The Influence of Intracellular Glutathione Levels on the Induction of Nrf2-Mediated Gene Expression by α-Dicarbonyl Precursors of Advanced Glycation End Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Lines
2.3. Kinetic Study of the Reaction between α-Dicarbonyl Compounds and GSH
2.4. LC-TOF-MS Analysis
2.5. LC-TQ-MS Analysis
2.6. Nrf2 CALUX Assay
2.7. Cytotox CALUX Assay
2.8. Cell Viability Assay
2.9. Measurement of Intracellular ROS Levels
2.10. Investigation of the Effects of Altered Intracellular GSH Levels on the Induction of Nrf2-Mediated Gene Expression by the α-Dicarbonyl Compounds
2.11. Quantification of the Intracellular GSH Levels
2.12. Statistical Analysis
3. Results
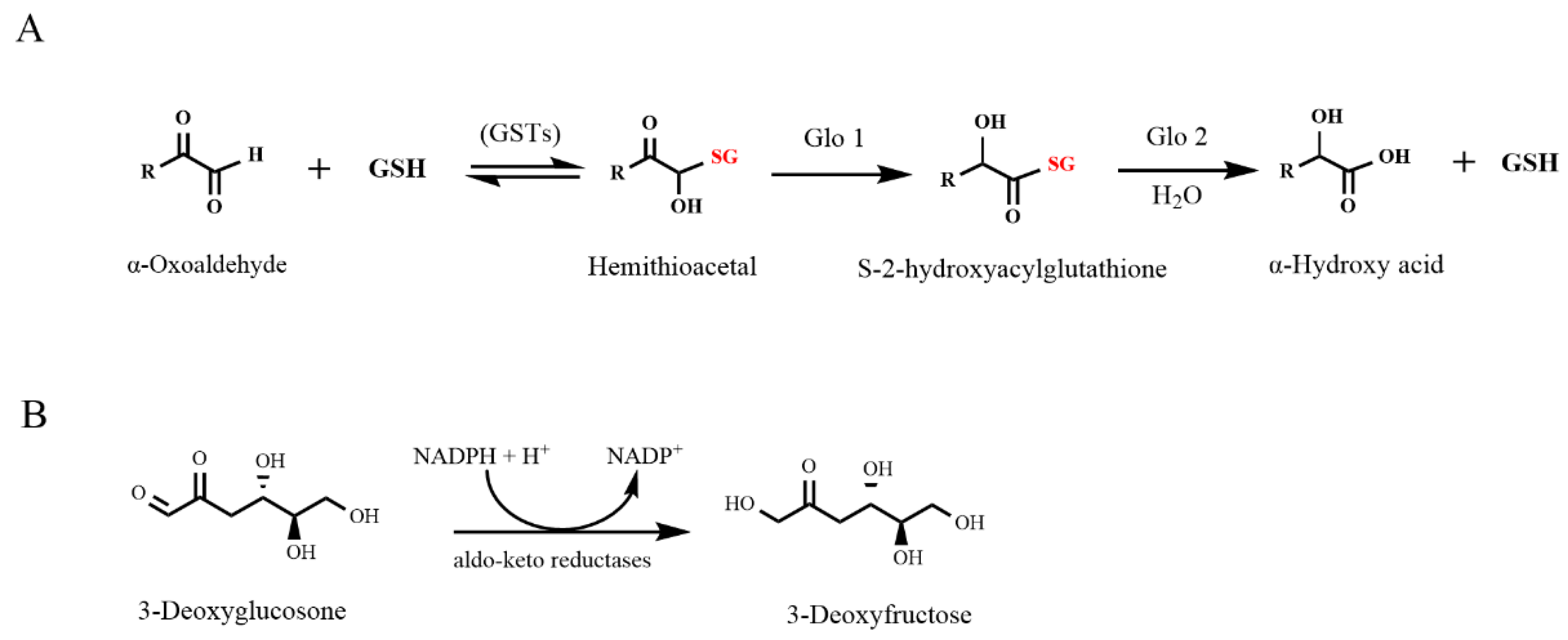
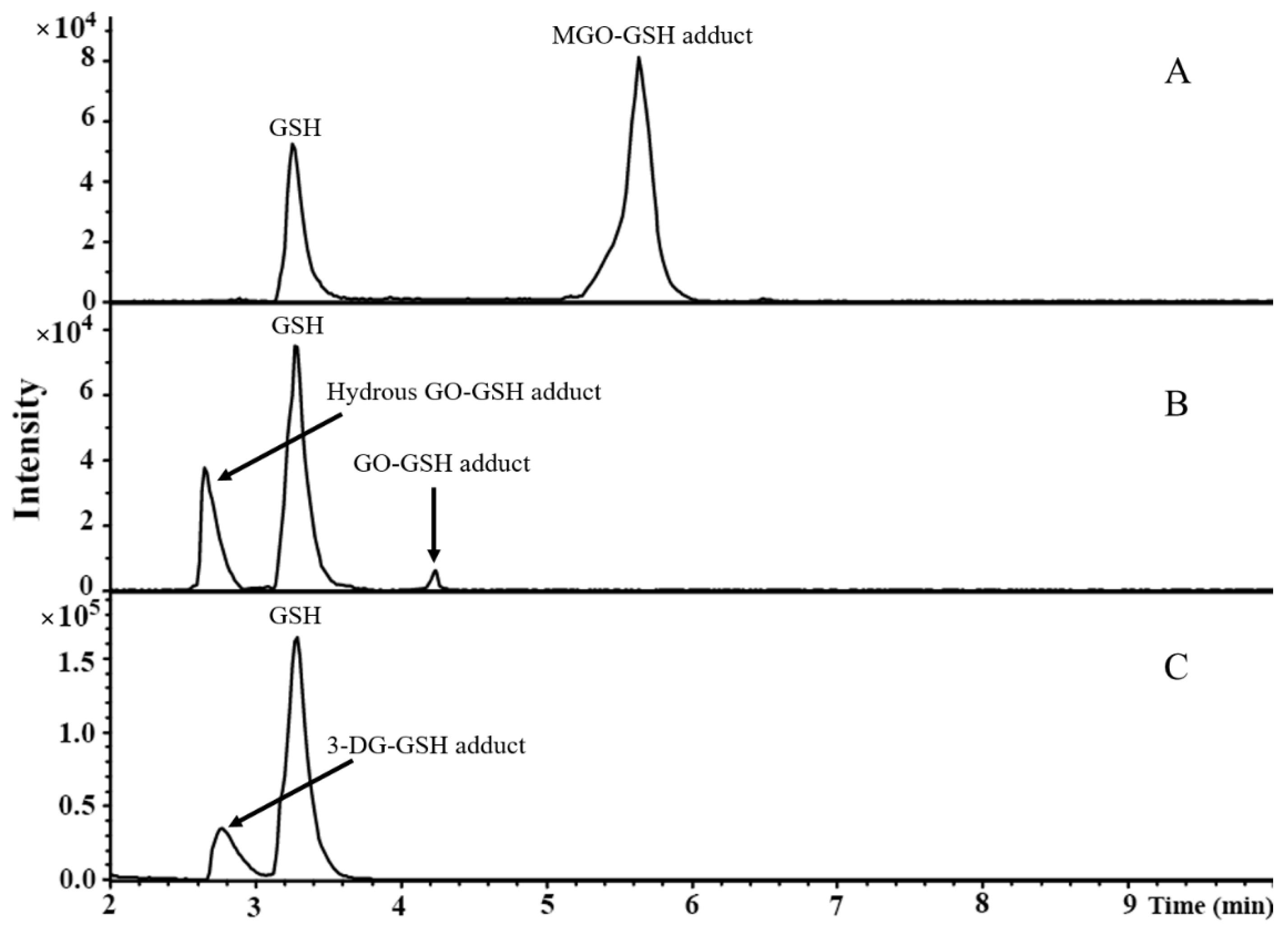
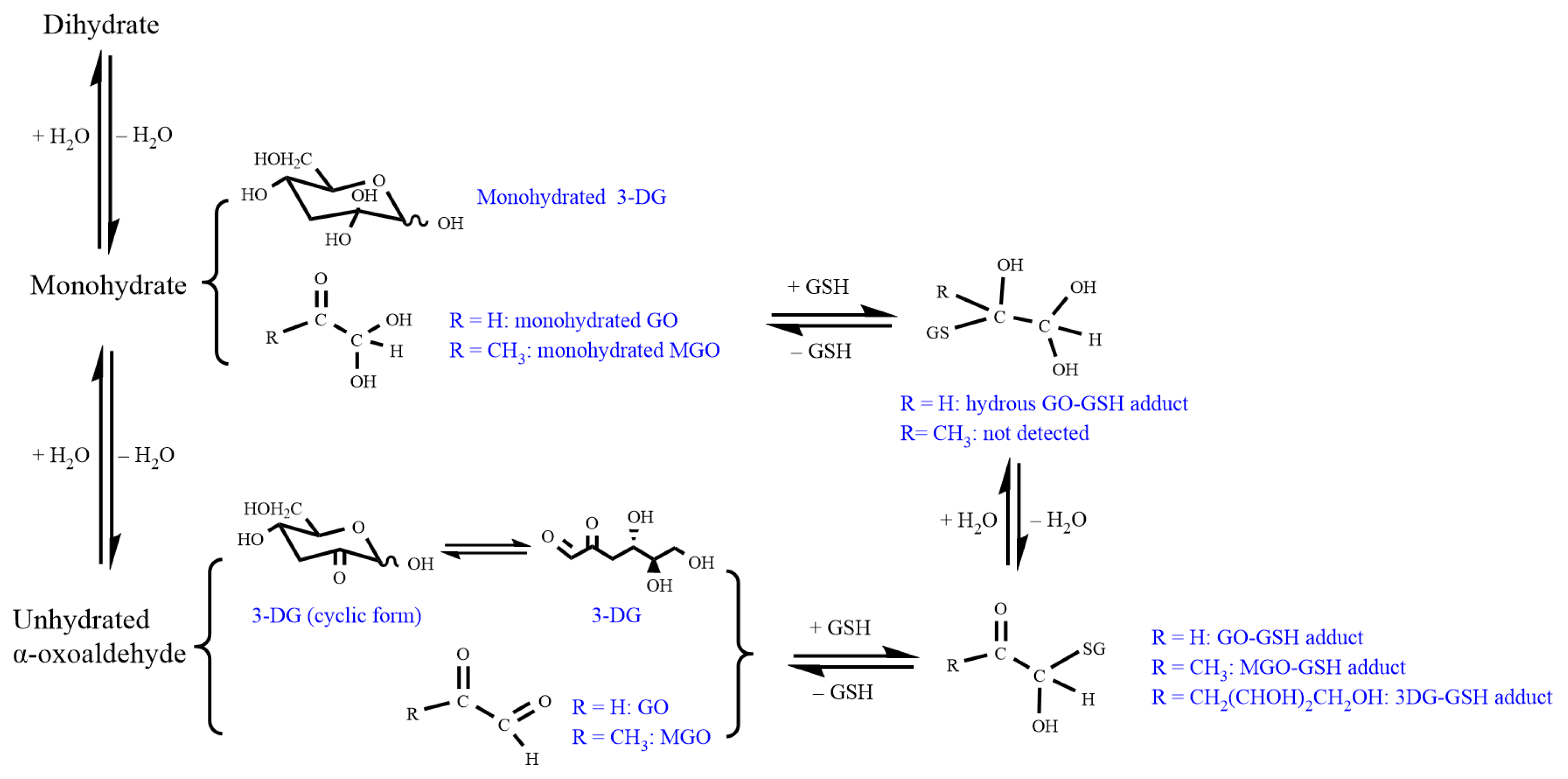
3.1. Identification of Reaction Products between α-Dicarbonyl Compounds and GSH by LC-TOF- MS
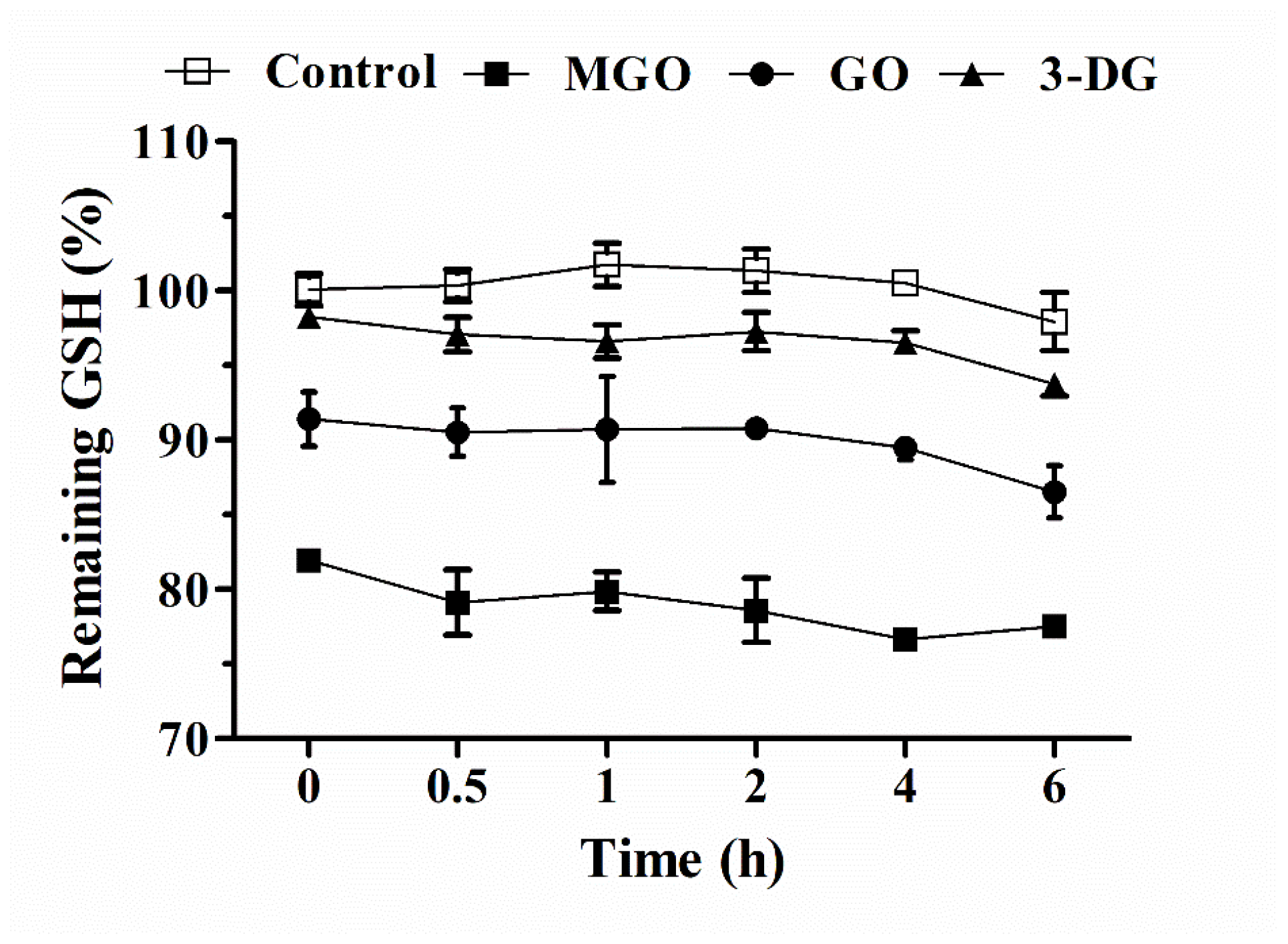
3.2. Kinetic Study of the Reaction between α-Dicarbonyl Compounds and GSH
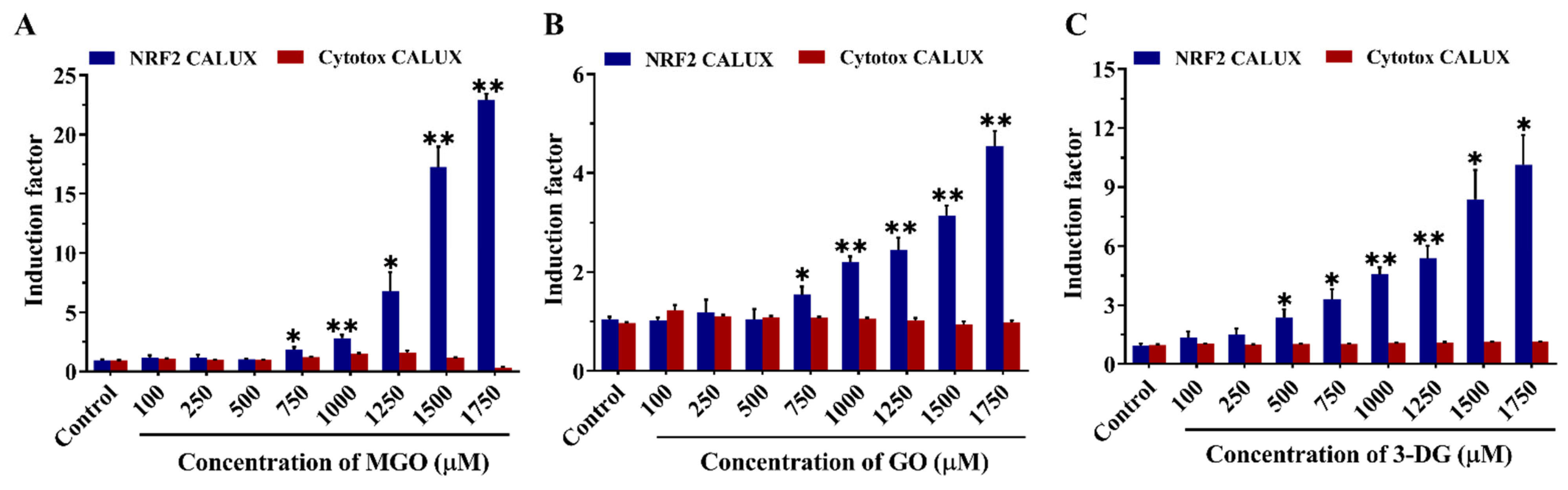
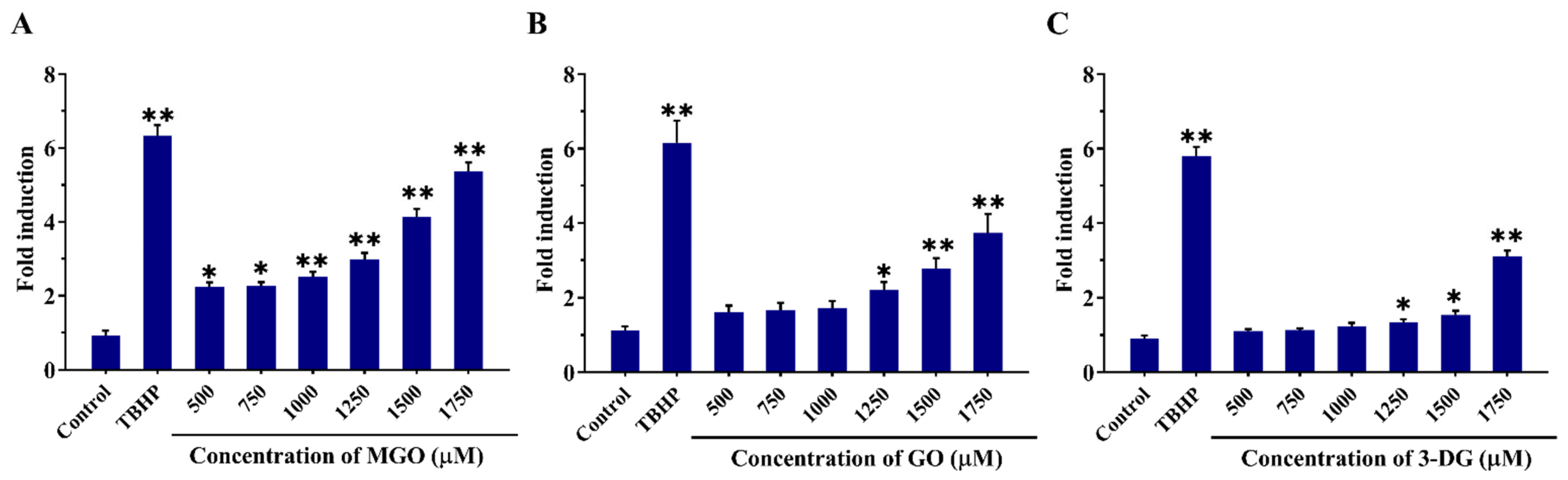
3.3. Effects of α-Dicarbonyl Compounds on the Viability of Nrf2 CALUX Cells and Induction of Nrf2-Mediated Gene Expression
3.4. Effects of α-Dicarbonyl Compounds on the Production of Intracellular ROS
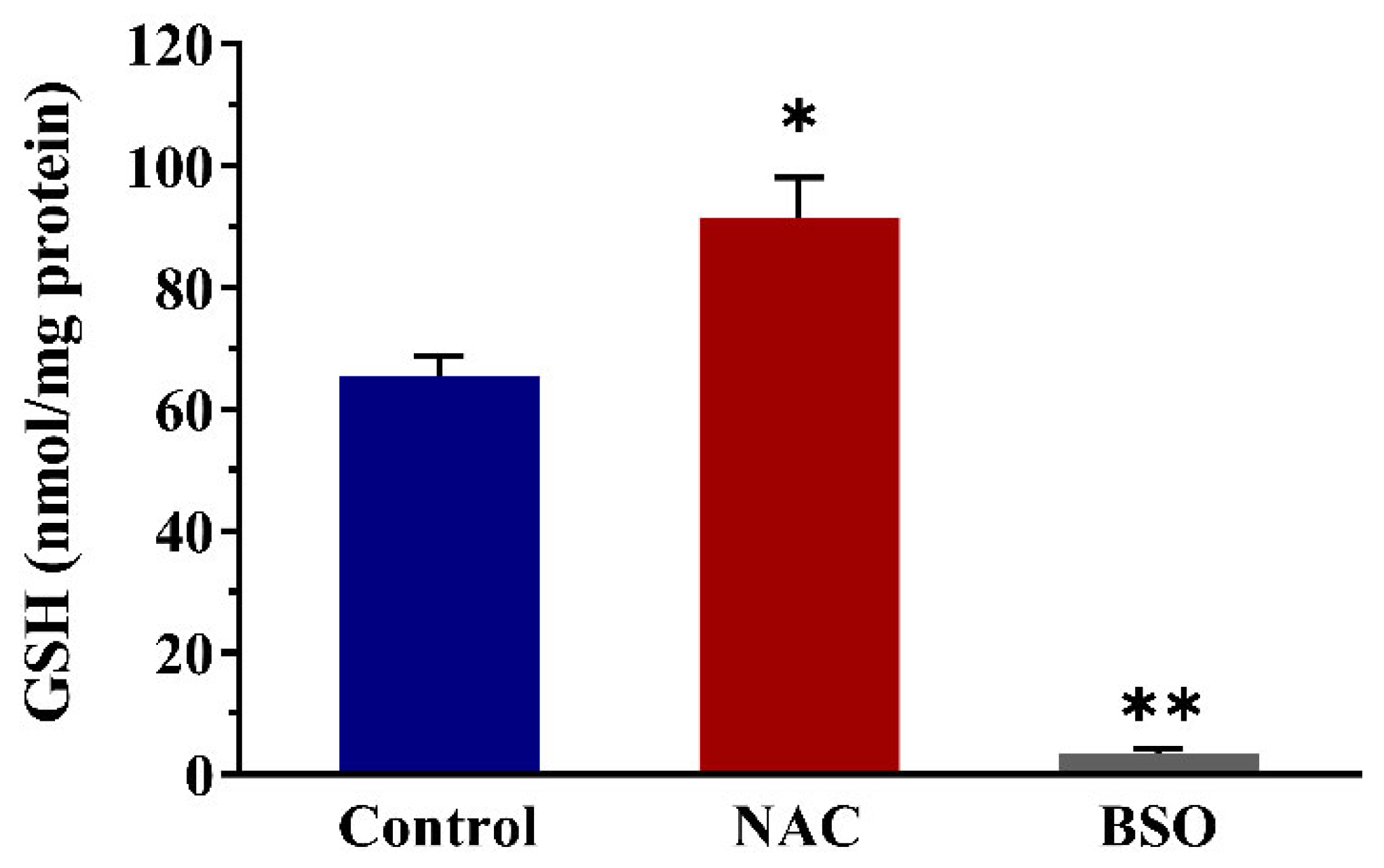
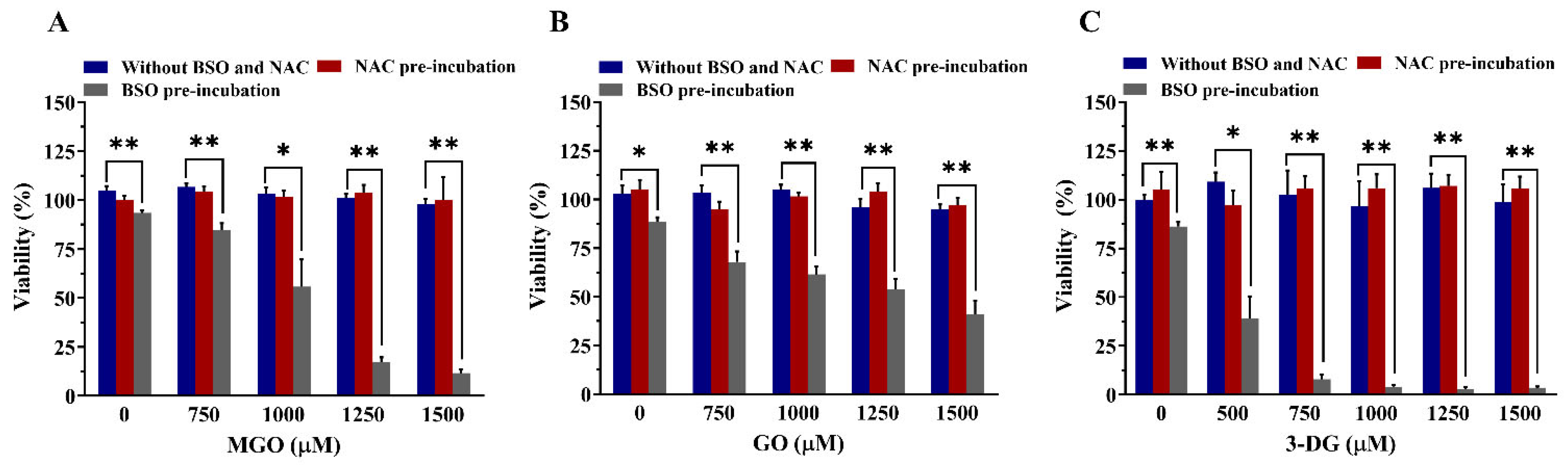
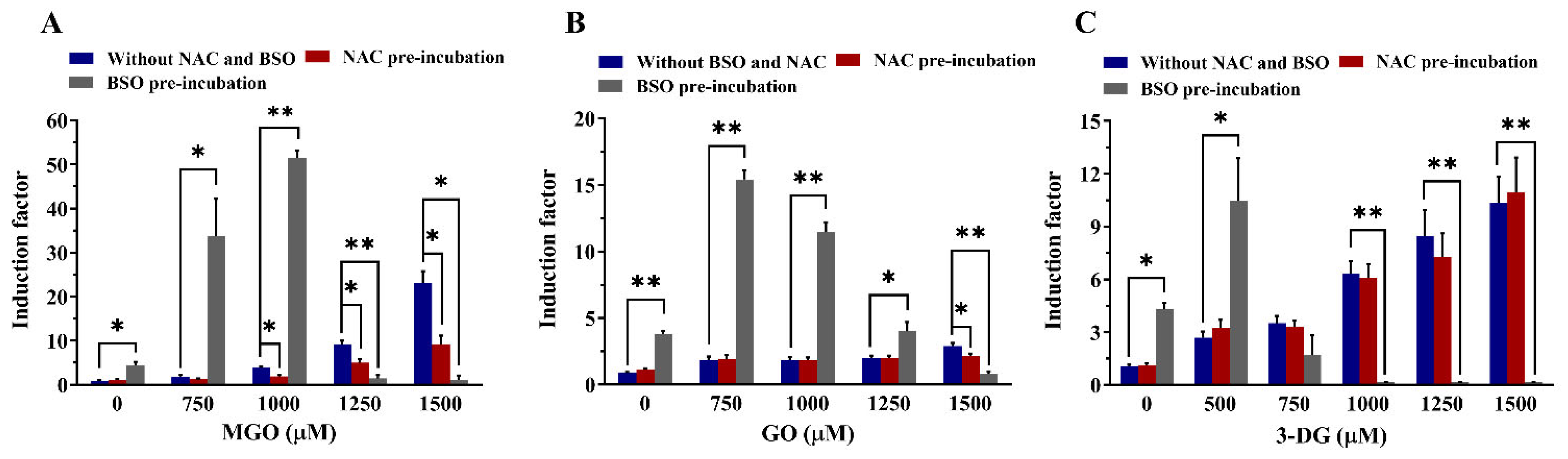
