Abstract
(1) Background: To investigate the association between plasma fatty acids (FAs) and dry eye disease (DED) in an elderly population; (2) Methods: We conducted a population-based study, the Montrachet study, in individuals older than 75 years. DED was evaluated using the Schirmer I test without anesthesia, tear film breakup time (TFBUT) measurement and fluorescein corneal staining. Plasma FAs were measured in fasting blood using gas chromatography; (3) Results: A total of 740 subjects with a plasma measurement of 25 FAs were included in this study. The mean age was 82.2 ± 3.7 years, and 62.7% were women. DED was present in 35.0% of participants. We identified a plasma FAs pattern positively associated with DED, characterized by low polyunsaturated fatty acids (PUFAs), high monounsaturated fatty acids (MUFAs) and low saturated fatty acids (SFAs) levels. After adjustment for major confounders, individuals in the upper quartile of the FAs pattern scores compared with those in the lower quartile were more likely to present DED (OR 2.46 (95% CI 1.51–4.01), p = 0.001); (4) Conclusion: In this study, we found that a plasma FAs pattern characterized by low PUFAs, high MUFAs and low SFAs was significantly associated with DED in elderly participants.
1. Introduction
Dry eye disease (DED) is one of the most common ocular surface diseases in the elderly [1]. DED prevalence rates range from 4.3% to 34.5%, depending on the age group and definition [2,3,4,5,6]. DED leads to discomfort and visual disturbances, impacting the quality of life of patients [7]. DED presents with two types of clinical manifestations: tear deficiency dry eye and evaporative dry eye, which leads to tear impairment and tear lipid layer instability [8]. These disturbances are more frequent in women than in men [9]. Several risk factors were shown to be associated with DED, including age, gender, smoking, autoimmune diseases, corneal dysfunction, menopausal status and environmental factors such as light and sun exposure and some medications [3,8].
Oxidative stress has been hypothesized to play a key role in DED by producing reactive oxygen species that induce damage on corneal and conjunctival epithelial cells and promote ocular surface inflammation [10,11]. Hyperosmolarity and inflammation also play a key role in the pathogenesis of DED, making DED to be considered as a chronic inflammatory disease [12]. Among inflammatory mediators, polyunsaturated fatty acids (PUFAs) have been associated with different ocular diseases involving inflammation pathways [13,14]. PUFAs have been used through dietary, systemic or topical delivery as a potential treatment of DED and appeared to be effective on both signs and symptoms of DED [15,16,17]. Indeed, a higher dietary intake of n-3 PUFAs was associated with the decrease of DED symptoms, while an unbalanced n-6 to n-3 PUFAs ratio was associated with a higher risk of DED [18]. However, as DED is a multifactorial disease, the potential role of plasma fatty acids (FAs) should be considered among other risk factors, especially in the elderly population [19,20].
Therefore, we aimed to investigate the association of plasma FAs and DED in a population-based study of participants aged 75 years and over and then to identify a FAs pattern more prone to be associated with DED.
2. Methods
2.1. Population Study
The Montrachet study (Maculopathy Optic Nerve and nuTRition neurovAsCular and HEarT) is an ancillary study of the population-based Three Cities (3C) study, which has previously been described [21]. Briefly, the 3C cohort study was undertaken to assess the relationship between vascular risk factors and aging disorders. Overall, 9294 persons aged 65 years and over, selected from the electoral rolls of three French urban cities (Bordeaux, Dijon and Montpellier), were included (n = 4931 living in Dijon). Ten years later, the subgroup of participants from Dijon were asked to participate in the Montrachet study in order to assess associations between age-related eye disorders and neurologic and heart diseases in the elderly. The methodology of the Montrachet study and the baseline characteristics of participants have already been thoroughly described [22].
