Do MAFLD Patients with Harmful Alcohol Consumption Have a Different Dietary Intake?
Abstract
:1. Introduction
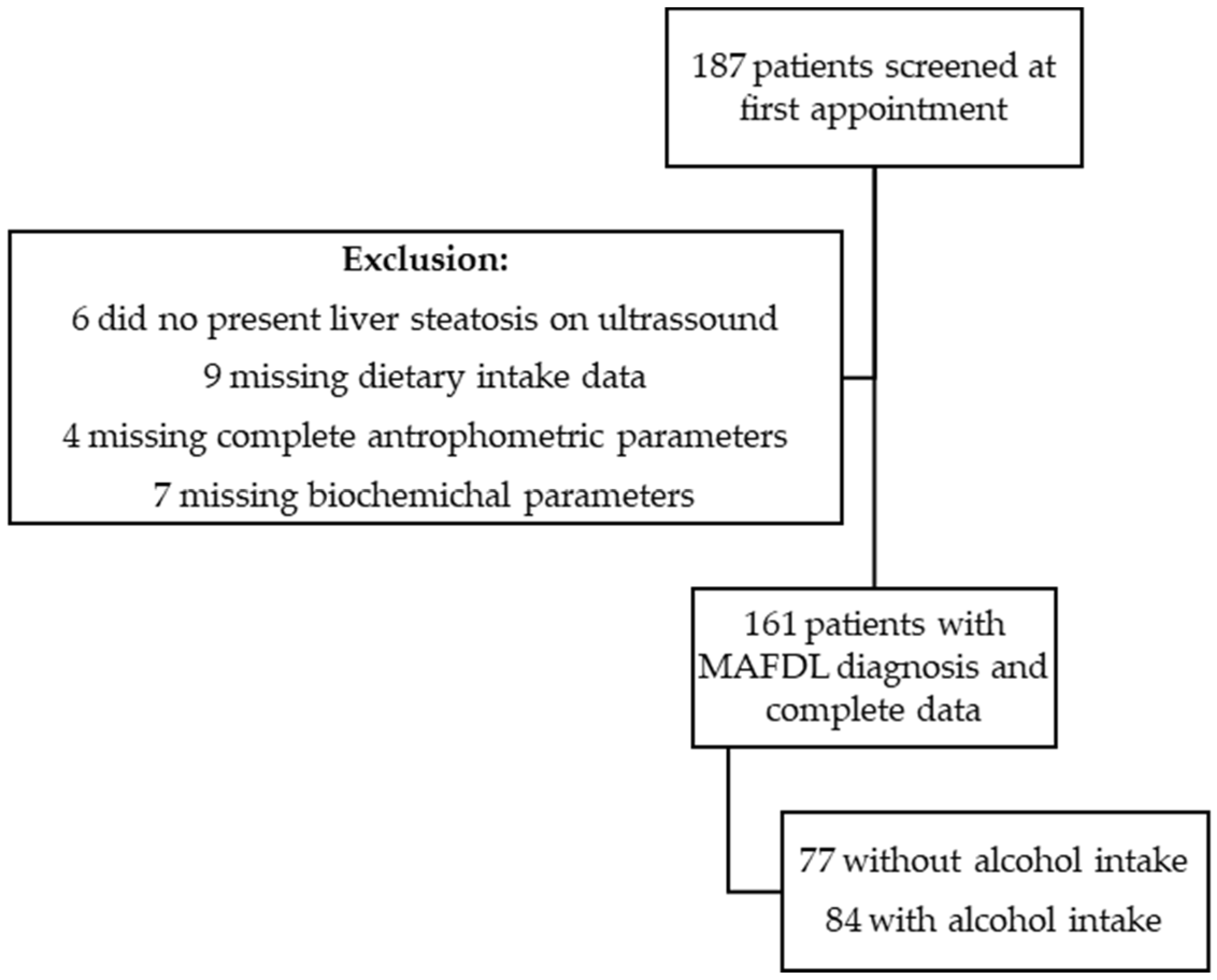
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Parameters
2.3. Dietary Intake and DII®
2.4. Assessment of MAFLD
2.5. Statistical Analysis
3. Results
3.1. Dietary Intake and DII®
3.2. Liver Disease
3.3. Dietary Intake, DII®, and Liver Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundi, M.S.; Velapati, S.; Patel, J.; Kellogg, T.A.; Abu Dayyeh, B.K.; Hurt, R.T. Evolution of NAFLD and Its Management. Nutr. Clin. Pract. 2019, 35, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef]
- Cheng, Y.-M.; Kao, J.-H.; Wang, C.-C. The metabolic profiles and body composition of lean metabolic associated fatty liver disease. Hepatol. Int. 2021, 15, 405–412. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol. Commun. 2018, 2, 48–57. [Google Scholar] [CrossRef]
- Fouad, Y.; Elwakil, R.; Elsahhar, M.; Said, E.; Bazeed, S.; Ali Gomaa, A.; Hashim, A.; Kamal, E.; Mehrez, M.; Attia, D. The NAFLD-MAFLD debate: Eminence vs. evidence. Liver Int. 2021, 41, 255–260. [Google Scholar] [CrossRef]
- Parry, S.A.; Turner, M.; Hodson, L. Lifestyle interventions affecting hepatic fatty acid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 373–379. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef] [Green Version]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascaró, C.M.; Bouzas, C.; Tur, J.A. Association between Non-Alcoholic Fatty Liver Disease and Mediterranean Lifestyle: A Systematic Review. Nutrients 2022, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.J.; George, E.; Roberts, S.K.; Tierney, A.C. Effect of dietary intervention, with or without co-interventions, on inflammatory markers in patients with nonalcoholic fatty liver disease: A systematic literature review. Nutr. Rev. 2019, 77, 765–786. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Corella, D.; Castañer, O.; Lamuela-Raventos, R.-M.; Salas-Salvadó, J.; Martínez-González, M.-A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvención con DIeta MEDiterránea (PREDIMED) Randomized Controlled Trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef]
