Optimal Obesity- and Lipid-Related Indices for Predicting Metabolic Syndrome in Chronic Kidney Disease Patients with and without Type 2 Diabetes Mellitus in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participant
- Male: VAI = WC (cm)/[39.68 + 1.88 × BMI (Kg/m2)] × [TG (mmol/L)/1.03] × [1.31/HDL (mmol/L)]
- 2.
- Female: VAI = WC (cm)/[36.58 + 1.89 × BMI (Kg/m2)] × [TG (mmol/L)/0.81] × [1.52/HDL (mmol/L)]
2.2. Biochemical Measurements
2.3. Definition of Variables
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of Patients with CKD
3.2. Different Characteristics of Obesity- and Lipid-Related Indices in Male and Female CKD Patients with and without T2DM
3.3. Association of Obesity- and Lipid-Related Indices with Prevalence of MetS
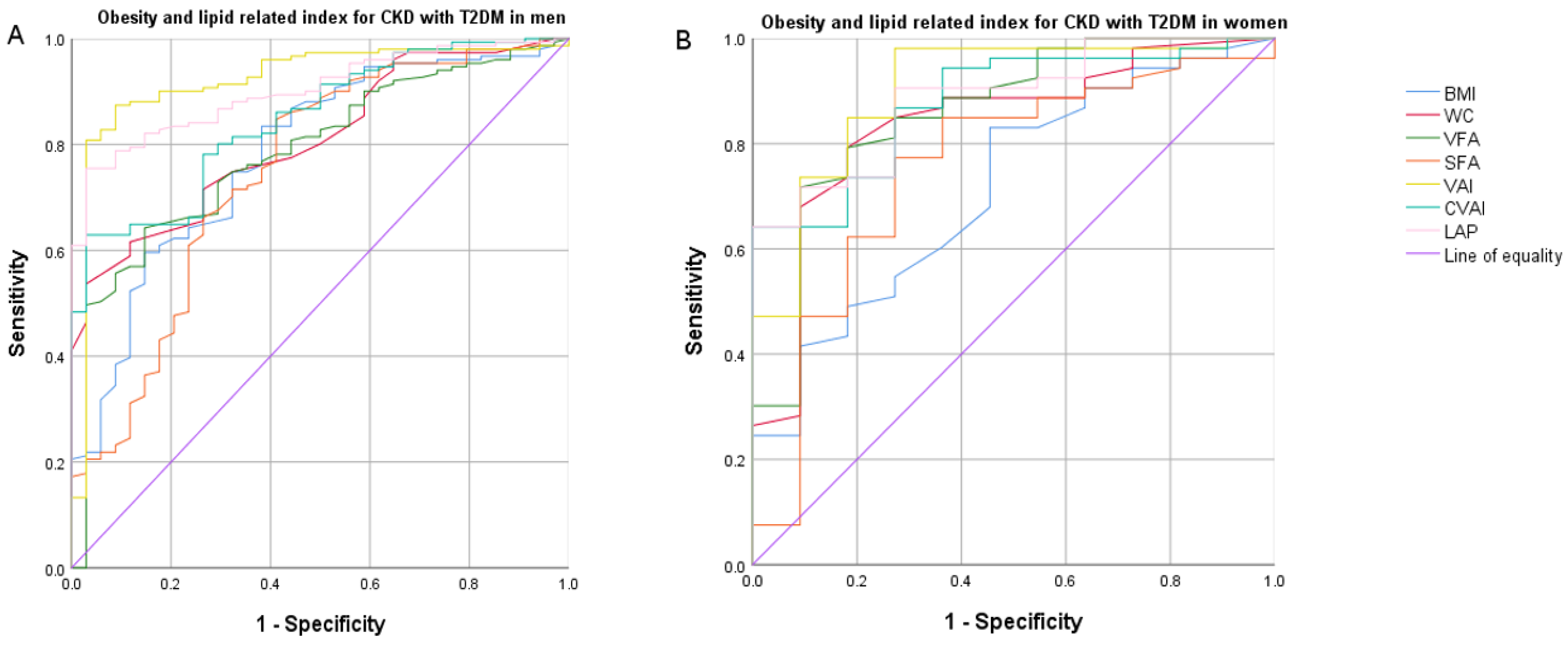
3.4. Receiver Operating Characteristic (ROC) Curve Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusis, A.J.; Attie, A.D.; Reue, K. Metabolic syndrome: From epidemiology to systems biology. Nat. Rev. Genet. 2008, 9, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallari, I.; Cannon, C.P.; Braunwald, E.; Goodrich, E.L.; Im, K.; Lukas, M.A.; O’Donoghue, M.L. Metabolic syndrome and the risk of adverse cardiovascular events after an acute coronary syndrome. Eur. J. Prev. Cardiol. 2018, 25, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Qin, Y.; Chen, S.; Yu, C.; Xu, Y.; Zhao, J.; Yang, X.; Li, N.; Pan, X.F. Association between metabolic syndrome and incident chronic kidney disease among Chinese: A nation-wide cohort study and updated meta-analysis. Diabetes Metab. Res. Rev. 2021, 37, e3437. [Google Scholar] [CrossRef]
- Tsai, H.J.; Wu, P.Y.; Huang, J.C.; Chen, S.C. Environmental Pollution and Chronic Kidney Disease. Int. J. Med. Sci. 2021, 18, 1121–1129. [Google Scholar] [CrossRef]
- Lunyera, J.; Mohottige, D.; Von Isenburg, M.; Jeuland, M.; Patel, U.D.; Stanifer, J.W. CKD of Uncertain Etiology: A Systematic Review. Clin. J. Am. Soc. Nephrol. 2016, 11, 379–385. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [Green Version]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Kari, J.A. Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 198–203. [Google Scholar] [CrossRef]
- Ma, A.; Liu, F.; Wang, C.; Liang, K.; Yan, F.; Hou, X.; Liu, J.; Chen, L. Both insulin resistance and metabolic syndrome accelerate the progression of chronic kidney disease among Chinese adults: Results from a 3-year follow-up study. Int. Urol. Nephrol. 2018, 50, 2239–2244. [Google Scholar] [CrossRef]
