Plasma Retinoid Concentrations Are Altered in Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Participants
2.3. Quantification of Retinoids in Plasma
2.4. Quantification of Retinol-Binding Protein 4 (RBP4) and Transthyretin (TTR) in Plasma
2.5. Statistical Methods
3. Results
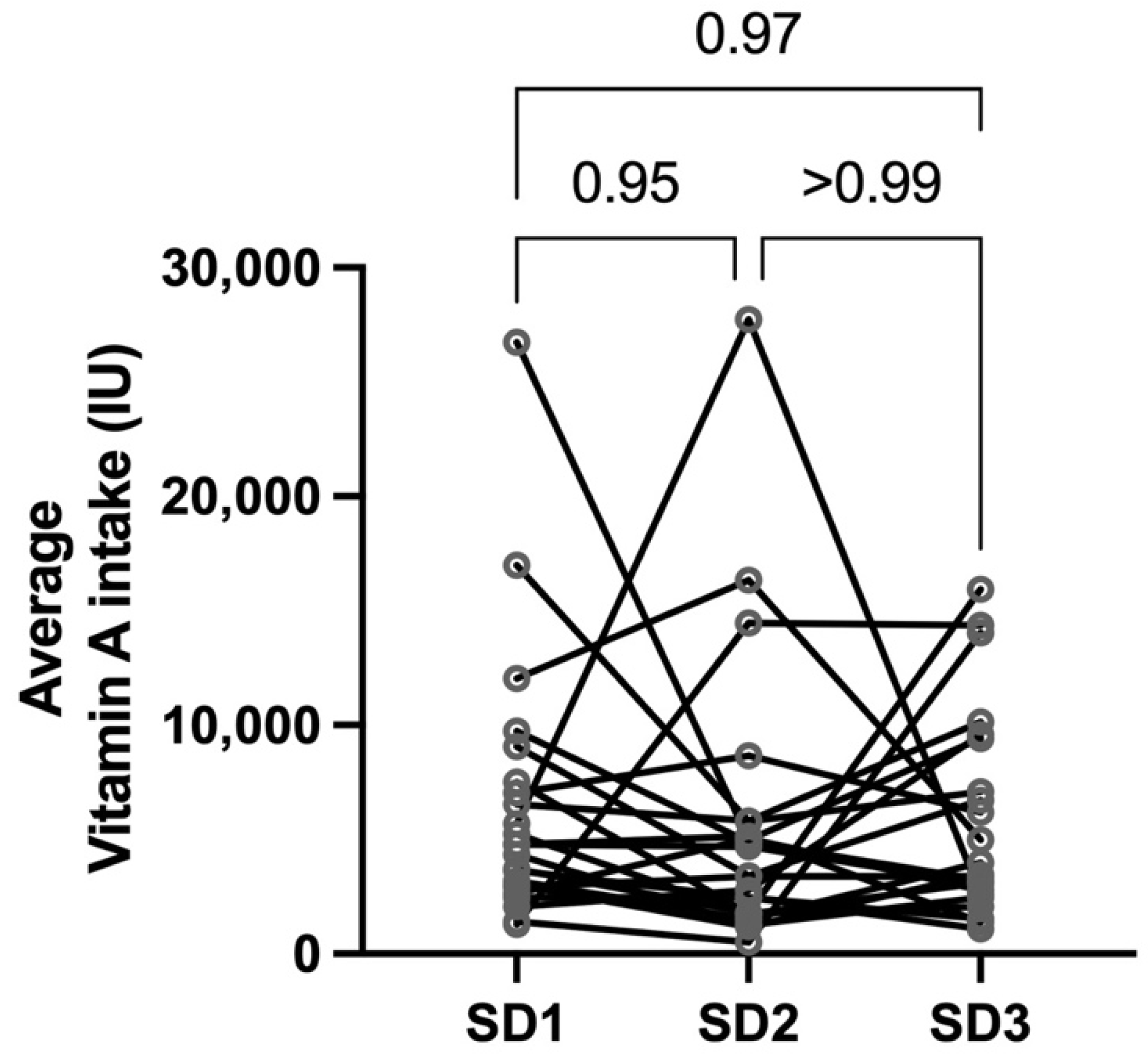
3.1. Human Subjects
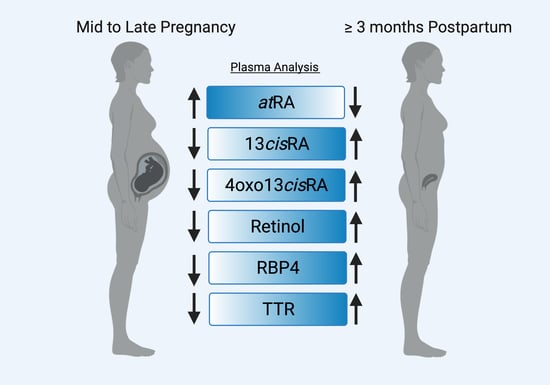
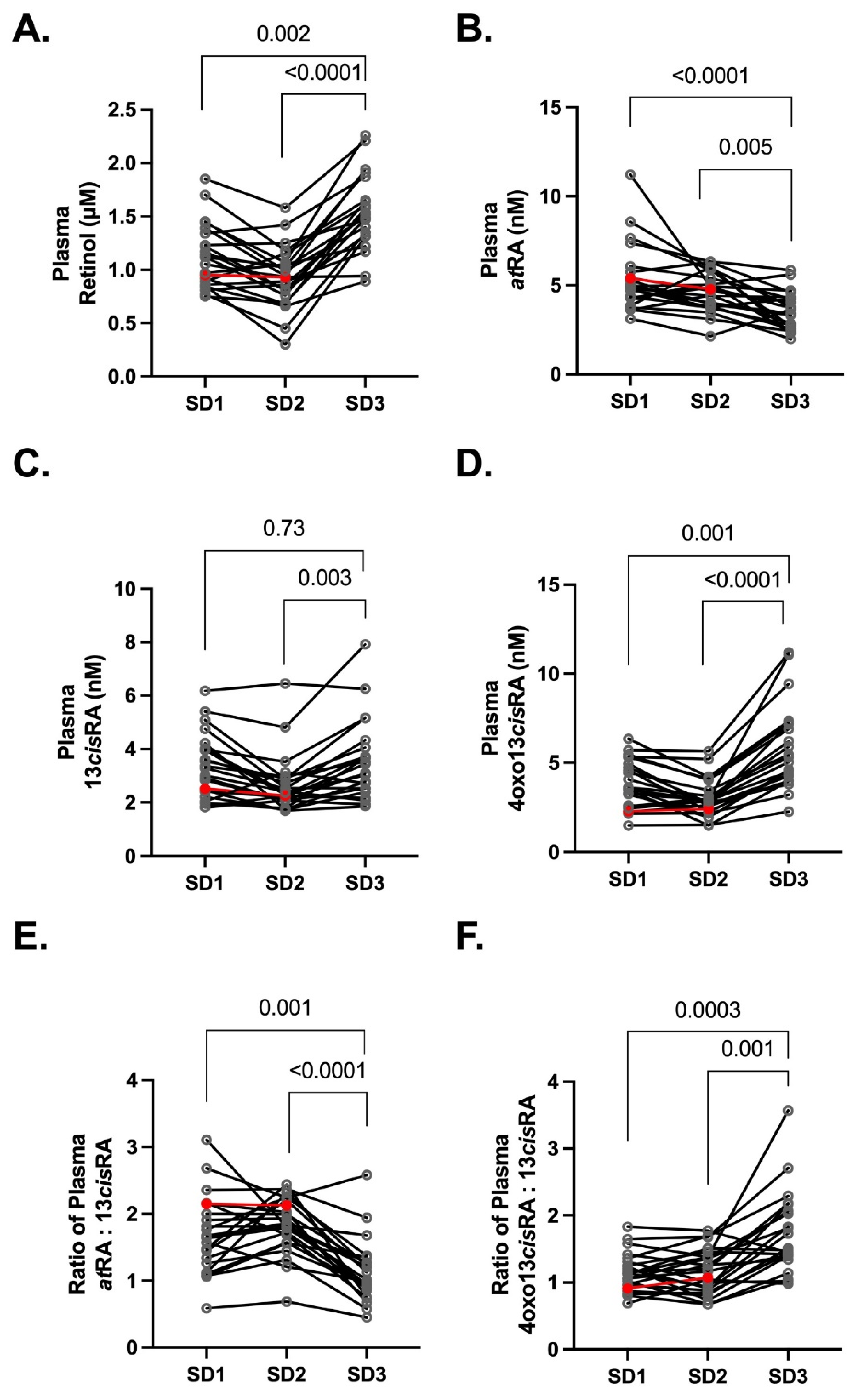
3.2. Plasma Retinoids during Normal Healthy Pregnancies
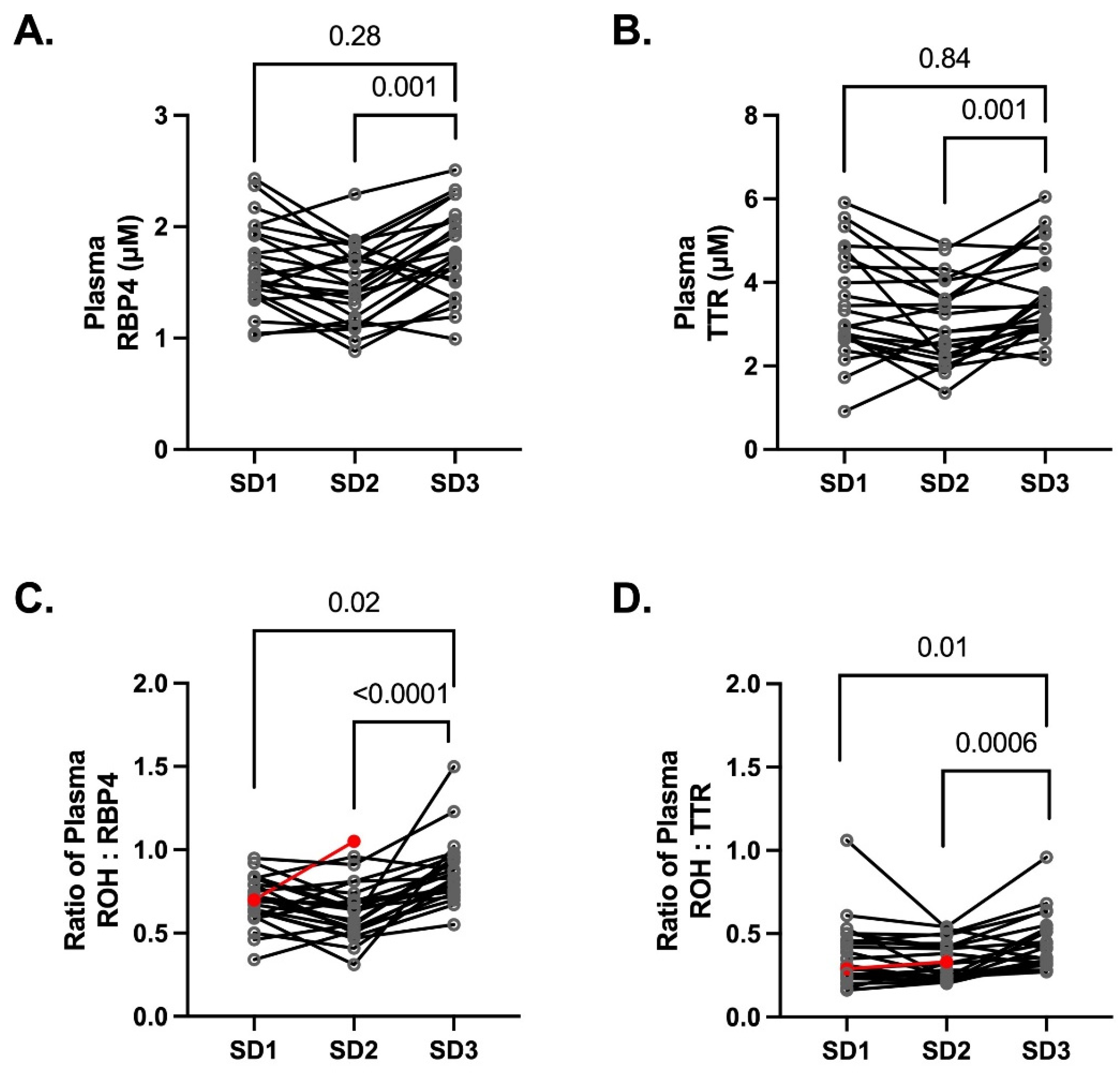
3.3. Plasma Concentrations of Retinol Binding Protein 4 (RBP4) and the Binding Partner Transthyretin (TTR)
3.4. Free atRA Concentrations during Pregnancy and Postpartum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin a Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wongsiriroj, N.; Blaner, W.S. The multifaceted nature of retinoid transport and metabolism. Hepatobiliary Surg. Nutr. 2014, 3, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Isoherranen, N.; Robinson-Cohen, C.; Petrie, I.; Kestenbaum, B.R.; Yeung, C.K. Chronic Kidney Disease Alters Vitamin A Homeostasis via Effects on Hepatic RBP4 Protein Expression and Metabolic Enzymes. Clin. Transl. Sci. 2016, 9, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Appleby, P.N.; Allen, N.E.; Travis, R.C.; Roddam, A.W.; Jenab, M.; Egevad, L.; Tjønneland, A.; Johnsen, N.F.; Overvad, K.; et al. Plasma Carotenoids, Retinol, and Tocopherols and the Risk of Prostate Cancer in the European Prospective Investigation into Cancer and Nutrition Study. Am. J. Clin. Nutr. 2007, 86. [Google Scholar] [CrossRef]
- Bell, E.C.; John, M.; Hughes, R.J.; Pham, T. Ultra-Performance Liquid Chromatographic Determination of Tocopherols and Retinol in Human Plasma. J. Chromatogr. Sci. 2014, 52, 1065–1070. [Google Scholar] [CrossRef][Green Version]