A comprehensive ocular surface assessment (namely fluorescein tear film breakup time (TFBUT), Schirmer I test and fluorescein corneal staining) was performed [23], and plasma FAs were measured. Subjects with one parameter missing were excluded. Our main variable of interest was the presence of DED. Secondary variables of interest corresponded to plasma FAs composition. Covariates, including demographic, lifestyle, clinical and treatment variables, were documented in another recent paper [6]. More specifically, oral medication use (anxiolytic drugs and global psychotropics) was defined from an anatomic therapeutic chemical classification for drug use. Written informed consent was obtained from all participants. The study followed the tenets of the Declaration of Helsinki and was approved by the regional ethics committee and registered as 2009-A00448-49.
2.2. DED Evaluation and Definition
The evaluation and definition of DED signs were previously described in details [6]. In the present study, we only used clinical signs to define DED subjects, because signs and symptoms of DED are poorly correlated [24,25]. Briefly, participants presenting two out of the three following signs were considered as having DED: positive corneal staining, TFBUT < 5 s or Schirmer I test < 5 mm
2.3. Plasma Lipids Measurement
Lipids were extracted from plasma samples from fasted volunteers according to Moilanen and Nikkari [26]. Lipid extracts were stored under inert gas until further analyses. Total lipids from plasma were transmethylated using boron trifluoride in methanol according to Morrison and Smith [27]. Fatty acid methyl esters (FAMEs) were subsequently extracted with hexane and analyzed on a Hewlett Packard Model 5890 gas chromatograph (Palo Alto, CA, USA) using a CPSIL-88 column (100 m × 0.25 mm i.d., film thickness 0.20 µm) (Varian, Les Ulis, France) equipped with a flame ionization detector. Hydrogen was used as carrier gas (inlet pressure 210 kPa). The oven temperature was held at 60 °C for 5 min, increased to 165 °C at 15 °C/min and held for 1 min, and then, it was increased to 225 °C at 2 °C/min and finally held at 225 °C for 17 min. The injector and the detector were maintained at 250 °C. FAMEs were identified by comparison with commercial and synthetic standards. The data were processed using the EZChrom Elite software (Agilent Technologies, Massy, France) and reported as a percentage of the total FAs. For analysis, 25 plasma FAs (saturated, monounsaturated and polyunsaturated) levels were considered. Long-chain FAs were synthesized through desaturation and elongation; then, enzymatic activities indexes were estimated through the calculation of the precursor to product ratios of individuals FAs as follows: Δ5 desaturase (C20:4 n-6 to C20:3 n-6) and ELOVL2/5 (C22:4 n-6 to C20:4 n-6 and C22:5 n-3 to C20:5 n-3).
2.4. Statistics
Continuous variables were expressed as the mean (SD) or median (interquartile range) according to their distribution and categorical variables as a number (%). Bivariate comparisons were performed with Student’s t-test or ANOVA test for continuous variables and Pearson’s Chi-squared or Fisher’s exact tests for categorical variables when appropriate. To assess a potential non-responders bias, we compared characteristics of participants and non-participants. In order to identify the FAs pattern associated with the presence of DED, the analysis was performed in two steps. First, we used the partial least squares regression generalized linear models method (PLS) [28]. Similar to a principal component analysis (PCA), PLS is a dimension reduction method that extracts a set of orthogonal factors called latent variables, which are used as predictors in the regression model [29,30]. PLS aims at reducing the dimension of predictor variables Xi (i.e., concentrations of 25 FAs) with the constraint of maximizing the covariance between Xi and response variable Y (i.e., the presence of DED). We retained the PLS component that was significantly associated with the presence of DED [31]. The identified FAs pattern was constructed further to a score by weighting each FA concentration with factor weight values. Individual factor scores were then categorized into quartiles. To interpret the FA pattern, we kept those FAs with absolute values of weights ≥ 0.20 [32]. In the first step of analysis, we performed a distribution of socio-demographics, lifestyle and medication use by quartiles of the FAs pattern scores. Second, a multivariable logistic regression analysis expressed as ORs and their 95% confidence interval was performed to determine whether the FAs pattern scores were significantly associated with the presence of DED. The lowest quartile of FAs pattern scores was defined as the reference group. Models were systematically adjusted for age and sex. A stepwise procedure was used to select a final model after adjustment for age, sex, educational level, iris color, best-corrected visual acuity (BCVA), age-related macular degeneration (AMD), systemic hypertension, anxiolytics, antihistamine eye drops, lipid-lowering drugs use, plasma HDL cholesterol and triglycerides.