- Gomez-Huelgas, R.; Martinez-Castelao, A.; Artola, S.; Gorriz, J.L.; Menendez, E.; en nombre del Grupo de Trabajo para el Documento de Consenso sobre el tratamiento de la diabetes tipo 2 en el paciente con enfermedad renal crónica. Treatment of type 2 diabetes mellitus in patients with chronic kidney disease. Med. Clin. 2014, 142, 85.e1–85.e10. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-Related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Furler, J.; Paul, T.V.; Thomas, N.; Oldenburg, B. Normal Weight Obesity: An Underrecognized Problem in Individuals of South Asian Descent. Clin. Ther. 2019, 41, 1638–1642. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Leonard, M.B.; Shults, J.; Zemel, B.S. A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J. Clin. Endocrinol. Metab. 2014, 99, 3208–3216. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Liu, Y.; Ding, C.; Liu, S.; Chen, X.; Bian, X. Comparison of visceral fat area measured by CT and bioelectrical impedance analysis in Chinese patients with gastric cancer: A cross-sectional study. BMJ Open 2020, 10, e036335. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Funahashi, T.; Matsuzawa, Y.; Shimomura, I. Visceral adiposity as a target for the management of the metabolic syndrome. Ann. Med. 2012, 44, 233–241. [Google Scholar] [CrossRef]
- Xia, M.F.; Chen, Y.; Lin, H.D.; Ma, H.; Li, X.M.; Aleteng, Q.; Li, Q.; Wang, D.; Hu, Y.; Pan, B.S.; et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci. Rep. 2016, 6, 38214. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study, G. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord 2005, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Society, C.D. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Diabetes Mellit. 2021, 13, 315–409. [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseini-Esfahani, F.; Azizi, F. Fast food consumption and the risk of metabolic syndrome after 3-years of follow-up: Tehran Lipid and Glucose Study. Eur. J. Clin. Nutr. 2013, 67, 1303–1309. [Google Scholar] [CrossRef]
- Gallardo-Alfaro, L.; Bibiloni, M.D.M.; Mascaro, C.M.; Montemayor, S.; Ruiz-Canela, M.; Salas-Salvado, J.; Corella, D.; Fito, M.; Romaguera, D.; Vioque, J.; et al. Leisure-Time Physical Activity, Sedentary Behaviour and Diet Quality are Associated with Metabolic Syndrome Severity: The PREDIMED-Plus Study. Nutrients 2020, 12, 1013. