Efficacy of the Nutritional Risk Index, Geriatric Nutritional Risk Index, BMI, and GLIM-Defined Malnutrition in Predicting Survival of Patients with Head and Neck Cancer Patients Qualified for Home Enteral Nutrition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Institutional Approach to Home Enteral Nutrition Procedure
2.3. Data Collection and Nutritional Status Evaluation
Ideal body weight (women) = height − 100 − [(height − 150)/2]
2.4. Statistical Analysis
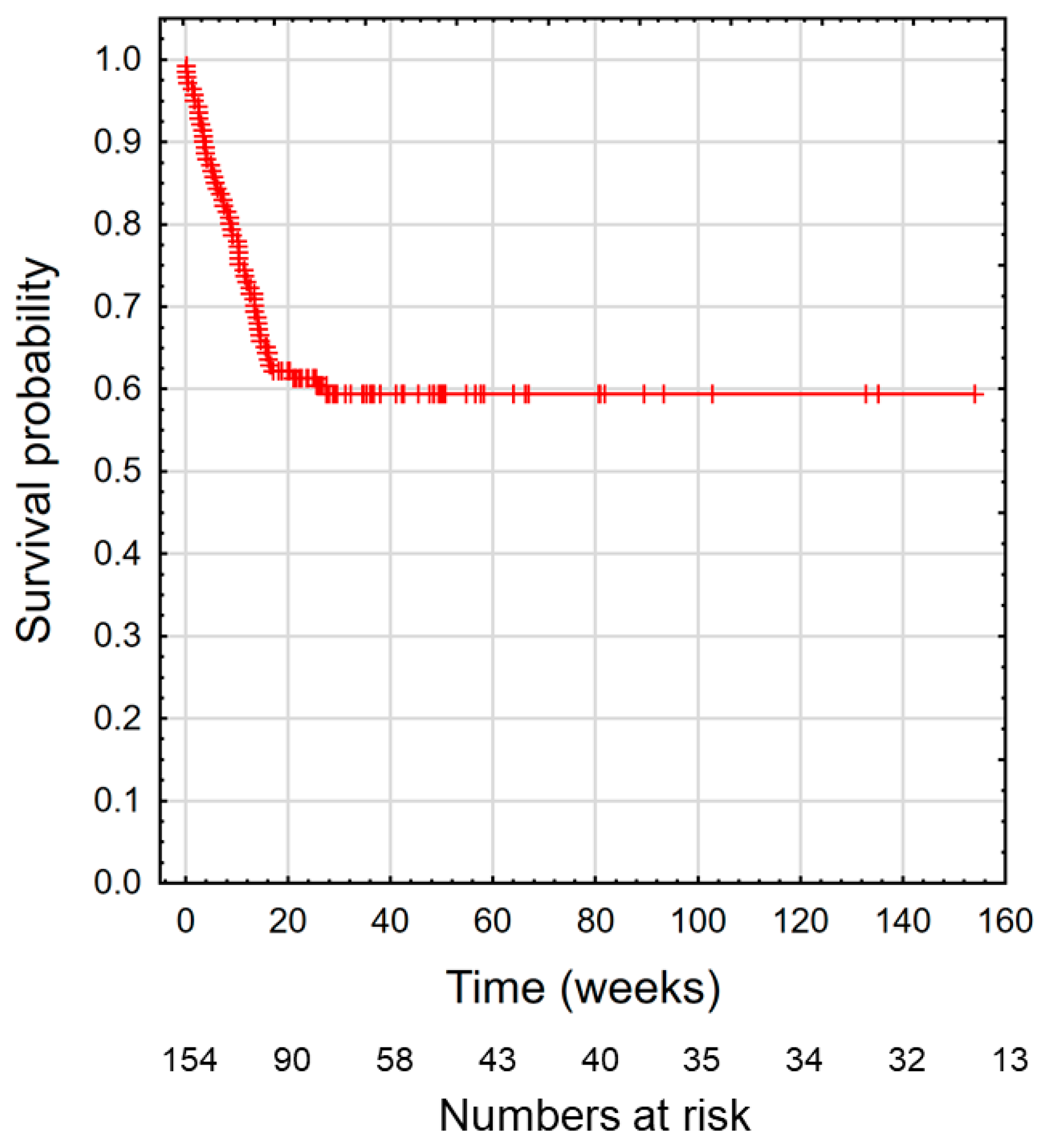
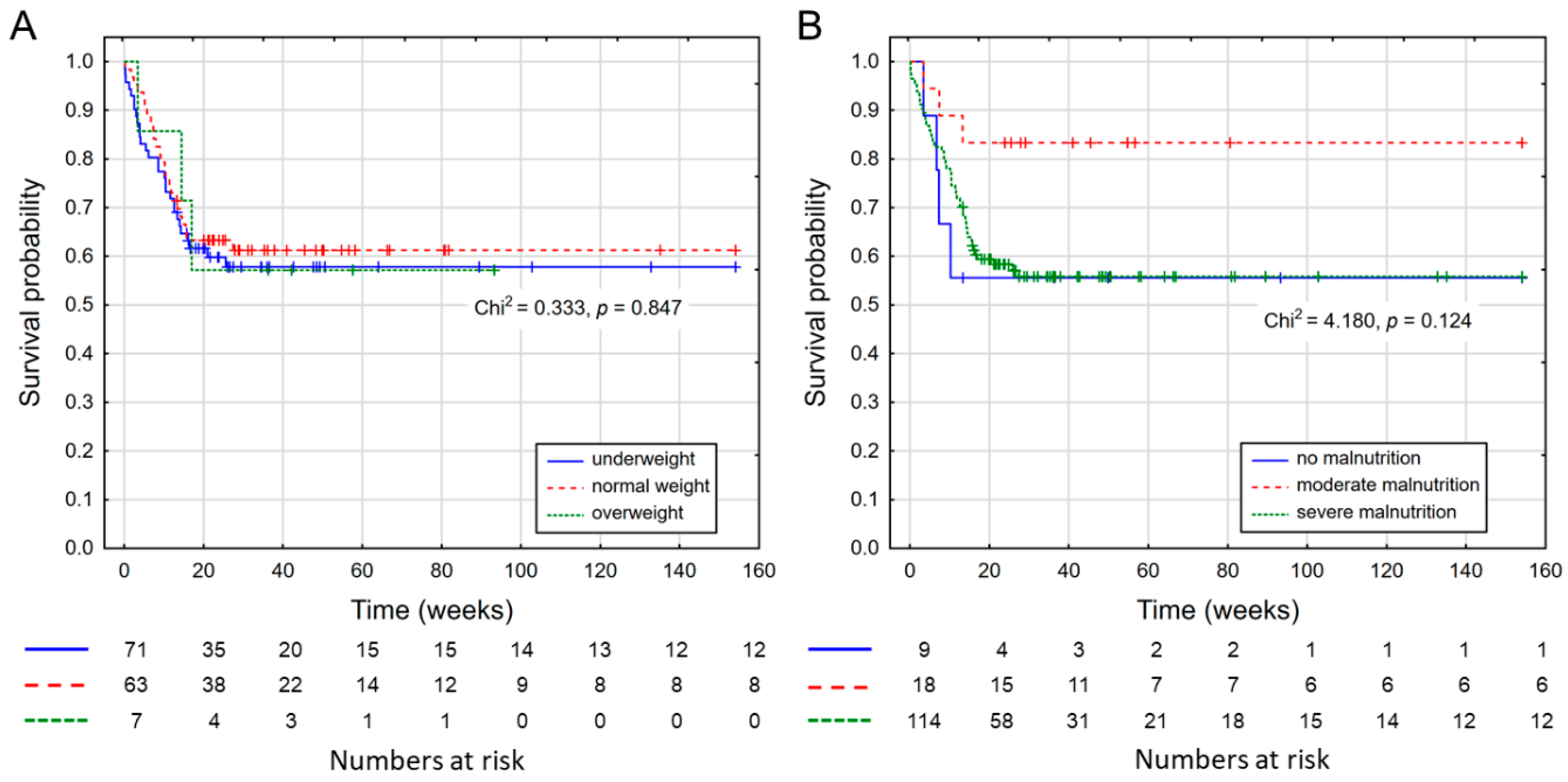
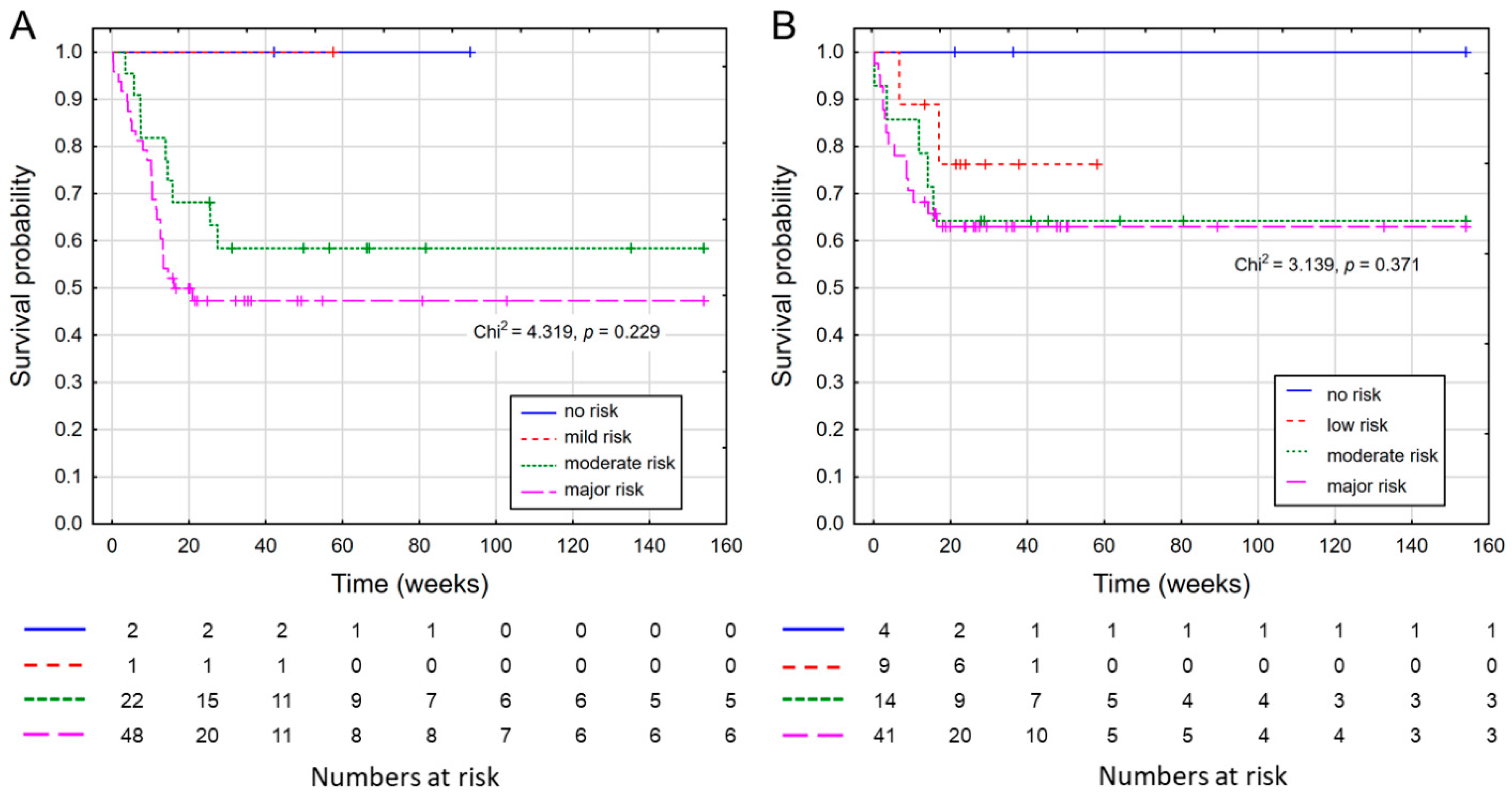
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, L.; Brandl, A.; Guiral, D.C.; Hoogwater, F.; Lundon, D.; Marano, L.; Montagna, G.; Polom, K.; Primavesi, F.; Schrage, Y.; et al. Nutritional assessment in surgical oncology: An ESSO-EYSAC global survey. Eur. J. Surg. Oncol. 