Finally, in order to evaluate the contribution of our plasma FAs pattern approach, we performed an analysis between clinically relevant FAs considered individually and DED using multivariable logistic regression models. For all analyses, the tests were two-sided, and results were considered significant when p < 0.05. Analyses were performed using SAS software (version 9.4; SAS institute Inc.; Cary, NC, USA) and R version 3.4.2 (plsRglm package) (http://www.R-project.org/, accessed on 20 April 2022).
3. Results
3.1. Demographic, Lifestyle and Clinical Characteristics of Participants
Among the 1153 participants of the Montrachet study, 740 subjects (mean [SD] age 82.2 [3.7] years, 62.7% women) had available clinical data and plasma FAs data for analysis (Fig.). The participants and non-participants presented the same demographic, lifestyle and clinical characteristics (Table 1). DED according to our definition (clinical sign-based DED) was present in 35.0% [95% CI 31.6–38.4] of participants. Table 2 presents the characteristics of participants and the age- and sex-adjusted associations according to DED presence. Participants with a short secondary school level, with a dark iris, using antianxiolytic drugs and with artificial tears were more likely to present DED, whereas participants with AMD were less likely to present DED.

Table 1.
Comparison of participants and non-participants for the association between plasma fatty acids and dry eye disease in the Montrachet Study, n = 1153.

Table 2.
Characteristics of participants and age- and sex-adjusted associations according to the presence of dry eye disease, n = 740.
3.2. Plasma Fatty Acids and DED
Table 3 presents the levels of plasma FAs and DED at inclusion (percentage of the total fatty acids). The highest levels of FAs found in the study participants were linoleic acid C18:2 n-6 (25.2%), palmitic acid C16:0 (22.3%), oleic acid C18:1 n-9 (21.4%) and arachidonic acid C20:4n-6 (AA, 7.7%). In the bivariate analysis, no difference in plasma FAs concentrations was found between subjects with and without DED. We identified a plasma FAs pattern associated with the presence of DED. This pattern explained 10.7% of the total variance in the original set of FAs and displayed high negative weights of gamma-linoleic acid (GLA, C18:3 n-6), arachidonic acid (AA, C20:4 n-6) and eicosapentaenoic acid (EPA, C20:5 n-3) PUFAs and stearic acid SFAs (C18:0). These FAs concentrations decreased significantly across quartiles of pattern scores except GLA and AA. On the contrary, this pattern presented high positive weights of DGLA (C20:3 n-6), DTA (C22:4 n-6), DPA n-6 (C22:5 n-6) PUFAs, vaccenic acid (C18:1 n-7) and oleic acid (C18:1 n-9) MUFAs and behenic acid (C22:0) SFAs. The concentrations of these FAs increased significantly across the quartiles of pattern scores (Table 4 and Supplementary Materials Table S1).

Table 3.
Percentages of plasma fatty acids according to the presence of dry eye disease, n = 740.

Table 4.
Factor weight values of plasma fatty acids obtained by the partial least squares regression generalized linear models associated with the presence of dry eye disease in the Montrachet Study.
3.3. Demographic, Lifestyle and Medical Characteristics, DED and FAs Pattern Scores
The distribution of potential confounders by quartiles of the FAs pattern scores is shown in Table S2. Individuals in upper quartiles of pattern scores were more likely men, overweight, smokers, with AMD diagnosis, with more systemic drugs (diuretics, beta-blockers and lipid-lowering drug), a with higher plasma level of triglycerides and with lower plasma level of HDL cholesterol compared to those in lower quartiles.
