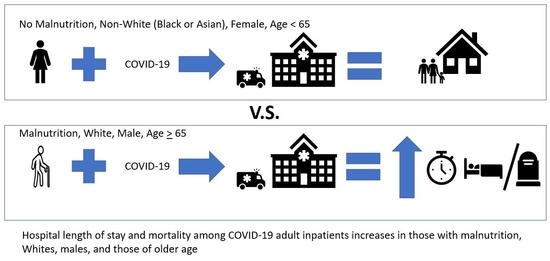
Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
2.2.1. Descriptive Analysis
2.2.2. Multivariate Analysis
2.2.3. Sensitivity Analysis
2.2.4. Software
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halaji, M.; Heiat, M.; Faraji, N.; Ranjbar, R. Epidemiology of COVID-19: An updated review. J. Res. Med. Sci. 2021, 26, 82. [Google Scholar] [CrossRef] [PubMed]
- Malviya, A.; Ahirwar, A.K.; Chandra Tripathi, S.; Asia, P.; Gopal, N.; Kaim, K. COVID-19: A review on SARS-CoV-2 origin, epidemiology, virology, clinical manifestations and complications with special emphasis on adverse outcome in Bhopal Gas Tragedy survivor. Horm. Mol. Biol. Clin. Investig. 2021, 42, 63–68. [Google Scholar] [CrossRef]
- Martinot, M.; Eyriey, M.; Gravier, S.; Bonijoly, T.; Kayser, D.; Ion, C.; Mohseni-Zadeh, M.; Camara, S.; Dubois, J.; Haerrel, E.; et al. Predictors of mortality, ICU hospitalization, and extrapulmonary complications in COVID-19 patients. Infect. Dis. Now 2021, 51, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; Senmaru, T.; et al. Sarcopenia Is Associated With a Risk of Mortality in People with Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 783363. [Google Scholar] [CrossRef] [PubMed]
- An, J.N.; Kim, J.K.; Lee, H.S.; Kim, S.G.; Kim, H.J.; Song, Y.R. Late stage 3 chronic kidney disease is an independent risk factor for sarcopenia, but not proteinuria. Sci. Rep. 2021, 11, 18472. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Arango-Lopera, V.E.; Arroyo, P.; Gutierrez-Robledo, L.M.; Perez-Zepeda, M.U.; Cesari, M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health Aging 2013, 17, 259–262. [Google Scholar] [CrossRef]
- Antwi, J.; Appiah, B.; Oluwakuse, B.; Abu, B.A.Z. The Nutrition-COVID-19 Interplay: A Review. Curr. Nutr. Rep. 2021, 10, 364–374. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, B.; Dang, Q.; Chen, Z.; Zhou, Q.; Luo, H.; Yuan, W.; Sun, Z. Pathogenesis and Mechanism of Gastrointestinal Infection with COVID-19. Front. Immunol. 2021, 12, 674074. [Google Scholar] [CrossRef]
- Somanchi, M.; Tao, X.; Mullin, G.E. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. J. Parenter. Enter. Nutr. 2011, 35, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Compher, C.; Sullivan, D.H.; Mullin, G.E. Recognizing malnutrition in adults: Definitions and characteristics, screening, assessment, and team approach. J. Parenter. Enter. Nutr. 2013, 37, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Ziegler, T.R.; Matheson, E.M.; Matarese, L.E.; Tappenden, K.A.; Baggs, G.E.; Nelson, J.L.; Luo, M.; Hegazi, R.; Jonnalagadda, S.S.; et al. Reduced mortality risk in malnourished hospitalized older adult patients with COPD treated with a specialized oral nutritional supplement: Sub-group analysis of the NOURISH study. Clin. Nutr. 2021, 40, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Rathinasabapathy, A.; Liu, D.; Zha, L.; Liu, X.; Tang, Y.; Li, F.; Lin, W.; Yu, Z.; Chen, H. Association of cardiac injury with hypertension in hospitalized patients with COVID-19 in China. Sci. Rep. 2021, 11, 22389. [Google Scholar] [CrossRef]