3.5. Effects of Altered Intracellular GSH Levels on the Viability of Nrf2 CALUX Cells and the Induction of Nrf2-Mediated Gene Expression by α-Dicarbonyl Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Richard, J.P. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans. 1993, 21, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piskorska, D.; Kopieczna-Grzebieniak, E. Participation of glyoxalases and methylglyoxal in diabetic complication development. Pol. Merkur Lekarski. 1998, 4, 342–344. [Google Scholar] [PubMed]
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys Res. Commun. 2015, 458, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj. J. 2016, 33, 513–525. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.H.; Wu, H.J.; Hsuuw, Y.D. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann. N. Y. Acad. Sci. 2005, 1042, 372–378. [Google Scholar] [CrossRef]
- Furstenau, C.R.; de Souza, I.C.C.; de Oliveira, M.R. Tanshinone I Induces Mitochondrial Protection by a Mechanism Involving the Nrf2/GSH Axis in the Human Neuroblastoma SH-SY5Y Cells Exposed to Methylglyoxal. Neurotox. Res. 2019, 36, 491–502. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Ehl, J.; Bretzel, R.G.; Kunz, C. Food derived carbonyl compounds affect basal and stimulated secretion of interleukin-6 and -8 in Caco-2 cells. Eur. J. Nutr. 2009, 48, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8. [Google Scholar] [PubMed]
- Salahuddin, P.; Rabbani, G.; Khan, R.H. The role of advanced glycation end products in various types of neurodegenerative disease: A therapeutic approach. Cell Mol. Biol. Lett. 2014, 19, 407–437. [Google Scholar] [CrossRef] [PubMed]