Table 5 presents the associations of FAs pattern scores by quartiles and the presence of DED. We observed a positive and significant association between FAs pattern scores and DED (ORcrude 2.53 [95% CI 1.60–3.99], p-trend < 0.001). These findings remained statistically significant after controlling for age and sex (OR 2.45 [95% CI 1.55–3.88], p-trend < 0.001). Similar results were found after further adjustment for potential confounders (p-trend = 0.001). Indeed, individuals in the upper quartiles compared with those in the lower quartiles of FAs pattern scores were more likely to present DED (ORadj 2.46 [95% CI 1.51–4.01]). Finally, when FAs were considered individually, associations appeared weaker than the relation between the pattern score and DED (Table S3), suggesting that our pattern approach with the FAs combination captured more information for the prediction of DED.

Table 5.
Multivariable associations of plasma fatty acids pattern scores across quartiles and the presence of dry eye disease in the Montrachet study.
4. Discussion
While FAs plasma levels taken individually were not different in DED patients compared to non-DED subjects, we identified a plasma FAs pattern positively and significantly associated with DED: low concentrations of PUFAs (GLA, AA and EPA), high MUFAs (vaccenic and oleic acids) and low SFAs (i.e., stearic acid). Individuals in the upper quartiles of the FAs pattern scores were more likely to present DED than those in the lower quartiles. We found that the FAs combination depicted by this pattern was significantly associated with DED in our old population.
In a recent observational longitudinal study, the nutrients pattern characterized by low plasma PUFAs (LA, AA and EPA), low vitamin D and carotenes was significantly associated with a higher risk of dementia in elderly patients [33]. Observational studies have reported that n-3 PUFAs intake is implicated in the prevention of age-related macular degeneration [34,35]. A good balance between higher n-3 PUFAs and lower linoleic acid intakes could improve the incorporation rate in the neurosensory retina [36]. Moreover, a potential interaction between the complement system and lipoprotein homeostasis has been hypothesized [37]. In DED, the rationale for dietary recommendations with n-3 PUFAs is based on different features: (1) n-3 and n-6 PUFAs play an important role in inflammatory pathways [38,39]; (2) there is a competition between n-3 and n-6 long chain PUFAs metabolism with n-3 acting as competitor to decrease AA production [36]; (3) an increased production of inflammatory mediators like cytokines has been demonstrated in DED [40]. The American Academy of Ophthalmology and the guidelines from the DEWS still stated that nutritional supplementation with n-3 and n-6 PUFAs can be recommended in DED [17]. However these practices remain controversial: while a meta-analysis reported a discrete improvement in the parameters of tear function, a recent multicenter, double-blind clinical trial did not find better outcomes with n-3 fatty acid supplementation [41]. Indeed, as observed in age-related macular degeneration, lipid supplementation failed to demonstrate clinical efficacy in DED [42]. However, rather than the effect of one specific member of the PUFAs family, the anti-inflammatory effect in DED is probably the result of a subtle balance between three FAs that are precursors of bioactive molecules belonging to eicosanoids: dGLA, AA and EPA [18,43,44].
The FAs pattern in this study is characterized by low PUFAs, especially low plasma concentrations of GLA, AA and EPA, and high concentrations of dGLA. GLA, AA and EPA have been reported to have implications in the DED pathogenesis when considered individually [20,45,46]. Additionally, it has been shown that these PUFAs improved the clinical signs of DED in an experimental rat model [44]. LA and ALA are the primary metabolites of n-6 and n-3 PUFAs, respectively (Figure 1). They are further elongated into dGLA and EPA, the last ones being precursors of anti-inflammatory eicosanoids from series-1 and series-2, respectively [47]. A lower plasma levels of GLA found in DED subjects can result either from the reduction of LA metabolization into GLA by Δ6-desaturase or from an increased conversion of GLA into dGLA by ELOVL5. Whereas the involvement of Δ6-desaturase and ELOVL5 activities remain unclear regarding the levels of GLA, the increased concentrations of dGLA are probably subsequent to the known inhibitory action of EPA on Δ5-desaturase. It can result in the accumulation of dGLA in the detriment of AA. Such a pattern may have consequences on the synthesis of dGLA-, AA- and EPA-derived eicosanoids, by favoring the synthesis of anti-inflammatory series-1 eicosanoids (derived from dGLA). On the contrary, it limits anti-inflammatory synthesis series-3 and pro-inflammatory series-2 eicosanoids (derived from EPA and AA, respectively). As inflammation plays a key role in DED pathophysiology [40], one can hypothesize that the increased conversion of dGLA into anti-inflammatory eicosanoids is not sufficient to balance the effects of the AA-derived pro-inflammatory eicosanoids. However, further investigations are needed.