- Suzuki, M.; Wang, T.; Garretto, D.; Isasi, C.; Cardoso, W.; Greally, J.; Quadro, L. Disproportionate Vitamin A Deficiency in Women of Specific Ethnicities Linked to Differences in Allele Frequencies of Vitamin A-Related Polymorphisms. Nutrients 2021, 13, 1743. [Google Scholar] [CrossRef]
- Söderlund, M.B.; Fex, G.; Nilsson-Ehle, P. Decreasing serum concentrations of all-trans, 13-cis retinoic acids and retinol during fasting and caloric restriction. J. Intern. Med. 2003, 253, 375–380. [Google Scholar] [CrossRef]
- Bastos Maia, S.; Rolland Souza, A.S.; Costa Caminha, M.D.; Lins da Silva, S.; Callou Cruz, R.D.; Carvalho dos Santos, C.; Batista Filho, M. Vitamin A and Pregnancy: A Narrative Review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef]
- Arnold, S.L.; Amory, J.; Walsh, T.J.; Isoherranen, N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J. Lipid Res. 2012, 53, 587–598. [Google Scholar] [CrossRef]
- N’Soukpoé-Kossi, C.; Sedaghat-Herati, R.; Ragi, C.; Hotchandani, S.; Tajmir-Riahi, H. Retinol and retinoic acid bind human serum albumin: Stability and structural features. Int. J. Biol. Macromol. 2007, 40, 484–490. [Google Scholar] [CrossRef]
- Stevison, F.; Kosaka, M.; Kenny, J.R.; Wong, S.; Hogarth, C.; Amory, J.; Isoherranen, N. Does In Vitro Cytochrome P450 Downregulation Translate to In Vivo Drug-Drug Interactions? Preclinical and Clinical Studies with 13-cis-retinoic acid. Clin. Transl. Sci. 2019, 12, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Jones, C.; Mehta, S. Vitamin A Requirements in Pregnancy and Lactation. Curr. Dev. Nutr. 2020, 4, nzaa142. [Google Scholar] [CrossRef] [PubMed]