- Matafome, P.; Sena, C.; Seica, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R. AGEs and neurodegeneration: The Nrf2/glyoxalase-1 interaction. Oncotarget 2017, 8, 5645. [Google Scholar]
- Yoon, K.D.; Yamamoto, K.; Zhou, J.; Sparrow, J.R. Photo-products of retinal pigment epithelial bisretinoids react with cellular thiols. Mol. Vis. 2011, 17, 1839–1849. [Google Scholar]
- Kammerscheit, X.; Hecker, A.; Rouhier, N.; Chauvat, F.; Cassier-Chauvat, C. Methylglyoxal Detoxification Revisited: Role of Glutathione Transferase in Model Cyanobacterium Synechocystis sp. Strain PCC 6803. mBio 2020, 11, e00882-20. [Google Scholar] [CrossRef]
- Abordo, E.A.; Minhas, H.S.; Thornalley, P.J. Accumulation of α-oxoaldehydes during oxidative stress: A role in cytotoxicity. Biochem. Pharmacol. 1999, 58, 641–648. [Google Scholar]
- Masterjohn, C.; Mah, E.; Park, Y.; Pei, R.; Lee, J.; Manautou, J.E.; Bruno, R.S. Acute glutathione depletion induces hepatic methylglyoxal accumulation by impairing its detoxification to D-lactate. Exp. Biol. Med. 2013, 238, 360–369. [Google Scholar] [CrossRef]
- Lee-Hilz, Y.Y.; Boerboom, A.M.; Westphal, A.H.; Berkel, W.J.; Aarts, J.M.; Rietjens, I.M. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem. Res. Toxicol. 2006, 19, 1499–1505. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Custodio de Souza, I.C.; Furstenau, C.R. Promotion of mitochondrial protection by naringenin in methylglyoxal-treated SH-SY5Y cells: Involvement of the Nrf2/GSH axis. Chem. Biol. Interact. 2019, 310, 108728. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Koike, S.; Inoue, N.; Suzuki, T.; Ogasawara, Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: Increase in GSH levels is a critical event for the detoxification mechanism. Biochem. Biophys Res. Commun. 