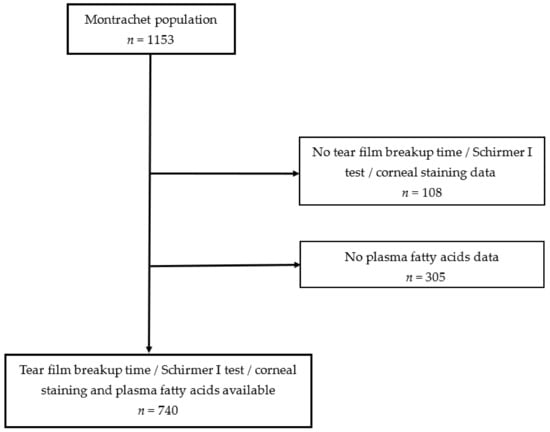
Figure 1.
Workflow of fatty acids and dry eye disease in the Montrachet study.
Apart from the main PUFAs associated with DED above, our FAs pattern was also characterized by low plasma stearic acid, which has not been reported before, followed by high oleic acid and high vaccenic acid levels. These FAs are mainly dependent on a rich saturated and monounsaturated intake. The effect of SFAs on inflammation remain controversial [48,49]. The inverse association between stearic acid and inflammation had already been reported in young adults in whom circulating stearic acid was inversely associated with high-sensitivity C-reactive protein [50]. Indeed, a recent study conducted with in vitro and in vivo models of joint inflammation, showed anti-inflammatory effects of copolymers of palmitic, oleic and stearic acids on cartilage disease [51].
The statistically significant association between DED and AMD presented in Table 2 (p = 0.037) was not reported in other population-based studies. Besides the age-related nature of both conditions, AMD and DED, we do not think this represents a relevant clinical association.
We acknowledge several limitations in this study. First, the cross-sectional design of the study did not allow us to determine longitudinal associations between alterations of plasma FAs levels and DED. Second, the corneal staining was not measured quantitatively as in the Oxford classification. Third, some biological markers assessment could have been of special interest, for example, the PGE1 essay or enzyme expression analysis through an mRNA assay, but they were not explored in our study. Fourth, these results were found in our urban and healthy elderly population and cannot be generalized to other populations.
The strengths of this study include its large population sample size on the population-based design. Second, major confounding factors were taken into account in the multivariable analysis. Third, we investigated the association of a combination of plasma FAs with DED in this elderly population, while the previous studies were often based on specific FAs.
5. Conclusions
In conclusion, our work highlights that a plasma FAs pattern characterized by low PUFAs (GLA, AA, EPA), high MUFAs (vaccenic and oleic acids) and low saturated FAs (i.e., stearic acid) was significantly associated with DED in our elderly population after taking into account major known confounding factors. We showed that the PUFAs metabolism, as expressed by their estimated enzymatic activities indexes, has a clinical implication in DED.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14112290/s1: Table S1. Mean percentages of fatty acids by quartiles of fatty acids pattern scores (n = 740). Table S2. Demographics and lifestyle characteristics of study participants and fatty acids pattern scores distribution (n = 740). Table S3. Associations of plasma fatty acids and Dry Eye Disease in the Montrachet Study.