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wang, L.; Li, M.; Xu, Y.; Jiang, Y.; Wang, W.; Li, J.; Mi, S.; Zhang, M.; Li, Y.; et al. Metabolic Syndrome Among Adults in China: The 2010 China Noncommunicable Disease Surveillance. J. Clin. Endocrinol. Metab. 2017, 102, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Chang, F.C.; Lee, M.C.; Chiang, C.K.; Liu, J.S.; Lai, T.S.; Chiang, W.C.; Chen, Y.M.; Chu, T.S. Angiopoietin-2 is associated with metabolic syndrome in chronic kidney disease. J. Formos. Med. Assoc. 2021, 120, 2113–2119. [Google Scholar] [CrossRef]
- Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition). Zhonghua Nei Ke Za Zhi 2022, 61, 12–50. [Google Scholar] [CrossRef]
- Morange, P.E.; Alessi, M.C. Thrombosis in central obesity and metabolic syndrome: Mechanisms and epidemiology. Thromb. Haemost. 2013, 110, 669–680. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Thokanit, N.S.; Katavetin, P.; Susanthitaphong, P.; Srisawat, N.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S. The magnitude of obesity and metabolic syndrome among diabetic chronic kidney disease population: A nationwide study. PLoS ONE 2018, 13, e0196332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, C.P.S.; Diniz, A.D.S.; Arruda, I.K.G.; Leite, A.; Petribu, M.M.V.; Rodrigues, I.G. Waist circumference measurement sites and their association with visceral and subcutaneous fat and cardiometabolic abnormalities. Arch. Endocrinol. Metab. 2018, 62, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. The concept of metabolic syndrome: Contribution of visceral fat accumulation and its molecular mechanism. J. Atheroscler. Thromb. 2011, 18, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Biyik, Z.; Guney, I. Lipid accumulation product and visceral adiposity index: Two new indices to predict metabolic syndrome in chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhan, L.; Yuan, J.; Tong, X.; Peng, Y.; Zha, Y. Comparison of visceral, general and central obesity indices in the prediction of metabolic syndrome in maintenance hemodialysis patients. Eat. Weight Disord. 2020, 25, 727–734. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Y.; Xiang, Q.; Fang, S.; Chen, Y.; Chen, C.; Zhang, W.; Zhang, H.; Xia, F.; Wang, N.; et al. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc. Diabetol. 2020, 19, 118. [Google Scholar] [CrossRef]