2017, 483, 874–879. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, S.C.; von Bergh, A.R.; van Vught-Lussenburg, B.M.; Jonker, L.R.; Teunis, M.; Krul, C.A.; van der Burg, B. Development of a panel of high-throughput reporter-gene assays to detect genotoxicity and oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 760, 23–32. [Google Scholar] [CrossRef]
- Sonneveld, E.; Jansen, H.J.; Riteco, J.A.; Brouwer, A.; van der Burg, B. Development of androgen-and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol. Sci. 2005, 83, 136–148. [Google Scholar]
- Sonneveld, E.; van den Brink, C.E.; van der Leede, B.J.M.; Schulkes, R.K.A.M.; Petkovich, M.; van der Burg, B.; van der Saag, P.T. Human retinoic acid (RA) 4-hydroxylase (CYP26) is highly specific for all-trans-RA and can be induced through RA receptors in human breast and colon carcinoma cells. Cell Growth Differ. 1998, 9, 629–637. [Google Scholar]
- Gijsbers, L.; van Eekelen, H.D.; Nguyen, T.H.; de Haan, L.H.; van der Burg, B.; Aarts, J.M.; Rietjens, I.M.; Bovy, A.G. Induction of electrophile-responsive element (EpRE)-mediated gene expression by tomato extracts in vitro. Food Chem. 2012, 135, 1166–1172. [Google Scholar] [CrossRef]
- Boerboom, A.M.; Vermeulen, M.; van der Woude, H.; Bremer, B.I.; Lee-Hilz, Y.Y.; Kampman, E.; van Bladeren, P.J.; Rietjens, I.M.; Aarts, J.M. Newly constructed stable reporter cell lines for mechanistic studies on electrophile-responsive element-mediated gene expression reveal a role for flavonoid planarity. Biochem. Pharmacol. 2006, 72, 217–226. [Google Scholar] [CrossRef]