- Quadro, L.; Spiegler, E.K. Maternal-Fetal Transfer of Vitamin A and Its Impact on Mammalian Embryonic Development. In The Biochemistry of Retinoid Signaling III: Vitamin A and Retinoic Acid in Embryonic Development; Asson-Batres, M.A., Rochette-Egly, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 27–55. ISBN 978-3-030-42282-0. [Google Scholar]
- Roberts, C. Regulating Retinoic Acid Availability during Development and Regeneration: The Role of the CYP26 Enzymes. J. Dev. Biol. 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Topletz, A.R.; Zhong, G.; Isoherranen, N. Scaling in vitro activity of CYP3A7 suggests human fetal livers do not clear retinoic acid entering from maternal circulation. Sci. Rep. 2019, 9, 4620. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, E.; Kim, Y.-K.; Wassef, L.; Shete, V.; Quadro, L. Maternal–fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tajima, A.; Mattie, F.J.; Green, M.H.; Ross, A.C. Pregnancy and Lactation Alter Vitamin A Metabolism and Kinetics in Rats under Vitamin A-Adequate Dietary Conditions. Nutrients 2021, 13, 2853. [Google Scholar] [CrossRef] [PubMed]
- Radhika, M.S.; Bhaskaram, P.; Balakrishna, N.; Ramalakshmi, B.A.; Devi, S.; Siva Kumar, B. Effects of Vitamin A Deficiency during Pregnancy on Maternal and Child Health. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 689–693. [Google Scholar] [CrossRef]
- Hanson, C.; Lyden, E.; Abresch, C.; Anderson-Berry, A. Serum Retinol Concentrations, Race, and Socioeconomic Status in of Women of Childbearing Age in the United States. Nutrients 2016, 8, 508. [Google Scholar] [CrossRef]