- De Blasio, F.; Di Gregorio, A.; de Blasio, F.; Bianco, A.; Bellofiore, B.; Scalfi, L. Malnutrition and sarcopenia assessment in patients with chronic obstructive pulmonary disease according to international diagnostic criteria, and evaluation of raw BIA variables. Respir. Med. 2018, 134, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mete, B.; Pehlivan, E.; Gulbas, G.; Gunen, H. Prevalence of malnutrition in COPD and its relationship with the parameters related to disease severity. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3307–3312. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Shi, X.; Fu, W.; Xiang, F.; He, X.; Yang, B.; Wang, X.; Ma, W.L. Gut Microbiota Dysbiosis Correlates with Abnormal Immune Response in Moderate COVID-19 Patients with Fever. J. Inflamm. Res. 2021, 14, 2619–2631. [Google Scholar] [CrossRef]
- Megyeri, K.; Dernovics, A.; Al-Luhaibi, Z.I.I.; Rosztoczy, A. COVID-19-associated diarrhea. World J. Gastroenterol. 2021, 27, 3208–3222. [Google Scholar] [CrossRef]
- Faxen-Irving, G.; Luiking, Y.; Gronstedt, H.; Franzen, E.; Seiger, A.; Vikstrom, S.; Wimo, A.; Bostrom, A.M.; Cederholm, T. Do Malnutrition, Sarcopenia and Frailty Overlap in Nursing-Home Residents? J. Frailty Aging 2021, 10, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Mullin, G.E.; Fan, L.; Sulo, S.; Partridge, J. The Association between Oral Nutritional Supplements and 30-Day Hospital Readmissions of Malnourished Patients at a US Academic Medical Center. J. Acad. Nutr. Diet. 2019, 119, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, G.C.; Munsell, M.; Brant, S.R.; LaVeist, T.A. Racial and geographic disparities in the use of parenteral nutrition among inflammatory bowel disease inpatients diagnosed with malnutrition in the United States. J. Parenter. Enter. Nutr. 2009, 33, 563–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spring, A.M.; Catalano, M.A.; Rutkin, B.; Hartman, A.; Yu, P.J. Racial and socioeconomic disparities in urgent transcatheter mitral valve repair: A National Inpatient Sample analysis. J. Card. Surg. 2021, 36, 3224–3229. [Google Scholar] [CrossRef]
- Nagarajan, N.; Rahman, S.; Boss, E.F. Are There Racial Disparities in Family-Reported Experiences of Care in Inpatient Pediatrics? Clin. Pediatr. 2017, 56, 619–626. [Google Scholar] [CrossRef]
- Vilar-Compte, M.; Burrola-Mendez, S.; Lozano-Marrufo, A.; Ferre-Eguiluz, I.; Flores, D.; Gaitan-Rossi, P.; Teruel, G.; Perez-Escamilla, R. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: A global systematic literature review. Int. J. Equity Health 2021, 20, 40. [Google Scholar] [CrossRef]
- Sheean, P.; Farrar, I.C.; Sulo, S.; Partridge, J.; Schiffer, L.; Fitzgibbon, M. Nutrition risk among an ethnically diverse sample of community-dwelling older adults. Public Health Nutr. 2019, 22, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Allard, L.; Ouedraogo, E.; Molleville, J.; Bihan, H.; Giroux-Leprieur, B.; Sutton, A.; Baudry, C.; Josse, C.; Didier, M.; Deutsch, D.; et al. Malnutrition: Percentage and Association with Prognosis in Patients Hospitalized for Coronavirus Disease 2019. Nutrients 2020, 12, 3679. [Google Scholar] [CrossRef]
- Da Porto, A.; Tascini, C.; Peghin, M.; Sozio, E.; Colussi, G.; Casarsa, V.; Bulfone, L.; Graziano, E.; De Carlo, C.; Catena, C.; et al. Prognostic Role of Malnutrition Diagnosed by Bioelectrical Impedance Vector Analysis in Older Adults Hospitalized with COVID-19 Pneumonia: A Prospective Study. Nutrients 2021, 13, 4085. [Google Scholar] [CrossRef]