- Garcia-Mendoza, D.; Han, B.; van den Berg, H.; van den Brink, N.W. Cell-specific immune-modulation of cadmium on murine macrophages and mast cell lines in vitro. J. Appl. Toxicol. 2019, 39, 992–1001. [Google Scholar] [CrossRef]
- Qanungo, S.; Wang, M.; Nieminen, A.L. N-Acetyl-L-cysteine enhances apoptosis through inhibition of nuclear factor-kappaB in hypoxic murine embryonic fibroblasts. J. Biol. Chem. 2004, 279, 50455–50464. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.M.; Watson, W.H.; Jones, D.P. Compartmentation of Nrf-2 redox control: Regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol. Sci. 2004, 82, 308–317. [Google Scholar] [CrossRef]
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.N.; Chen, D.; Zhang, T.Y.; Ding, J.; Feng, Y.Q. Use of ammonium sulfite as a post-column derivatization reagent for rapid detection and quantification of aldehydes by LC-MS. Talanta 2020, 206, 120172. [Google Scholar] [CrossRef] [PubMed]
- Tamotsu, W.; Soichiro, M. Polarographic Behavior of Glyoxal and Its Related Compounds. In Bulletin of University of Osaka Prefecture. Series A, Engineering and Nat Ural Sciences; University of Osaka Prefecture: Sakai, Japan, 1970; Volume 19, pp. 169–180. [Google Scholar]