2020, 46, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.; Laszlo, M.; Provisor, A.; Yu, A. Nutrition Management for the Head and Neck Cancer Patient. Cancer Treat. Res. 2018, 174, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Bressan, V.; Stevanin, S.; Bianchi, M.; Aleo, G.; Bagnasco, A.; Sasso, L. The effects of swallowing disorders, dysgeusia, oral mucositis and xerostomia on nutritional status, oral intake and weight loss in head and neck cancer patients: A systematic review. Cancer Treat. Rev. 2016, 45, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Alshadwi, A.; Nadershah, M.; Carlson, E.R.; Young, L.S.; Burke, P.A.; Daley, B.J. Nutritional Considerations for Head and Neck Cancer Patients: A Review of the Literature. J. Oral Maxillofac. Surg. 2013, 71, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2020, 39, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.; Deutz, N.; Erickson, N.; Laviano, A.; Lisanti, M.; Lobo, D. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, E.; Giannantonio, M.; Ostan, R.; Agostini, F.; Sasdelli, A.S.; Valeriani, L.; Pironi, L.; Pannuti, R. Choice of access route for artificial nutrition in cancer patients: 30 y of activity in a home palliative care setting. Nutrition 2021, 90, 111264. [Google Scholar] [CrossRef]
- Nakayama, M.; Ohnishi, K.; Adachi, M.; Ii, R.; Matsumoto, S.; Nakamura, M.; Miyamoto, H.; Hirose, Y.; Nishimura, B.; Tanaka, S.; et al. Efficacy of the pretreatment geriatric nutritional risk index for predicting severe adverse events in patients with head and neck cancer treated with chemoradiotherapy: Efficacy of the pretreatment Geriatric Nutritional Risk Index for predicting severe adverse events. Auris Nasus Larynx 2021, 49, 279–285. [Google Scholar] [CrossRef]
- Caburet, C.; Farigon, N.; Mulliez, A.; Mom, T.; Boirie, Y.; Gilain, L.; Saroul, N. Impact of nutritional status at the outset of assessment on postoperative complications in head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 393–398. [Google Scholar] [CrossRef]
- Yamahara, K.; Mizukoshi, A.; Lee, K.; Ikegami, S. Pretherapeutic nutritional/inflammatory factors as predictors for survival of both early and advanced staged head and neck cancer patients. Auris Nasus Larynx 2021, 48, 731–737. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examination Survey (NHANES). Anthropometry Procedures Manual. 2017. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2017_Anthropometry_Procedures_Manual.pdf (accessed on 1 March 2022).