- Bedock, D.; Bel Lassen, P.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J. Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balci, C.; Bolayir, B.; Esme, M.; Arik, G.; Kuyumcu, M.E.; Yesil, Y.; Varan, H.D.; Kara, O.; Gungor, A.E.; Dogu, B.B.; et al. Comparison of the Efficacy of the Global Leadership Initiative on Malnutrition Criteria, Subjective Global Assessment, and Nutrition Risk Screening 2002 in Diagnosing Malnutrition and Predicting 5-Year Mortality in Patients Hospitalized for Acute 1Illnesses. J. Parenter. Enter. Nutr. 2021, 45, 1172–1180. [Google Scholar] [CrossRef]
- Moriana, M.; Civera, M.; Artero, A.; Real, J.T.; Caro, J.; Ascaso, J.F.; Martinez-Valls, J.F. Validity of subjective global assessment as a screening method for hospital malnutrition. Prevalence of malnutrition in a tertiary hospital. Endocrinol. Nutr. 2014, 61, 184–189. [Google Scholar] [CrossRef]
- Sauer, A.C.; Goates, S.; Malone, A.; Mogensen, K.M.; Gewirtz, G.; Sulz, I.; Moick, S.; Laviano, A.; Hiesmayr, M. Prevalence of Malnutrition Risk and the Impact of Nutrition Risk on Hospital Outcomes: Results From nutritionDay in the U.S. J. Parenter. Enter. Nutr. 2019, 43, 918–926. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, I.M.Y.; Gomes-Neto, A.W.; de Jong, M.F.C.; Jager-Wittenaar, H.; Navis, G.J. High prevalence of malnutrition both on hospital admission and predischarge. Nutrition 2020, 77, 110814. [Google Scholar] [CrossRef]
- Sobestiansky, S.; Aberg, A.C.; Cederholm, T. Sarcopenia and malnutrition in relation to mortality in hospitalised patients in geriatric care-predictive validity of updated diagnoses. Clin. Nutr. ESPEN 2021, 45, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Zhu, S.; Wang, H.; Chen, J.; Zhang, X.; Xu, P.; Xie, Y.; Zhu, X.; Zhu, W.; Sun, W.; et al. Association between malnutrition and long-term mortality in older adults with ischemic stroke. Clin. Nutr. 2021, 40, 2535–2542. [Google Scholar] [CrossRef]
- Czapla, M.; Juarez-Vela, R.; Gea-Caballero, V.; Zielinski, S.; Zielinska, M. The Association between Nutritional Status and In-Hospital Mortality of COVID-19 in Critically-Ill Patients in the ICU. Nutrients 2021, 13, 3302. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.; De Lorenzo, R.; D’Amico, M.; Sofia, V.; Roveri, L.; Mele, R.; Saibene, A.; Rovere-Querini, P.; Conte, C. COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: A post-hoc analysis of a prospective cohort study. Clin. Nutr. 2021, 40, 2420–2426. [Google Scholar] [CrossRef]
- Recinella, G.; Marasco, G.; Serafini, G.; Maestri, L.; Bianchi, G.; Forti, P.; Zoli, M. Prognostic role of nutritional status in elderly patients hospitalized for COVID-19: A monocentric study. Aging Clin. Exp. Res. 2020, 32, 2695–2701. [Google Scholar] [CrossRef]
- Valladares, A.F.; Kilgore, K.M.; Partridge, J.; Sulo, S.; Kerr, K.W.; McCauley, S. How a Malnutrition Quality Improvement Initiative Furthers Malnutrition Measurement and Care: Results From a Hospital Learning Collaborative. J. Parenter. Enter. Nutr. 2021, 45, 366–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, N.M.; Chen, H.C.; Lechner, M.G.; Su, M.A. Sex Differences in Immunity. Annu. Rev. Immunol. Jan. 5 2022, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Chen, D.; Xie, X.H.; Zhang, J.E.; Zeng, Y.; Cheng, A.S. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, N.; Koike, T.; Kamiya, K.; Hamazaki, N.; Nozaki, K.; Ichikawa, T.; Matsunaga, A.; Kuroiwa, M.; Arai, M. Assessment of Sarcopenia in the Intensive Care Unit and 1-Year Mortality in Survivors of Critical Illness. Nutrients 2021, 13, 2726. [Google Scholar] [CrossRef] [PubMed]