- Rae, C.; Bernersprice, S.J.; Bulliman, B.T.; Kuchel, P.W. Kinetic-Analysis of the Human Erythrocyte Glyoxalase System Using H-1-Nmr and a Computer-Model. Eur. J. Biochem. 1990, 193, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Vikic-Topic, D.; Varga-Defterdarovic, L. Spectroscopic studies of methylglyoxal in water and dimethylsulfoxide. Bioorg. Chem. 2004, 32, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Weenen, H.; Tjan, S.B. Analysis, Structure, and Reactivity of 3-Deoxyglucosone. In Flavor Precursors; ACS Symposium Series; American Chemical Society: Washington, DC, 1992; pp. 217–231. [Google Scholar]
- Wang, Y.; Ho, C.T. Flavour chemistry of methylglyoxal and glyoxal. Chem. Soc. Rev. 2012, 41, 4140–4149. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Yurek-George, A.; Argirov, O.K. Kinetics and mechanism of the reaction of aminoguanidine with the alpha-oxoaldehydes glyoxal, methylglyoxal, and 3-deoxyglucosone under physiological conditions. Biochem. Pharm. 2000, 60, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef] [Green Version]
- Kold-Christensen, R.; Johannsen, M. Methylglyoxal Metabolism and Aging-Related Disease: Moving from Correlation toward Causation. Trends Endocrinol. Metab. 2020, 31, 81–92. [Google Scholar] [CrossRef]
- Li, D.; Ma, S.; Ellis, E.M. Nrf2-mediated adaptive response to methyl glyoxal in HepG2 cells involves the induction of AKR7A2. Chem.-Biol. Interact. 2015, 234, 366–371. [Google Scholar]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Bollong, M.J.; Lee, G.; Coukos, J.S.; Yun, H.; Zambaldo, C.; Chang, J.W.; Chin, E.N.; Ahmad, I.; Chatterjee, A.K.; Lairson, L.L.; et al. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature 2018, 562, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Ding, Y.; Wang, Q.; Chenheng, W.; Qiao, X. Effects of buthionine sulfoxine on the redox state of human umbilical vein endothelial cells cultured with K562 cells conditioned media. Chin. Pharmacol. Bull. 2005, 21, 450–453. [Google Scholar]