- Dai, D.; Chang, Y.; Chen, Y.; Chen, S.; Yu, S.; Guo, X.; Sun, Y. Visceral Adiposity Index and Lipid Accumulation Product Index: Two Alternate Body Indices to Identify Chronic Kidney Disease among the Rural Population in Northeast China. Int. J. Environ. Res. Public Health 2016, 13, 1231. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lai, S.H.; Tsai, Y.W.; Chang, S.S. Visceral Adiposity Index as a Predictor of Chronic Kidney Disease in a Relatively Healthy Population in Taiwan. J. Ren. Nutr. 2018, 28, 91–100. [Google Scholar] [CrossRef]
- Bellafronte, N.T.; Sizoto, G.R.; Vega-Piris, L.; Chiarello, P.G.; Cuadrado, G.B. Bed-Side measures for diagnosis of low muscle mass, sarcopenia, obesity, and sarcopenic obesity in patients with chronic kidney disease under non-dialysis-dependent, dialysis dependent and kidney transplant therapy. PLoS ONE 2020, 15, e0242671. [Google Scholar] [CrossRef]

| Variable | CKD without T2DM (n = 158) | CKD with T2DM (n = 214) | ||||
|---|---|---|---|---|---|---|
| MetS− (n = 79) | MetS+ (n = 79) | p-Value | MetS− (n = 42) | MetS+ (n = 172) | p-Value | |
| Age (years) | 41.05 ± 14.86 | 45.58 ± 15.59 | 0.112 | 57.36 ± 8.36 | 53.56 ± 10.57 | 0.041 |
| BMI (Kg/m2) | 23.9 (21.6, 25.9) | 27.3 (25.1, 29.8) | <0.001 | 22.85 (21.45, 25.63) | 26.6 (24.46, 28.7) | <0.001 |
| Waist circumference (cm) | 81.33 ± 8.53 | 93.01 ± 9.33 | <0.001 | 81 (75, 85.5) | 90 (83, 95) | <0.001 |
| Systolic blood pressure (mmHg) | 124.8 ± 13.29 | 133.54 ± 14.29 | <0.001 | 149.55 ± 24.67 | 152.99 ± 22.83 | 0.371 |
| Diastolic blood pressure (mmHg) | 74.82 ± 9.49 | 80.72 ± 11.09 | 0.001 | 84.74 ± 12.41 | 89.52 ± 12.64 | 0.041 |
| FBG (mmol/L) | 4.48 (4.17, 4.74) | 4.56 (4.3, 5.45) | 0.015 | 5.32 (4.46, 6.71) | 5.61 (4.69, 6.89) | 0.463 |
| TC (mmol/L) | 4.21 (3.49, 4.83) | 4.19 (3.65, 5.18) | 0.688 | 3.94 (3.31, 4.99) | 4.44 (3.58, 5.61) | 0.463 |
| TG (mmol/L) | 1.37 (1.07, 1.75) | 2.41 (1.91, 3.22) | <0.001 | 1.17 (0.95, 1.32) | 2.06 (1.55, 2.96) | <0.001 |
| HDL-C (mmol/L) | 1.16 (0.93, 1.41) | 0.86 (0.76, 0.98) | <0.001 | 1.24 (1.12, 1.39) | 0.88 (0.75, 1.02) | <0.001 |
| LDL-C (mmol/L) | 2.75 (2.15, 3.14) | 2.55 (1.98, 3.22) | 0.279 | 2.53 (1.91, 3.53) | 2.62 (1.97, 3.48) | 0.706 |
| eGFR, mL/min per 1.73 m2 | 55.75 (35.84, 95.25) | 43.59 (26.86, 61.16) | 0.003 | 34.85 (18.33, 69.97) | 34.96 (15.86, 64.71) | 0.853 |
| Hemoglobin (g/L) | 127.15 ± 20.55 | 131.43 ± 22.45 | 0.213 | 115.36 ± 22.35 | 122.39 ± 25.146 | 0.099 |
| Creatinine (mmol/L) | 107.6 (80.9, 159.3) | 135.8 (107.1, 192) | 0.003 | 147.15 (81.83, 275.2) | 159.55 (94.68, 285.93) | 0.753 |
| Uric acid (mmol/L) | 404.28 ± 100.38 | 434.56 ± 113.14 | 0.077 | 381.28 ± 99.64 | 420.95 ± 113.17 | 0.038 |