- Dotson, J.L.; Kappelman, M.D.; Chisolm, D.J.; Crandall, W.V. Racial disparities in readmission, complications, and procedures in children with Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 801–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damayanthi, H.; Prabani, K.I.P. Nutritional determinants and COVID-19 outcomes of older patients with COVID-19: A systematic review. Arch. Gerontol. Geriatr. 2021, 95, 104411. [Google Scholar] [CrossRef]
- Nobel, Y.R.; Su, S.H.; Anderson, M.R.; Luk, L.; Small-Saunders, J.L.; Reyes-Soffer, G.; Gallagher, D.; Freedberg, D.E. Relationship Between Body Composition and Death in Patients with COVID-19 Differs Based on the Presence of Gastrointestinal Symptoms. Dig. Dis. Sci. 2021, 1–8. [Google Scholar] [CrossRef]
- Aguila-Gordo, D.; Martinez-Del Rio, J.; Mazoteras-Munoz, V.; Negreira-Caamano, M.; Nieto-Sandoval Martin de la Sierra, P.; Piqueras-Flores, J. Mortality and associated prognostic factors in elderly and very elderly hospitalized patients with respiratory disease COVID-19. Rev. Esp. Geriatr. Gerontol. 2021, 56, 259–267. [Google Scholar] [CrossRef]
- Snider, J.T.; Jena, A.B.; Linthicum, M.T.; Hegazi, R.A.; Partridge, J.S.; LaVallee, C.; Lakdawalla, D.N.; Wischmeyer, P.E. Effect of hospital use of oral nutritional supplementation on length of stay, hospital cost, and 30-day readmissions among Medicare patients with COPD. Chest 2015, 147, 1477–1484. [Google Scholar] [CrossRef] [Green Version]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R.; Group, N.S. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Moran-Lopez, J.M. Malnutrition and nutrition support in COVID-19: The results of a nutrition support protocol. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, R.F.W.; Silva, A.P.; Santana, A.I.C.; Silva, D.S.E.; Ramos, M.S.; Souza, M.C.; Marques Miguel Suen, V.; Maduro, I.; Ribas Filho, D.; D’Oliveira Junior, A.; et al. Effect of immunonutrition on serum levels of C-reactive protein and lymphocytes in patients with COVID-19: A randomized, controlled, double-blind clinical trial. Nutr. Hosp. 2022, 39, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Lengfelder, L.; Mahlke, S.; Moore, L.; Zhang, X.; Williams, G., 3rd; Lee, J. Prevalence and impact of malnutrition on length of stay, readmission, and discharge destination. J. Parenter. Enter. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Nowicki, M.; Mullin, G.E.; Abraham, A.; Rodriguez-Roman, E.; Petzold, M.B.; Bendau, A.; Sahu, K.K.; Ather, A.; Naviaux, A.F.; et al. Quantity does not equal quality: Scientific principles cannot be sacrificed. Int. Immunopharmacol. 2020, 86, 106711. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Fiksel, J.; Muschelli, J.; Robinson, M.L.; Rouhizadeh, M.; Perin, J.; Schumock, G.; Nagy, P.; Gray, J.H.; Malapati, H.; et al. Patient Trajectories Among Persons Hospitalized for COVID-19: A Cohort Study. Ann. Intern. Med. 2021, 174, 33–41. [Google Scholar] [CrossRef]
| No Malnutrition n = 3908; 90.7% | Malnutrition n = 403; 9.3% | ||
|---|---|---|---|
| Characteristic | Mean (SD) | Mean (SD) | T-Test p-Value |
| Age (years) | 58.78 (19.3) | 68.05 (17.2) | <0.0001 |
| Characteristic | Median (IQR) | Median (IQR) | Wilcoxon Test p-Value |
| Body mass index (kg/m2) * | 29.0 (25.0–34.3) | 24.1 (20.2–29.9) | <0.0001 |
| Characteristic | n (%) | n (%) | Chi-Squared Test p-Value |
| Male gender | 1909 (48.9) | 210 (52.1) | 0.213 |
| Race | <0.0001 | ||
| Asian | 184 (4.7) | 13 (3.2) | |
| Black | 1435 (36.7) | 140 (34.7) | |
| Other | 936 (24.0) | 49 (12.2) | |