- Zemva, J.; Fink, C.A.; Fleming, T.H.; Schmidt, L.; Loft, A.; Herzig, S.; Kniess, R.A.; Mayer, M.; Bukau, B.; Nawroth, P.P.; et al. Hormesis enables cells to handle accumulating toxic metabolites during increased energy flux. Redox. Biol. 2017, 13, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Ferlita, S.; Yegiazaryan, A.; Noori, N.; Lal, G.; Nguyen, T.; To, K.; Venketaraman, V. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium tuberculosis. J. Clin. Med. 2019, 8, 2219. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013, 14, 21021–21044. [Google Scholar] [CrossRef] [Green Version]
- Cepas, V.; Manig, F.; Mayo, J.C.; Hellwig, M.; Collotta, D.; Sanmartino, V.; Carrocera-Pumarino, R.; Collino, M.; Henle, T.; Sainz, R.M. In Vitro Evaluation of the Toxicological Profile and Oxidative Stress of Relevant Diet-Related Advanced Glycation End Products and Related 1,2-Dicarbonyls. Oxidative Med. Cell. Longev. 2021, 2021, 9912240. [Google Scholar] [CrossRef]
- Rogatsky, I.; Trowbridge, J.M.; Garabedian, M.J. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Mol. Cell. Biol. 1997, 17, 3181–3193. [Google Scholar]
- Jeong, S.R.; Lee, K.W. Methylglyoxal-Derived Advanced Glycation End Product (AGE4)-Induced Apoptosis Leads to Mitochondrial Dysfunction and Endoplasmic Reticulum Stress through the RAGE/JNK Pathway in Kidney Cells. Int. J. Mol. Sci. 2021, 22, 6530. [Google Scholar] [CrossRef]