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care Clin. Off. Pract. 1994, 21, 55–67. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Gascón-Ruiz, M.; Casas-Deza, D.; Torres-Ramón, I.; Zapata-García, M.; Alonso, N.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quílez, E.; Isla, D.; et al. GLIM vs ESPEN criteria for the diagnosis of early malnutrition in oncological outpatients. Clin. Nutr. 2021, 40, 3741–3747. [Google Scholar] [CrossRef]
- Paccagnella, A.; Baruffi, C.; Pizzolato, D.; Favaro, V.; Marcon, M.L.; Morello, M.; Semenzin, M.; Rebuffi, S.; Fossa, E.; Faronato, P.; et al. Home enteral nutrition in adults: A five-year (2001–2005) epidemiological analysis. Clin. Nutr. 2008, 27, 378–385. [Google Scholar] [CrossRef]
- Nazari, V.; Pashaki, A.S.; Hasanzadeh, E. The reliable predictors of severe weight loss during the radiotherapy of Head and Neck Cancer. Cancer Treat. Res. Commun. 2021, 26, 100281. [Google Scholar] [CrossRef]
- Yin, L.; Song, C.; Cui, J.; Wang, N.; Fan, Y.; Lin, X.; Zhang, L.; Zhang, M.; Wang, C.; Liang, T.; et al. Low fat mass index outperforms handgrip weakness and GLIM-defined malnutrition in predicting cancer survival: Derivation of cutoff values and joint analysis in an observational cohort. Clin. Nutr. 2022, 41, 153–164. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Tang, M.; Song, M.; Zhang, Q.; Zhang, K.; Ruan, G.; Zhang, X.; Ge, Y.; Yang, M.; et al. Different muscle mass indices of the Global Leadership Initiative on Malnutrition in diagnosing malnutrition and predicting survival of patients with gastric cancer. Nutrition 2021, 89, 111286. [Google Scholar] [CrossRef]
- Tapia, M.J.; Ocón, J.; Cabrejas-Gómez, C.; Ballesteros-Pomar, M.D.; Vidal-Casariego, A.; Arraiza-Irigoyen, C.; Olivares, J.; Conde-García, M.C.; García-Manzanares, Á.; Botella-Romero, F.; et al. Nutrition-related risk indexes and long-term mortality in noncritically ill inpatients who receive total parenteral nutrition (prospective multicenter study). Clin. Nutr. 2015, 34, 962–967. [Google Scholar] [CrossRef]
- Adejumo, O.L.; Koelling, T.M.; Hummel, S.L. Nutritional Risk Index predicts mortality in hospitalized advanced heart failure patients. J. Heart Lung Transplant. 2015, 34, 1385–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundararajan, S.K.; Garacci, Z.; Saltzberg, M.; Gaglianello, N.; Walker, R.; Williams, J.S.; Joyce, D.; Mohammed, A. Assessing Malnutrition Using Nutritional Risk Index Predicts Mortality after Left Ventricular Assist Device Implantation in Patients with End Stage D Heart Failure. J. Heart Lung Transplant. 2019, 38, S441–S442. [Google Scholar] [CrossRef]
- Ha, J.W.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.-B.; Lee, S.-W. Nutrition Risk Index Score at Diagnosis Can Effectively Predict Poor Prognosis in Patients With Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. J. Ren. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Liu, F.; Lin, J.; Chen, Q.; Chen, L.; Chen, F.; Wang, J.; Qiu, Y.; Shi, B.; Pan, L.; et al. Nutritional assessment and prognosis of oral cancer patients: A large-scale prospective study. BMC Cancer 2020, 20, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Total Group | Female (n = 40) | p-Value * | Male (n = 117) | p-Value * | p Difference between Genders * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 (n = 21) | ≥65 (n = 19) | <65 (n = 62) | ≥65 (n = 55) | ||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | ||||