| White | 1353 (34.6) | 201 (49.9) | |
| Comorbidity | |||
| Diabetes | 1618 (41.4) | 191 (47.4) | 0.020 |
| Hypertension | 2639 (67.53) | 341 (84.6) | <0.0001 |
| Diarrhea | 759 (19.42) | 116 (28.8) | <0.0001 |
| COPD | 308 (7.9) | 46 (11.4) | 0.014 |
| Admission Source | 3022 (77.4) | 236 (58.6) | <0.0001 |
| Home or workplace or non-health care facility; court/law enforcement | |||
| Physician’s office or clinic; other health care facility | 249 (6.4) | 27 (6.7) | |
| Skilled nursing facility, intermediate care facility or assisted living facility | 437 (11.2) | 104 (25.8) | |
| Transfers from another acute care hospital or ED | 195 (5) | 36 (8.9) | |
| Outcomes | |||
| Mortality | 453 (11.6) | 102 (25.3) | <0.0001 |
| ICU Patients | N (%); N = 2956; 90.4% | N (%); N = 313; 9.6% | Chi-Squared Test p-Value |
| WHO Score ** | <0.0001 | ||
| 3 (hospitalized, no oxygen therapy) | 561 (19.0) | 38 (12.1) | |
| 4 (oxygen) | 1389 (47.0) | 106 (33.9) | |
| 5 (NIPPV or hi-flow oxygen) | 264 (8.9) | 16 (5.1) | |
| 6 (intubation and mechanical ventilation) | 66 (2.2) | 5 (1.6) | |
| 7 (ventilation and additional organ support) | 289 (9.8) | 73 (23.3) | |
| 8 (death) | 387 (13.1) | 75 (24.0) | |
| Median (IQR) | Median (IQR) | Wilcoxon Test p-Value | |
| Length of hospital stay (days) | 5.63 (2.73–13.19) | 16.08 (6.32–56.73) | <0.0001 |
| Alive | Deceased | |||
|---|---|---|---|---|
| n = 3756; 87.1% | n = 555; 12.9% | |||
| Variables | Mean (SD) | Mean (SD) | Statistical Test | p-Value |
| Age (years) | 57.42 (18.9) | 74.76 (14.3) | T-test | <0.0001 |
| Body mass index (kg/m2) * | 30.34 (8.2) | 27.66 (7.9) | Wilcoxon | <0.0001 |
| n (%) | n (%) | |||
| Gender | ||||
| Female | 1933 (51.5) | 259 (46.7) | Chi-square | 0.034834 |
| Male | 1823 (48.5) | 296 (53.3) | ||
| Race | ||||
| Asian | 169 (4.5) | 28 (5.1) | Chi-square | <0.0001 |
| Black | 1395 (37.1) | 180 (32.4) | ||
| Other | 917 (24.4) | 68 (12.3) | ||
| White | 1275 (34.0) | 279 (50.3) | ||
| Black | ||||
| No | 2361 (62.9) | 375 (67.6) | Chi-square | 0.031551 |
| Yes | 1395 (37.1) | 180 (32.4) | ||
| Comorbidity | ||||
| Diabetes | 1537 (40.9) | 272 (49.0) | Chi-square | 0.000314 |
| Hypertension | 2501 (66.6) | 479 (86.6) | Chi-square | <0.0001 |
| Diarrhea | 766 (20.4) | 109 (19.6) | Chi-square | 0.680012 |
| COPD | 249 (6.6) | 105 (18.9) | Chi-square | <0.0001 |
| Malnutrition | 301 (8.0) | 102 (18.4) | Chi-square | <0.0001 |
| WHO Score (Grouped) ** | ||||
| Death | 0 | 462 (93.7) | NA | |
| Mild/moderate | 2077 (74.8) | 17 (3.5) | ||
| Severe | 699 ((25.2) | 14 (2.8) | ||
| Admission Source (Grouped) *** | ||||
| Home or workplace or non-health care facility; court/law enforcement | 2946 (78.5) | 312 (56.2) | Chi-square | <0.0001 |
| Physician’s office or clinic; other health care facility | 245 (6.5) | 31 (5.6) | ||
| Skilled nursing facility, intermediate care facility or assisted living facility | 360 (9.6) | 181 (32.6) | ||
| Transfers from another acute care hospital or ED | 200 (5.3) | 31 (5.6) | ||
| Parameter | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Malnutrition | 1.76 | 1.34–2.30 | <0.0001 |
| Age 61–74 (vs. age ≤ 60; years) | 2.98 | 2.22–3.99 | <0.0001 |
| Age ≥ 75 (vs. age ≤ 60; years) | 5.55 | 4.07–7.57 | <0.0001 |
| Body mass index (kg/m2) | 0.99 | 0.98–1.01 | 0.3551 |
| Female gender | 0.76 | 0.63–0.93 | 0.0065 |
| White race | 1.09 | 0.89–1.34 | 0.3871 |
| Diabetes | 1.12 | 0.91–1.38 | 0.2707 |