| Hypertension, n (%) | 38 (48.1) | 64 (81) | <0.001 | 33 (78.6) | 162 (94.2) | 0.004 |
| Cardiovascular disease, n (%) | 9 (11.4) | 8 (10.1) | 1 | 17 (40.5) | 63 (36.6) | 0.385 |
| VFA (cm2) | 79.54 ± 30.23 | 134.58 ± 41.27 | <0.001 | 64 (43, 99) | 119 (90, 148) | <0.001 |
| SFA (cm2) | 174 (130, 204) | 235 (205, 276) | <0.001 | 141 (115, 184) | 204 (168, 239) | <0.001 |
| VAI | 1.5 (1.04, 2.13) | 3.48 (2.75, 4.64) | <0.001 | 0.99 (0.88, 1.39) | 2.97 (1.98, 4.89) | <0.001 |
| CVAI | 69.26 ± 38.33 | 130.06 ± 38.94 | <0.001 | 80.34 (50.96, 104.24) | 120.36 (94.43, 143.42) | <0.001 |
| LAP | 24.65 (12.06, 33.75) | 67.41 (47.32, 93.21) | <0.001 | 18.53 (10.71, 23.98) | 46.38 (30.77, 81) | <0.001 |
| Variable | CKD without T2DM (n = 95) | CKD with T2DM (n = 70) | ||||
|---|---|---|---|---|---|---|
| MetS− (n = 69) | MetS+ (n = 26) | p-Value | MetS− (n = 11) | MetS+ (n = 59) | p-Value | |
| Age (years) | 46.26 ± 11.89 | 49.35 ± 13.28 | 0.169 | 56.82 ± 10.69 | 58.86 ± 9.11 | 0.462 |
| BMI (Kg/m2) | 22.91 ± 3.78 | 26.16 ± 3.94 | 0.001 | 23.31 ± 2.65 | 26.06 ± 3.75 | 0.03 |
| Waist circumference (cm) | 76 (70, 81.5) | 88.5 (80.75, 91.25) | <0.001 | 77.64 ± 6.31 | 87.19 ± 7.95 | <0.001 |
| Systolic blood pressure (mmHg) | 119 (108, 135) | 129 (118.5, 139) | 0.048 | 134.55 ± 23.36 | 151.9 ± 24.07 | 0.026 |
| Diastolic blood pressure (mmHg) | 76 (68.5, 83.5) | 78 (71.5, 88.25) | 0.185 | 77.64 ± 12.53 | 85.63 ± 11.69 | 0.026 |
| FBG (mmol/L) | 4.43 (4.03, 4.78) | 4.57 (4.13, 4.87) | 0.339 | 5.35 (4.79, 7.03) | 5.91 (4.94, 7.1) | 0.508 |
| TC (mmol/L) | 4.85 (4.15, 5.59) | 4.34 (3.44, 5.15) | 0.021 | 4.2 (3.77, 4.83) | 5.11 (4.07, 6.17) | 0.11 |
| TG (mmol/L) | 1.53 (1.17, 2.17) | 2.39 (1.81, 3.39) | <0.001 | 1.2 (1, 1.64) | 2.43 (1.83, 3.72) | <0.001 |
| HDL-C (mmol/L) | 1.37 (1.17, 1.65) | 0.91 (0.87, 1.09) | <0.001 | 1.26 (1.07, 1.54) | 1.04 (0.85, 1.18) | 0.006 |
| LDL-C (mmol/L) | 2.95 (2.48, 3.67) | 2.69 (1.74, 3.45) | 0.072 | 2.71 (2.14, 3.23) | 3.02 (2.1, 3.96) | 0.366 |
| eGFR, mL/min per 1.73 m2 | 53.43 (31.44, 95.07) | 33.76 (24.07, 51.46) | 0.005 | 70.41 ± 32.05 | 59 ± 33.59 | 0.313 |
| Hemoglobin (g/L) | 112.58 ± 15.83 | 108.12 ± 16.47 | 0.229 | 117.09 ± 18.43 | 115.71 ± 20.86 | 0.838 |
| Creatinine (mmol/L) | 112.2 (68.5, 165.65) | 159.75 (116.55, 215.63) | 0.008 | 89.9 (59.1, 128.5) | 103.5 (70.8, 156.3) | 0.415 |
| Uric acid (mmol/L) | 349.19 ± 96.79 | 382.84 ± 92.22 | 0.129 | 348 (233.4, 385.6) | 358.6 (287.7, 441.1) | 0.255 |