- Hanson, C.; Lyden, E.; Anderson-Berry, A.; Kocmich, N.I.; Rezac, A.; Delair, S.; Furtado, J.; Van Ormer, M.; Izevbigie, N.; Olateju, E.K.; et al. Status of Retinoids and Carotenoids and Associations with Clinical Outcomes in Maternal-Infant Pairs in Nigeria. Nutrients 2018, 10, 1286. [Google Scholar] [CrossRef]
- Baker, H.; DeAngelis, B.; Holland, B.; Gittens-Williams, L.; Barrett, T., Jr. Vitamin Profile of 563 Gravidas during Trimesters of Pregnancy. J. Am. Coll. Nutr. 2002, 21, 33–37. [Google Scholar] [CrossRef]
- Krzyzanowska, K.; Zemany, L.; Krugluger, W.; Schernthaner, G.H.; Mittermayer, F.; Schnack, C.; Rahman, R.; Brix, J.; Kahn, B.B.; Schernthaner, G. Serum concentrations of retinol-binding protein 4 in women with and without gestational diabetes. Diabetologia 2008, 51, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Sapin, V.; Alexandre, M.C.; Chaïb, S.; Bournazeau, J.A.; Sauvant, P.; Borel, P.; Jacquetin, B.; Grolier, P.; Lémery, D.; Dastugue, B.; et al. Effect of Vitamin A Status at the End of Term Pregnancy on Thesaturation of Retinol Binding Protein with Retinol. Am. J. Clin. Nutr. 2000, 71, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Takamoto, N.; Akahori, Y.; Masumoto, A.; Nakatsukasa, H.; Msuyama, H.; Hiramatsu, Y. Elevated level of serum retinol-binding protein 4 in pregnancy-induced hypertension. J. Obstet. Gynaecol. Res. 2009, 35, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Dalsoren, T.; Voldner, N.; Godang, K.; Henriksen, T.; Bollerslev, J. Retinol-binding protein-4 is not strongly associated with insulin sensitivity in normal pregnancies. Eur. J. Endocrinol. 2008, 159, 49–54. [Google Scholar] [CrossRef]
- Mendola, P.; Ghassabian, A.; Mills, J.; Zhang, C.; Tsai, M.; Liu, A.; Yeung, E.H. Retinol-Binding Protein 4 and Lipids Prospectively Measured During Early to Mid-Pregnancy in Relation to Preeclampsia and Preterm Birth Risk. Am. J. Hypertens. 2017, 30, 569–576. [Google Scholar] [CrossRef]