| Hypertension | 1.33 | 0.99–1.77 | 0.0567 |
| Diarrhea | 0.89 | 0.70–1.14 | 0.3619 |
| COPD | 2.04 | 1.56–2.66 | <0.0001 |
| Admission from nursing home | 2.02 | 1.61–2.55 | <0.0001 |
| Parameter | Beta Estimate | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Malnutrition | 0.72 | 0.587–0.854 | <0.0001 |
| Age (years) | 0.01 | 0.004–0.009 | <0.0001 |
| (log) Body mass index (kg/m2) | −0.08 | −0.247–0.088 | 0.3536 |
| Female gender | −0.12 | −0.197–−0.045 | 0.0019 |
| Black race | −0.12 | −0.216–−0.032 | 0.0082 |
| Asian race | −0.17 | −0.363–0.014 | 0.0704 |
| Other race | −0.17 | −0.277–−0.06 | 0.0024 |
| White race | reference | ||
| Diabetes | 0.27 | 0.188–0.354 | <0.0001 |
| Hypertension | 0.32 | 0.218–0.417 | <0.0001 |
| Diarrhea | 0.27 | 0.174–0.365 | <0.0001 |
| COPD | 0.56 | 0.416–0.698 | <0.0001 |
| Admission from nursing home | −0.27 | −0.397–−0.149 | <0.0001 |
| Parameters | Estimate | Standard Error | Wald Chi-Square | Pr > ChiSq | |
|---|---|---|---|---|---|
| Intercept | 3.1011 | 0.3822 | 65.84 | <0.0001 | |
| Age (years) | 0.0008 | 0.0017 | 0.22 | 0.6425 | |
| (log) Body mass index (kg/m2) | −0.3779 | 0.1017 | 13.81 | 0.0002 | |
| Male | 0.0036 | 0.0472 | 0.01 | 0.9391 | |
| Female | reference | 0 | |||
| Race | |||||
| Asian | −0.342 | 0.1147 | 8.9 | 0.0029 | |
| Black | −0.2073 | 0.057 | 13.24 | 0.0003 | |
| Other | −0.4063 | 0.0674 | 36.4 | <0.0001 | |
| White | reference | 0 | |||
| Comorbidity | |||||
| Hypertension | Yes | 0.4205 | 0.0615 | 46.76 | <0.0001 |
| No | reference | 0 | |||
| Diabetes | Yes | 0.2177 | 0.0511 | 18.12 | <0.0001 |
| No | reference | 0 | |||
| Diarrhea | Yes | 0.3053 | 0.0574 | 28.25 | <0.0001 |
| No | reference | 0 | |||
| COPD | Yes | 0.4743 | 0.0828 | 32.83 | <0.0001 |
| No | reference | 0 | |||
| Malnutrition | Yes | 0.6305 | 0.0811 | 60.41 | <0.0001 |
| No | reference | 0 | |||
| WHO Score Grouped | |||||
| Death | 0.4346 | 0.0742 | 34.29 | <0.0001 | |
| Severe | 0.8204 | 0.0583 | 197.74 | <0.0001 | |
| Mild/moderate | reference | 0 | |||
| Admission Source Grouped | |||||
| Physician’s office or clinic; other health care facility | 0.0638 | 0.0955 | 0.45 | 0.5045 | |
| Skilled nursing facility, intermediate care facility or assisted living facility | −0.353 | 0.0787 | 20.14 | <0.0001 | |
| Transfers from another acute care hospital or ED | 0.0631 | 0.0999 | 0.4 | 0.5277 | |
| Home or workplace or non-health care facility; court/law enforcement | reference | 0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vong, T.; Yanek, L.R.; Wang, L.; Yu, H.; Fan, C.; Zhou, E.; Oh, S.J.; Szvarca, D.; Kim, A.; Potter, J.J.; et al. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients 2022, 14, 1310. https://doi.org/10.3390/nu14061310
Vong T, Yanek LR, Wang L, Yu H, Fan C, Zhou E, Oh SJ, Szvarca D, Kim A, Potter JJ, et al. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients. 2022; 14(6):1310. https://doi.org/10.3390/nu14061310
Chicago/Turabian StyleVong, Tyrus, Lisa R. Yanek, Lin Wang, Huimin Yu, Christopher Fan, Elinor Zhou, Sun Jung Oh, Daniel Szvarca, Ahyoung Kim, James J. Potter, and et al. 2022. "Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19" Nutrients 14, no. 6: 1310. https://doi.org/10.3390/nu14061310
APA StyleVong, T., Yanek, L. R., Wang, L., Yu, H., Fan, C., Zhou, E., Oh, S. J., Szvarca, D., Kim, A., Potter, J. J., & Mullin, G. E. (2022). Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients, 14(6), 1310. https://doi.org/10.3390/nu14061310