Author Contributions
Conceptualization, L.A., C.C.-G. and N.A.; methodology, A.M.B.; validation, formal analysis, A.S.; investigation, A.S. and F.B.; resources, C.C.-G.; writing—original draft preparation, L.A., A.S. and P.-H.G.; writing—review and editing, F.B. and I.B.G.; supervision, A.M.B., C.C.-G. and N.A.; project administration, C.C.-G.; funding acquisition, C.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by an inter-regional grant (PHRC) and the regional Council of Burgundy. This study was also funded by INRAE, CNRS, Burgundy-Franche-Comté University, Regional Council of Burgundy, France (PARI Agrale 1), FEDER (European Funding for Regional Economic Development), a French Government grant managed by the French National Research Agency (ANR) under the ‘‘Investissements d’Avenir’’ program with reference ANR-11-LABX-0021-01-LipSTIC Labex and Fondation de France/Fondation de l’Oeil. The funding organizations had no role in the design or conduct of this research. This study has also received financial support from Laboratoires Théa (Clermont-Ferrand, France). Laboratoires Théa did not participate in the design of the study, data collection, statistical analysis, interpretation of the data nor in the writing of the manuscript.
Institutional Review Board Statement
The study followed the tenets of the Declaration of Helsinki and was approved by the regional ethics committee (3City Study Committee) and registered as 2009-A00448-49.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data are available on reasonable request. A 3C study data agreement request should be sent to louis.arnould@chu-dijon.fr.
Acknowledgments
The authors thank Christine Binquet, Emilie Galizzi, Vanessa Cottet and Sandrine Daniel from the CIC-EC 1432 Dijon University of Burgundy for their methodological support and precious skills in the data management for the Montrachet study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schaumberg, D.A.; Sullivan, D.A.; Buring, J.E.; Dana, M.R. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003, 136, 318–326. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Dana, R.; Buring, J.E.; Sullivan, D.A. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Schein, O.D.; Muñoz, B.; Tielsch, J.M.; Bandeen-Roche, K.; West, S. Prevalence of dry eye among the elderly. Am. J. Ophthalmol. 1997, 124, 723–728. [Google Scholar] [CrossRef]
- Lee, A.J.; Lee, J.; Saw, S.-M.; Gazzard, G.; Koh, D.; Widjaja, D.; Tan, D.T. Prevalence and risk factors associated with dry eye symptoms: A population based study in Indonesia. Br. J. Ophthalmol. 2002, 86, 1347–1351. [Google Scholar] [CrossRef]
- Ferrero, A.; Alassane, S.; Binquet, C.; Bretillon, L.; Acar, N.; Arnould, L.; Muselier-Mathieu, A.; Delcourt, C.; Bron, A.M.; Creuzot-Garcher, C. Dry eye disease in the elderly in a French population-based study (the Montrachet study: Maculopathy, Optic Nerve, nuTRition, neurovAsCular and HEarT diseases): Prevalence and associated factors. Ocul. Surf. 2018, 16, 112–119. [Google Scholar] [CrossRef]
- Miljanović, B.; Dana, R.; Sullivan, D.A.; Schaumberg, D.A. Impact of dry eye syndrome on vision-related quality of life. Am. J. Ophthalmol. 2007, 143, 409–415. [Google Scholar] [CrossRef]
- The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 93–107. [CrossRef]
- Maïssa, C.; Guillon, M. Tear film dynamics and lipid layer characteristics-effect of age and gender. Cont. Lens Anterior Eye 2010, 33, 176–182. [Google Scholar] [CrossRef]
- Uchino, Y.; Kawakita, T.; Miyazawa, M.; Ishii, T.; Onouchi, H.; Yasuda, K.; Ogawa, Y.; Shimmura, S.; Ishii, N.; Tsubota, K. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS ONE 2012, 7, e45805. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Benlian, P.; Puche, N.; Bassols, A.; Delcourt, C.; Souied, E.H. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2010–2019. [Google Scholar] [CrossRef]