| Hypertension, n (%) | 40 (58) | 23 (88.5) | 0.007 | 6 (54.5) | 53 (89.8) | 0.011 |
| Cardiovascular disease, n(%) | 5 (7.2) | 3 (11.5) | 0.679 | 1 (9.1) | 18 (30.5) | 0.267 |
| VFA (cm2) | 66.22 ± 27.63 | 92.19 ± 31.32 | <0.001 | 51 (34, 82) | 99 (86.5, 119) | <0.001 |
| SFA (cm2) | 149 (102.5, 199.5) | 217.5 (162.75, 263.75) | <0.001 | 151.45 ± 56.73 | 204.26 ± 60.57 | 0.007 |
| VAI | 2.12 (1.36, 2.76) | 4.18 (3.16, 6.36) | <0.001 | 1.69 (1.3, 2.35) | 4.41 (2.69, 7.23) | <0.001 |
| CVAI | 68.55 ± 39.82 | 111.12 ± 34.13 | <0.001 | 85.61 ± 24.98 | 125.94 ± 27.93 | <0.001 |
| LAP | 28.88 (16.95, 43.88) | 72.17 (46.96, 90.56) | <0.001 | 26.5 (13, 47.12) | 72.9 (46.6, 103.04) | <0.001 |
| Variable | Optimal Cut-Offs | Youden Index | Sensitivity (%) | Specificity (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| CKD with T2DM (Men) | ||||||
| BMI | 23.6 | 0.4723 | 83.82 | 63.41 | 1.378 (1.187–1.600) | <0.001 |
| WC | 87 | 0.5048 | 61.3 | 89.2 | 1.160 (1.086–1.240) | <0.001 |
| VFA (cm2) | 101 | 0.5053 | 63.4 | 87.2 | 1.021 (1.010–1.032) | <0.001 |
| SFA (cm2) | 149 | 0.4539 | 83.85 | 61.54 | 1.019 (1.010–1.028) | <0.001 |
| VAI | 1.51 | 0.7802 | 86.84 | 91.18 | 40.585 (8.683–189.695) | <0.001 |
| CVAI | 111.21 | 0.5956 | 62.5 | 97.06 | 1.050 (1.030–1.071) | <0.001 |
| LAP | 31.44 | 0.7182 | 74.68 | 97.14 | 1.145 (1.083–1.209) | <0.001 |
| CKD with T2DM (Women) | ||||||
| BMI | 22.7 | 0.3421 | 79.66 | 54.55 | 1.320 (1.029–1.694) | 0.029 |
| WC | 81 | 0.6106 | 79.25 | 81.82 | 1.226 (1.070–1.405) | 0.003 |
| VFA (cm2) | 88 | 0.6284 | 71.93 | 90.91 | 1.066 (1.025–1.109) | 0.001 |
| SFA (cm2) | 159 | 0.4992 | 77.19 | 72.73 | 1.020 (1.004–1.035) | 0.012 |
| VAI | 1.806 | 0.7084 | 98.11 | 72.73 | 5.076 (1.247–20.657) | 0.023 |
| CVAI | 117.78 | 0.6415 | 64.15 | 100 | 1.069 (1.027–1.114) | 0.001 |
| LAP | 56.76 | 0.6415 | 64.15 | 100 | 1.100 (1.030–1.175) | 0.004 |
| CKD without T2DM (Men) | ||||||
| BMI | 26.9 | 0.4684 | 56.96 | 89.87 | 1.441 (1.247–1.664) | <0.001 |
| WC | 89 | 0.5823 | 69.62 | 88.61 | 1.186 (1.114–1.262) | <0.001 |
| VFA (cm2) | 113 | 0.6329 | 72.15 | 91.14 | 1.051 (1.033–1.070) | <0.001 |
| SFA (cm2) | 208 | 0.5316 | 74.68 | 78.48 | 1.027 (1.017–1.037) | <0.001 |
| VAI | 2.35 | 0.6962 | 83.54 | 86.08 | 7.514 (3.757–15.027) | <0.001 |
| CVAI | 113.09 | 0.6329 | 73.42 | 89.87 | 1.055 (1.036–1.075) | <0.001 |
| LAP | 39.82 | 0.7089 | 86.08 | 84.81 | 1.137 (1.083–1.193) | <0.001 |
| CKD without T2DM (Women) | ||||||
| BMI | 25.4 | 0.4365 | 65.38 | 78.26 | 1.305 (1.121–1.520) | 0.001 |