- Söderlund, M.B.; Fex, G.A.; Nilsson-Ehle, P. Concentrations of retinoids in early pregnancy and in newborns and their mothers. Am. J. Clin. Nutr. 2005, 81, 633–636. [Google Scholar] [CrossRef][Green Version]
- Frederiksen, M.C. Physiologic changes in pregnancy and their effect on drug disposition. Semin. Perinatol. 2001, 25, 120–123. [Google Scholar] [CrossRef]
- Czuba, L.C.; Zhong, G.; Yabut, K.C.; Isoherranen, N. Analysis of Vitamin A and Retinoids in Biological Matrices; Pohl, E., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 637, ISBN 9780128201442. [Google Scholar]
- U.S. Food and Drug Administration Center for Drug Evaluation and Research. Bioanalytical Method Validation Guidance for Industry; 2018; FDA-2013-D-1020-0039. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 10 February 2022).
- Jing, J.; Nelson, C.; Paik, J.; Shirasaka, Y.; Amory, J.; Isoherranen, N. Physiologically Based Pharmacokinetic Model of All-trans-Retinoic Acid with Application to Cancer Populations and Drug Interactions. J. Pharmacol. Exp. Ther. 2017, 361, 246–258. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Londero, A.P.; Driul, L.; Henze, A.; Tonutti, L.; Ceraudo, M.; Zanotti, G.; Berni, R.; Schweigert, F.J.; Raila, J. First trimester concentrations of the TTR-RBP4-retinol complex components as early markers of insulin-treated gestational diabetes mellitus. Clin. Chem. Lab. Med. 2015, 53, 1643–1651. [Google Scholar] [CrossRef]
- Belatik, A.; Hotchandani, S.; Bariyanga, J.; Tajmir-Riahi, H. Binding sites of retinol and retinoic acid with serum albumins. Eur. J. Med. Chem. 2012, 48, 114–123. [Google Scholar] [CrossRef]