| body weight (kg) | 59.7 | 13.23 | 52.5 | 11.31 | 50.3 | 10.90 | 0.529 | 61.3 | 11.23 | 64.0 | 14.25 | 0.260 | 0.000 |
| % loss of body weight | 16.3 | 9.11 | 15.7 | 10.56 | 14.6 | 8.32 | 0.718 | 16.9 | 7.99 | 16.3 | 10.08 | 0.733 | 0.408 |
| BMI (kg/m2) | 20.6 | 3.88 | 19.7 | 3.89 | 20.3 | 3.47 | 0.629 | 20.2 | 3.60 | 21.4 | 4.23 | 0.084 | 0.270 |
| BMI Categories | n | % | n | % | n | % | p-Value ** | n | % | n | % | p-Value * | p Difference between Genders ** |
| Normal weight | 70 | 46.5 | 10 | 47.6 | 7 | 36.8 | 0.120 | 35 | 56.5 | 18 | 32.7 | 0.024 | 0.854 |
| Underweight | 73 | 44.6 | 8 | 38.1 | 12 | 63.2 | 21 | 33.9 | 32 | 58.2 | |||
| Overweight | 14 | 8.9 | 3 | 14.3 | 0 | 0.0 | 6 | 9.7 | 5 | 9.1 | |||
| GLIM | |||||||||||||
| No | 9 | 5.7 | 2 | 9.5 | 1 | 5.3 | 0.841 | 3 | 4.8 | 3 | 5.5 | 0.564 | 0.538 |
| Moderate | 28 | 17.8 | 5 | 23.8 | 4 | 21.1 | 8 | 12.9 | 11 | 20.0 | |||
| Severe | 120 | 76.4 | 14 | 66.7 | 14 | 73.7 | 51 | 82.3 | 41 | 74.6 | |||
| CRP | |||||||||||||
| <10 mg/L | 28 | 17.8 | 8 | 38.1 | 2 | 10.5 | 0.044 | 12 | 19.4 | 6 | 10.9 | 0.206 | 0.170 |
| ≥10 mg/L | 129 | 82.2 | 13 | 61.9 | 17 | 89.5 | 50 | 80.7 | 49 | 89.1 | |||
| Variable | NRI (n = 83) | GNRI (n = 74) | ||
|---|---|---|---|---|
| n | % | n | % | |
| No risk | 2 | 2.4 | 5 | 6.8 |
| Mild/Low risk | 2 | 2.4 | 10 | 13.5 |
| Moderate risk | 28 | 33.7 | 17 | 23.0 |
| Major risk | 51 | 61.5 | 42 | 56.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przekop, Z.; Szostak-Węgierek, D.; Milewska, M.; Panczyk, M.; Zaczek, Z.; Sobocki, J. Efficacy of the Nutritional Risk Index, Geriatric Nutritional Risk Index, BMI, and GLIM-Defined Malnutrition in Predicting Survival of Patients with Head and Neck Cancer Patients Qualified for Home Enteral Nutrition. Nutrients 2022, 14, 1268. https://doi.org/10.3390/nu14061268
Przekop Z, Szostak-Węgierek D, Milewska M, Panczyk M, Zaczek Z, Sobocki J. Efficacy of the Nutritional Risk Index, Geriatric Nutritional Risk Index, BMI, and GLIM-Defined Malnutrition in Predicting Survival of Patients with Head and Neck Cancer Patients Qualified for Home Enteral Nutrition. Nutrients. 2022; 14(6):1268. https://doi.org/10.3390/nu14061268
Chicago/Turabian StylePrzekop, Zuzanna, Dorota Szostak-Węgierek, Magdalena Milewska, Mariusz Panczyk, Zuzanna Zaczek, and Jacek Sobocki. 2022. "Efficacy of the Nutritional Risk Index, Geriatric Nutritional Risk Index, BMI, and GLIM-Defined Malnutrition in Predicting Survival of Patients with Head and Neck Cancer Patients Qualified for Home Enteral Nutrition" Nutrients 14, no. 6: 1268. https://doi.org/10.3390/nu14061268
APA StylePrzekop, Z., Szostak-Węgierek, D., Milewska, M., Panczyk, M., Zaczek, Z., & Sobocki, J. (2022). Efficacy of the Nutritional Risk Index, Geriatric Nutritional Risk Index, BMI, and GLIM-Defined Malnutrition in Predicting Survival of Patients with Head and Neck Cancer Patients Qualified for Home Enteral Nutrition. Nutrients, 14(6), 1268. https://doi.org/10.3390/nu14061268