- Li, Z.; Choi, J.-H.; Oh, H.-J.; Park, S.-H.; Lee, J.-B.; Yoon, K.C. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr. Eye Res. 2014, 39, 871–878. [Google Scholar] [CrossRef]
- Rand, A.L.; Asbell, P.A. Nutritional supplements for dry eye syndrome. Curr. Opin. Ophthalmol. 2011, 22, 279–282. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar]
- Miljanović, B.; Trivedi, K.A.; Dana, M.R.; Gilbard, J.P.; Buring, J.E.; Schaumberg, D.A. The relationship between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr. 2005, 82, 887–893. [Google Scholar] [CrossRef]
- Barabino, S.; Horwath-Winter, J.; Messmer, E.M.; Rolando, M.; Aragona, P.; Kinoshita, S. The role of systemic and topical fatty acids for dry eye treatment. Prog. Retin. Eye Res. 2017, 61, 23–34. [Google Scholar] [CrossRef]
- Aragona, P.; Bucolo, C.; Spinella, R.; Giuffrida, S.; Ferreri, G. Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjögren’s syndrome patients. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4474–4479. [Google Scholar] [CrossRef]
- 3C Study Group. Vascular factors and risk of dementia: Design of the Three-City Study and baseline characteristics of the study population. Neuroepidemiology 2003, 22, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Creuzot-Garcher, C.; Binquet, C.; Daniel, S.; Bretillon, L.; Acar, N.; de Lazzer, A.; Arnould, L.; Tzourio, C.; Bron, A.M.; Delcourt, C. The Montrachet Study: Study design, methodology and analysis of visual acuity and refractive errors in an elderly population. Acta Ophthalmol. 2016, 94, e90–e97. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Foulks, G.N. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Nichols, K.K.; Nichols, J.J.; Mitchell, G.L. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004, 23, 762–770. [Google Scholar] [CrossRef]
- Alves, M.; Reinach, P.S.; Paula, J.S.; Vellasco e Cruz, A.A.; Bachette, L.; Faustino, J.; Aranha, F.P.; Vigorito, A.; de Souza, C.A.; Rocha, E.M. Comparison of diagnostic tests in distinct well-defined conditions related to dry eye disease. PLoS ONE 2014, 9, e97921. [Google Scholar] [CrossRef]
- Moilanen, T.; Nikkari, T. The effect of storage on the fatty acid composition of human serum. Clin. Chim. Acta 1981, 114, 111–116. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride--methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Bertrand, F.; Maumy, M.; Meyer, N. plsRglm, modèles linéaires généralisés PLS sous R. Chimiometrie 2009, 2009, 52–54. [Google Scholar]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- Kabir, A.; Rahman, M.J.; Shamim, A.A.; Klemm, R.D.W.; Labrique, A.B.; Rashid, M.; Christian, P.; West, K.P., Jr. Identifying maternal and infant factors associated with newborn size in rural Bangladesh by partial least squares (PLS) regression analysis. PLoS ONE 2017, 12, e0189677. [Google Scholar] [CrossRef]
- Bastien, P.; Vinzi, V.E.; Tenenhaus, M. PLS generalised linear regression. Comput. Stat. Data Anal. 2005, 48, 17–46. [Google Scholar] [CrossRef]
- Lo, Y.-L.; Pan, W.-H.; Hsu, W.-L.; Chien, Y.-C.; Chen, J.-Y.; Hsu, M.-M.; Lou, P.J.; Chen, I.H.; Hildesheim, A.; Chen, C.J. Partial Least Square Discriminant Analysis Discovered a Dietary Pattern Inversely Associated with Nasopharyngeal Carcinoma Risk. PLoS ONE 2016, 11, e0155892. [Google Scholar] [CrossRef] [PubMed]
- Amadieu, C.; Lefèvre-Arbogast, S.; Delcourt, C.; Dartigues, J.-F.; Helmer, C.; Féart, C.; Samieri, C. Nutrient biomarker patterns and long-term risk of dementia in older adults. Alzheimers Dement. 2017, 13, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Acar, N.; Merle, B.; Ajana, S.; He, Z.; Grégoire, S.; Hejblum, B.P.; Martine, L.; Buaud, B.; Bron, A.M.; Creuzot-Garcher, C.P.; et al. Predicting the Retinal Content in Omega-3 Fatty Acids for Age-Related Macular-Degeneration. Clin. Transl. Med. 2021, 11, e404. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.; Delyfer, M.N.; Korobelnik, J.F.; Rougier, M.B.; Malet, F.; Féart, C.; Le Goff, M.; Peuchant, E.; Letenneur, L.; Dartigues, J.F.; et al. High Concentrations of Plasma N3 Fatty Acids Are Associated with Decreased Risk for Late Age-Related Macular Degeneration. J. Nutr. 2013, 4, 505–511. [Google Scholar] [CrossRef]