| WC | 84 | 0.5474 | 69.23 | 85.51 | 1.127 (1.058–1.200) | <0.001 |
| VFA (cm2) | 71 | 0.3924 | 76.92 | 62.32 | 1.037 (1.015–1.060) | 0.001 |
| SFA (cm2) | 201 | 0.4509 | 65.38 | 79.71 | 1.011 (1.005–1.018) | 0.001 |
| VAI | 3.11 | 0.6844 | 84.62 | 83.82 | 3.008 (1.789–5.056) | <0.001 |
| CVAI | 75.708 | 0.4581 | 88.46 | 57.35 | 1.056 (1.029–1.084) | <0.001 |
| LAP | 34.92 | 0.6042 | 92.31 | 68.12 | 1.103 (1.054–1.154) | <0.001 |
| Variable | MetS-China (2020) Criterion | MetS-NCEP-ATPIII Criterion | MetS-IDF Criterion | |||
|---|---|---|---|---|---|---|
| AUC (95% CI) | p-Value | AUC (95% CI) | p-Value | AUC (95% CI) | p-Value | |
| CKD with T2DM (Men) | ||||||
| BMI | 0.782 (0.740–0.876) | <0.001 | 0.686 (0.597–0.776) | <0.001 | 0.897 (0.855–0.94) | <0.001 |
| WC | 0.808 (0.740–0.876) | <0.001 | 0.694 (0.610–0.778) | <0.001 | 0.998 (0.995–1) | <0.001 |
| VFA (cm2) | 0.785 (0.706–0.864) | <0.001 | 0.707 (0.622–0.792) | <0.001 | 0.915 (0.874–0.956) | <0.001 |
| SFA (cm2) | 0.750 (0.652–0.847) | <0.001 | 0.663 (0.569–0.757) | 0.001 | 0.928 (0.893–0.963) | <0.001 |
| VAI | 0.920 (0.864–0.976) | <0.001 | 0.957 (0.919–0.995) | <0.001 | 0.695 (0.619–0.771) | <0.001 |
| CVAI | 0.847 (0.785–0.908) | <0.001 | 0.765 (0.691–0.838) | <0.001 | 0.973 (0.953–0.992) | <0.001 |
| LAP | 0.902 (0.857–0.946) | <0.001 | 0.858 (0.806–0.911) | <0.001 | 0.860 (0.808–0.912) | <0.001 |
| CKD with T2DM (Women) | ||||||
| BMI | 0.715 (0.555–0.876) | 0.026 | 0.744 (0.610–0.879) | 0.036 | 0.906 (0.820–0.993) | <0.001 |
| WC | 0.839 (0.708–0.970) | <0.001 | 0.807 (0.676–0.938) | 0.008 | 1(1–1) | <0.001 |
| VFA (cm2) | 0.860 (0.734–0.987) | <0.001 | 0.882 (0.784–0.981) | 0.001 | 0.971 (0.936–1) | <0.001 |
| SFA (cm2) | 0.754 (0.586–0.921) | 0.008 | 0.773 (0.639–0.907) | 0.019 | 0.971 (0.928–1) | <0.001 |
| VAI | 0.902 (0.8–1) | <0.001 | 0.965 (0.912–1) | <0.001 | 0.760 (0.604–0.916) | 0.003 |
| CVAI | 0.878 (0.782–0.975) | <0.001 | 0.862 (0.752–0.972) | 0.002 | 0.913 (0.832–0.993) | <0.001 |
| LAP | 0.885 (0.794–0.976) | <0.001 | 0.865 (0.768–0.962) | 0.002 | 0.890 (0.776–1) | <0.001 |
| CKD without T2DM (Men) | ||||||
| BMI | 0.798 (0.729–0.867) | <0.001 | 0.704 (0.621–0.786) | <0.001 | 0.917 (0.875–0.959) | <0.001 |
| WC | 0.830 (0.765–0.894) | <0.001 | 0.717 (0.635–0.789) | <0.001 | 0.964 (0.937–0.991) | <0.001 |
| VFA (cm2) | 0.865 (0.807–0.922) | <0.001 | 0.741 (0.662–0.820) | <0.001 | 0.947 (0.915–0.979) | <0.001 |
| SFA (cm2) | 0.820 (0.756–0.885) | <0.001 | 0.727 (0.648–0.806) | <0.001 | 0.903 (0.857–0.949) | <0.001 |