- Garretto, D.; Kim, Y.-K.; Quadro, L.; Rhodas, R.R.; Pimentel, V.; Crnosija, N.A.; Nie, L.; Bernstein, P.; Tropper, P.; Neal-Perry, G.S. Vitamin A and β-carotene in pregnant and breastfeeding post-bariatric women in an urban population. J. Périnat. Med. 2019, 47, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Guo, F.; Zhang, Y.; Yuan, Y.; Chen, D.; Bai, G. Vitamin A, D, and E Levels and Reference Ranges for Pregnant Women: A Cross-Sectional Study 2017–2019. Front. Nutr. 2021, 8, 628902. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, J.; Liu, Z.; Yun, C.; Piao, J.; Yang, X. Prevalence and influence factors of vitamin A deficiency of Chinese pregnant women. Nutr. J. 2015, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.-D.; Mao, C.-Y.; Kang, X.; Zhang, S.-H. An investigation of the levels of vitamins A, D, and E in the serum of Chinese pregnant women. J. Clin. Lab. Anal. 2018, 32, e22176. [Google Scholar] [CrossRef]
- Liu, J.; Zhan, S.; Jia, Y.; Li, Y.; Liu, Y.; Dong, Y.; Tang, G.; Li, L.; Zhai, Y.; Cao, Z. Retinol and α-tocopherol in pregnancy: Establishment of reference intervals and associations with CBC. Matern. Child Nutr. 2020, 16, e12975. [Google Scholar] [CrossRef]
- Yliniemi, A.; Nurkkala, M.-M.; Kopman, S.; Korpimaki, T.; Kouru, H.; Ryynanen, M.; Marttala, J. First Trimester Placental Retinol-Binding Protein 4 (RBP4) and Pregnancy-Associated Placental Protein A (PAPP-A) in the Prediction of Early-Onset Severe Pre-Eclampsia. Metabolism 2015, 64, 521–526. [Google Scholar] [CrossRef]
- Saucedo, R.; Zarate, A.; Basurto, L.; Hernandez, M.; Puello, E.; Galvan, R.; Campos, S. Relationship Between Circulating Adipokines and Insulin Resistance During Pregnancy and Postpartum in Women with Gestational Diabetes. Arch. Med. Res. 2011, 42, 318–323. [Google Scholar] [CrossRef]