- Simon, E.; Bardet, B.; Grégoire, S.; Acar, N.; Bron, A.M.; Creuzot-Garcher, C.P.; Bretillon, L. Decreasing dietary linoleic acid promotes long chain omega-3 fatty acid incorporation into rat retina and modifies gene expression. Exp. Eye Res. 2011, 93, 628–635. [Google Scholar] [CrossRef]
- Van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res. 2018, 67, 56–86. [Google Scholar] [CrossRef]
- Schnebelen, C.; Grégoire, S.; Pasquis, B.; Joffre, C.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L.; Acar, N. Dietary n-3 and n-6 PUFA enhance DHA incorporation in retinal phospholipids without affecting PGE(1) and PGE (2) levels. Lipids 2009, 44, 465–470. [Google Scholar] [CrossRef]
- Schnebelen, C.; Viau, S.; Grégoire, S.; Joffre, C.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L.; Acar, N. Nutrition for the eye: Different susceptibility of the retina and the lacrimal gland to dietary omega-6 and omega-3 polyunsaturated fatty acid incorporation. Ophthalmic Res. 2009, 41, 216–224. [Google Scholar] [CrossRef]
- Baudouin, C.; Murat, I.; Messmer, E.; Benítez-Del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical Impact of Inflammation in Dry Eye Disease: Proceedings of the ODISSEY Group Meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef]
- Dry Eye Assessment and Management Study Research Group; Asbell, P.A.; Maguire, M.G.; Peskin, E.; Bunya, V.Y.; Kuklinksi, E.J. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Gorusupudi, A.; Nelson, K.; Bernstein, P.S. The Age-Related Eye Disease 2 Study: Micronutrients in the Treatment of Macular Degeneration. Adv. Nutr. 2017, 8, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Creuzot, C.; Passemard, M.; Viau, S.; Joffre, C.; Pouliquen, P.; Elena, P.P.; Pouliquen, P.; Baudouin, C.; Bron, A.M. Improvement of dry eye symptoms with polyunsaturated fatty acids. J. Fr. Ophtalmol. 2006, 29, 868–873. [Google Scholar] [CrossRef]
- Viau, S.; Maire, M.-A.; Pasquis, B.; Grégoire, S.; Acar, N.; Bron, A.M.; Bretillon, L.; Creuzot-Garcher, C.P.; Joffre, C. Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in a rat model. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Rolando, M.; Camicione, P.; Ravera, G.; Zanardi, S.; Giuffrida, S.; Calabria, S. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea 2003, 22, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.D.; Singh, R.; McClellan, A.J.; Weikert, M.P.; Scoper, S.V.; Joly, T.J.; Whitley, W.O.; Kakkar, E.; Pflugfelder, S.C. Long-term Supplementation With n-6 and n-3 PUFAs Improves Moderate-to-Severe Keratoconjunctivitis Sicca: A Randomized Double-Blind Clinical Trial. Cornea 2013, 32, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef]
- Fernández-Real, J.-M.; Broch, M.; Vendrell, J.; Ricart, W. Insulin resistance, inflammation, and serum fatty acid composition. Diabetes Care 2003, 26, 1362–1368. [Google Scholar] [CrossRef]
- Perreault, M.; Roke, K.; Badawi, A.; Nielsen, D.E.; Abdelmagid, S.A.; El-Sohemy, A.; Ma, D.W.; Mutch, D.M. Plasma levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are positively associated, but 18:0 and 18:2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids 2014, 49, 255–263. [Google Scholar] [CrossRef]
- Baugé, C.; Lhuissier, E.; Girard, N.; Quesnelle, C.; Ewert, G.; Boumediene, K. Anti-inflammatory effects of an injectable copolymer of fatty acids (Ara 3000 beta®) in joint diseases. J. Inflamm. 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).