| VAI | 0.910 (0.866–0.955) | <0.001 | 0.895 (0.844–0.945) | <0.001 | 0.776 (0.703–0.849) | <0.001 |
| CVAI | 0.879 (0.826–0.931) | <0.001 | 0.773 (0.7–0.847) | <0.001 | 0.980 (0.963–0.998) | <0.001 |
| LAP | 0.921 (0.881–0.961) | <0.001 | 0.848 (0.789–0.907) | <0.001 | 0.909 (0.863–0.954) | <0.001 |
| CKD without T2DM (Women) | ||||||
| BMI | 0.731 (0.618–0.844) | 0.001 | 0.751 (0.648–0.855) | <0.001 | 0.867 (0.794–0.940) | <0.001 |
| WC | 0.793 (0.689–0.897) | <0.001 | 0.778 (0.678–0.877) | <0.001 | 0.923 (0.868–0.978) | <0.001 |
| VFA (cm2) | 0.734 (0.617–0.852) | <0.001 | 0.679 (0.562–0.796) | 0.005 | 0.851 (0.773–0.930) | <0.001 |
| SFA (cm2) | 0.741 (0.633–0.850) | <0.001 | 0.734 (0.630–0.839) | <0.001 | 0.890 (0.826–0.955) | <0.001 |
| VAI | 0.881 (0.812–0.949) | <0.001 | 0.921 (0.869–0.973) | <0.001 | 0.824 (0.741–0.908) | <0.001 |
| CVAI | 0.782 (0.684–0.881) | <0.001 | 0.835 (0.753–0.918) | <0.001 | 0.896 (0.833–0.960) | <0.001 |
| LAP | 0.854 (0.769–0.939) | <0.001 | 0.891 (0.821–0.960) | <0.001 | 0.905 (0.845–0.965) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, Q.; Ke, J.; Lin, W.; Luo, Y.; Yao, J.; Zhang, W.; Zhang, L.; Duan, S.; Dong, Z.; et al. Optimal Obesity- and Lipid-Related Indices for Predicting Metabolic Syndrome in Chronic Kidney Disease Patients with and without Type 2 Diabetes Mellitus in China. Nutrients 2022, 14, 1334. https://doi.org/10.3390/nu14071334
Li H, Wang Q, Ke J, Lin W, Luo Y, Yao J, Zhang W, Zhang L, Duan S, Dong Z, et al. Optimal Obesity- and Lipid-Related Indices for Predicting Metabolic Syndrome in Chronic Kidney Disease Patients with and without Type 2 Diabetes Mellitus in China. Nutrients. 2022; 14(7):1334. https://doi.org/10.3390/nu14071334
Chicago/Turabian StyleLi, Hangtian, Qian Wang, Jianghua Ke, Wenwen Lin, Yayong Luo, Jin Yao, Weiguang Zhang, Li Zhang, Shuwei Duan, Zheyi Dong, and et al. 2022. "Optimal Obesity- and Lipid-Related Indices for Predicting Metabolic Syndrome in Chronic Kidney Disease Patients with and without Type 2 Diabetes Mellitus in China" Nutrients 14, no. 7: 1334. https://doi.org/10.3390/nu14071334
APA StyleLi, H., Wang, Q., Ke, J., Lin, W., Luo, Y., Yao, J., Zhang, W., Zhang, L., Duan, S., Dong, Z., & Chen, X. (2022). Optimal Obesity- and Lipid-Related Indices for Predicting Metabolic Syndrome in Chronic Kidney Disease Patients with and without Type 2 Diabetes Mellitus in China. Nutrients, 14(7), 1334. https://doi.org/10.3390/nu14071334