- Hébert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)—Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. Int. Rev. J. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Garcia-Arellano, A.; Martínez-González, M.A.; Ramallal, R.; Salas-Salvadó, J.; Hébert, J.R.; Corella, D.; Shivappa, N.; Forga, L.; Schröder, H.; Muñoz-Bravo, C.; et al. Dietary inflammatory index and all-cause mortality in large cohorts: The SUN and PREDIMED studies. Clin. Nutr. 2019, 38, 1221–1231. [Google Scholar] [CrossRef]
- Veronese, N.; Cisternino, A.M.; Shivappa, N.; Hebert, J.R.; Notarnicola, M.; Reddavide, R.; Inguaggiato, R.; Guerra, V.; Logroscino, A.; Rotolo, O.; et al. Dietary inflammatory index and mortality: A cohort longitudinal study in a Mediterranean area. J. Hum. Nutr. Diet. 2020, 33, 138–146. [Google Scholar] [CrossRef]
- Phillips, C.M.; Chen, L.-W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Kengne, A.P. Diet with greater inflammatory potential is associated with higher prevalence of fatty liver among US adults. Eur. J. Clin. Nutr. 2019, 73, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hennein, R.; Liu, C.; Long, M.T.; Hoffmann, U.; Jacques, P.F.; Lichtenstein, A.H.; Hu, F.B.; Levy, D. Improved Diet Quality Associates with Reduction in Liver Fat, Particularly in Individuals with High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantero, I.; Abete, I.; Babio, N.; Arós, F.; Corella, D.; Estruch, R.; Fitó, M.; Hebert, J.R.; Martínez-González, M.Á.; Pintó, X.; et al. Dietary Inflammatory Index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin. Nutr. 2018, 37, 1736–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of Nutritional Changes on Nonalcoholic Fatty Liver Disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.; Aro, A.; Azevedo, A.; Ramos, E.; Barros, H. Intake and Adipose Tissue Composition of Fatty Acids and Risk of Myocardial Infarction in a Male Portuguese Community Sample. J. Am. Diet. Assoc. 2007, 107, 276–286. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Cortez-Pinto, H. Detecting liver fat from viscoelasticity: How good is CAP in clinical practice? The need for universal cut-offs. J. Hepatol. 2017, 66, 886–887. [Google Scholar] [CrossRef]
- Gaia, S.; Carenzi, S.; Barilli, A.L.; Bugianesi, E.; Smedile, A.; Brunello, F.; Marzano, A.; Rizzetto, M. Reliability of transient elastography for the detection of fibrosis in Non-Alcoholic Fatty Liver Disease and chronic viral hepatitis. J. Hepatol. 2011, 54, 64–71. [Google Scholar] [CrossRef]
