelli, Micol Angelini, Catherine Klersy, Virginia Valeria Ferretti, Barbara Gardella, Giulia Vittoria Carletti, Arsenio Spinillo, Chryssoula Tzialla, and Stefano Ghirardello. 2022. "Leptin Levels of the Perinatal Period Shape Offspring’s Weight Trajectories through the First Year of Age" Nutrients 14, no. 7: 1451. https://doi.org/10.3390/nu14071451
APA StyleGarofoli, F., Mazzucchelli, I., Angelini, M., Klersy, C., Ferretti, V. V., Gardella, B., Carletti, G. V., Spinillo, A., Tzialla, C., & Ghirardello, S. (2022). Leptin Levels of the Perinatal Period Shape Offspring’s Weight Trajectories through the First Year of Age. Nutrients, 14(7), 1451. https://doi.org/10.3390/nu14071451