- Nasu, R.; Furukawa, A.; Suzuki, K.; Takeuchi, M.; Koriyama, Y. The Effect of Glyceraldehyde-Derived Advanced Glycation End Products on beta-Tubulin-Inhibited Neurite Outgrowth in SH-SY5Y Human Neuroblastoma Cells. Nutrients 2020, 12, 2958. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; van Dongen, K.C.W.; Bakker, W.; Miro Estruch, I.; Rietjens, I.M.C.M. The Influence of Intracellular Glutathione Levels on the Induction of Nrf2-Mediated Gene Expression by α-Dicarbonyl Precursors of Advanced Glycation End Products. Nutrients 2022, 14, 1364. https://doi.org/10.3390/nu14071364
Zheng L, van Dongen KCW, Bakker W, Miro Estruch I, Rietjens IMCM. The Influence of Intracellular Glutathione Levels on the Induction of Nrf2-Mediated Gene Expression by α-Dicarbonyl Precursors of Advanced Glycation End Products. Nutrients. 2022; 14(7):1364. https://doi.org/10.3390/nu14071364
Chicago/Turabian StyleZheng, Liang, Katja C. W. van Dongen, Wouter Bakker, Ignacio Miro Estruch, and Ivonne M. C. M. Rietjens. 2022. "The Influence of Intracellular Glutathione Levels on the Induction of Nrf2-Mediated Gene Expression by α-Dicarbonyl Precursors of Advanced Glycation End Products" Nutrients 14, no. 7: 1364. https://doi.org/10.3390/nu14071364
APA StyleZheng, L., van Dongen, K. C. W., Bakker, W., Miro Estruch, I., & Rietjens, I. M. C. M. (2022). The Influence of Intracellular Glutathione Levels on the Induction of Nrf2-Mediated Gene Expression by α-Dicarbonyl Precursors of Advanced Glycation End Products. Nutrients, 14(7), 1364. https://doi.org/10.3390/nu14071364