- Imajo, K.; Kessoku, T.; Honda, Y.; Tomeno, W.; Ogawa, Y.; Mawatari, H.; Fujita, K.; Yoneda, M.; Taguri, M.; Hyogo, H.; et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016, 150, 626–637.e7. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Kwon, M.; Shivappa, N.; Hébert, J.R.; Kim, M.K. Proinflammatory Dietary Intake is Associated with Increased Risk of Metabolic Syndrome and Its Components: Results from the Population-Based Prospective Study. Nutrients 2020, 12, 1196. [Google Scholar] [CrossRef] [PubMed]
- Taheri, E.; Bostick, R.M.; Hatami, B.; Pourhoseingholi, M.A.; Aghdaei, H.A.; Moslem, A.; Jarrahi, A.M.; Zali, M.R. Dietary and Lifestyle Inflammation Scores Are Inversely Associated with Metabolic-Associated Fatty Liver Disease among Iranian Adults: A Nested Case-Control Study. J. Nutr. 2021, 152, 559–567. [Google Scholar] [CrossRef]
- Pohl, K.; Moodley, P.; Dhanda, A.D. Alcohol’ Impact on the Gut and Liver. Nutrients 2021, 13, 3170. [Google Scholar] [CrossRef]
- Visontay, R.; Sunderland, M.; Slade, T.; Wilson, J.; Mewton, L. Are there non-linear relationships between alcohol consumption and long-term health?: A systematic review of observational studies employing approaches to improve causal inference. BMC Med. Res. Methodol. 2022, 22, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Tsompanaki, E.; Thanapirom, K.; Papatheodoridi, M.; Parikh, P.; de Lima, Y.C.; Tsochatzis, E.A. Systematic Review and Meta-analysis: The Role of Diet in the Development of Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 1–18. [Google Scholar] [CrossRef]
- Eslam, M.; Ahmed, A.; Després, J.-P.; Jha, V.; Halford, J.C.G.; Chieh, J.T.W.; Harris, D.C.H.; Nangaku, M.; Colagiuri, S.; Targher, G.; et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol. Hepatol. 2021, 6, 743–753. [Google Scholar] [CrossRef]

| MAFLD Patients (n = 161) | MAFLD without Alcohol (n = 77) | MAFLD with Alcohol (n = 84) | p-Value * | |||
|---|---|---|---|---|---|---|
| Sex (%) | Male | 59.6 (96) | 42.9 (33) | 75.0 (63) | <0.001 | |
| Female | 40.4 (65) | 57.1 (44) | 25.0 (21) | |||
| Age (years) | 55.9 ± 12.7 | 55.2 ± 13.3 | 56.5 ± 12.2 | 0.534 | ||
| Weight (kg) | 86.3 ± 16.5 | 83.5 ± 16.9 | 89.0 ± 15.7 | 0.035 | ||
| BMI (kg/m2) | 30.9 ± 5.3 | 30.9 ± 5.3 | 30.8 ± 5.3 | 0.909 | ||
| BMI | Normal (<25 kg/m2) | 8.1 (13) | 6.5 (5) | 9.5 (8) | 0.732 | |
| Pre-obese (≥25–29.9 kg/m2) | 42.2 (68) | 41.6 (32) | 42.9 (36) | |||
| Obese (≥30 kg/m2) | 49.7 (80) | 51.9 (40) | 47.6 (40) | |||
| Waist circumference (cm) | 102.9 ± 10.8 | 100.9 ± 9.8 | 104.6 ± 11.4 | 0.109 | ||