| Characteristic | Value (Mean ± SD) |
|---|---|
| Women (n = 23) | |
| Age (y) | 32.4 ± 3.1 A |
| Height (cm) | 161.9 ± 21.6 |
| Pre-pregnancy weight (kg) | 64.8 ± 10.7 |
| Pre-pregnancy BMI (kg/m2) | 25.5 ± 3.1 |
| Race B | % |
| White | 70 |
| Asian | 13 |
| Black | 9 |
| Pacific Islander | 4 |
| Mixed Asian and Caucasian | 4 |
| Study Days | Weeks (mean ± SD) |
| Study Day 1 | 26.8 ± 1.2 weeks gestation |
| Study Day 2 | 30.1 ± 1.2 weeks gestation |
| Study Day 3 | 15.3 ± 2.6 weeks postpartum |
| Prenatal vitamin supplementation | Frequency (%) |
| Study Day 1 | 87% |
| Study Day 2 | 0% |
| Study Day 3 | 57% |
| Lactating | 91% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czuba, L.C.; Fay, E.E.; LaFrance, J.; Smith, C.K.; Shum, S.; Moreni, S.L.; Mao, J.; Isoherranen, N.; Hebert, M.F. Plasma Retinoid Concentrations Are Altered in Pregnant Women. Nutrients 2022, 14, 1365. https://doi.org/10.3390/nu14071365
Czuba LC, Fay EE, LaFrance J, Smith CK, Shum S, Moreni SL, Mao J, Isoherranen N, Hebert MF. Plasma Retinoid Concentrations Are Altered in Pregnant Women. Nutrients. 2022; 14(7):1365. https://doi.org/10.3390/nu14071365
Chicago/Turabian StyleCzuba, Lindsay C., Emily E. Fay, Jeffrey LaFrance, Chase K. Smith, Sara Shum, Sue L. Moreni, Jennie Mao, Nina Isoherranen, and Mary F. Hebert. 2022. "Plasma Retinoid Concentrations Are Altered in Pregnant Women" Nutrients 14, no. 7: 1365. https://doi.org/10.3390/nu14071365
APA StyleCzuba, L. C., Fay, E. E., LaFrance, J., Smith, C. K., Shum, S., Moreni, S. L., Mao, J., Isoherranen, N., & Hebert, M. F. (2022). Plasma Retinoid Concentrations Are Altered in Pregnant Women. Nutrients, 14(7), 1365. https://doi.org/10.3390/nu14071365