| Body fat mass (kg) | 28.1 ± 11.4 | 29.4 ± 12.4 | 26.9 ± 10.5 | 0.228 | ||
| AST above threshold | 40.6 (65) | 39.0 (30) | 42.2 (35) | 0.680 | ||
| ALT above threshold | 59.4 (95) | 57.1 (44) | 61.4 (51) | 0.580 | ||
| GGT above threshold | 56.9 (87) | 58.9 (43) | 55.0 (44) | 0.626 | ||
| Type 2 diabetes mellitus (%) | 41.0 (66) | 45.5 (35) | 36.9 (31) | 0.271 | ||
| High blood pressure (%) | 58.4 (94) | 66.2 (51) | 51.2 (43) | 0.053 | ||
| Dyslipidemia (%) | 59.6 (96) | 57.1 (44) | 61.9 (52) | 0.538 | ||
| Metabolic Syndrome (%) | 50.3 (81) | 57.1 (44) | 44 (37) | 0.097 | ||
| MAFLD Patients (n = 161) | MAFLD without Alcohol (n = 77) | MAFLD with Alcohol (n = 84) | p-Value * | |
|---|---|---|---|---|
| Total energy intake (g/day) | 1868 ± 415 | 1777 ± 381 | 1945 ± 429 | 0.013 |
| Total fat intake (g/day) | 74.2 ± 19.0 | 74.6 ± 18.4 | 73.9 ± 19.6 | 0.861 |
| Saturated fat intake (g/day) | 21.4 ± 6.3 | 21.3 ± 6.7 | 21.5 ± 6.1 | 0.687 |
| MUFA intake (g/day) | 34.3 ± 9.5 | 34.7 ± 9.3 | 33.9 ± 9.7 | 0.828 |
| PUFA intake (g/day) | 12.6 ± 3.9 | 12.5 ± 13.9 | 12.6 ± 3.9 | 0.575 |
| Total protein intake (g/day) | 83.2 ± 18.6 | 83.6 ± 18.3 | 83.1 ± 18.9 | 0.719 |
| Total CHO intake (g/day) | 209.8 ± 56.8 | 201.6 ± 56.0 | 216.7 ± 56.8 | 0.096 |
| Sugar (g/day) | 89.9 ± 37.5 | 86.2 ± 39.7 | 92.9 ± 35.5 | 0.094 |
| Fiber intake (g/day) | 19.2 ± 6.4 | 18.9 ± 6.5 | 19.5 ± 6.3 | 0.425 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Policarpo, S.; Carvalhana, S.; Craciun, A.; Crespo, R.R.; Cortez-Pinto, H. Do MAFLD Patients with Harmful Alcohol Consumption Have a Different Dietary Intake? Nutrients 2022, 14, 1335. https://doi.org/10.3390/nu14071335
Policarpo S, Carvalhana S, Craciun A, Crespo RR, Cortez-Pinto H. Do MAFLD Patients with Harmful Alcohol Consumption Have a Different Dietary Intake? Nutrients. 2022; 14(7):1335. https://doi.org/10.3390/nu14071335
Chicago/Turabian StylePolicarpo, Sara, Sofia Carvalhana, Ana Craciun, Ricardo Rios Crespo, and Helena Cortez-Pinto. 2022. "Do MAFLD Patients with Harmful Alcohol Consumption Have a Different Dietary Intake?" Nutrients 14, no. 7: 1335. https://doi.org/10.3390/nu14071335
APA StylePolicarpo, S., Carvalhana, S., Craciun, A., Crespo, R. R., & Cortez-Pinto, H. (2022). Do MAFLD Patients with Harmful Alcohol Consumption Have a Different Dietary Intake? Nutrients, 14(7), 1335. https://doi.org/10.3390/nu14071335






