Association between Telomere Length and Pediatric Obesity: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Literature Search
2.4. Study Selection
3. Results
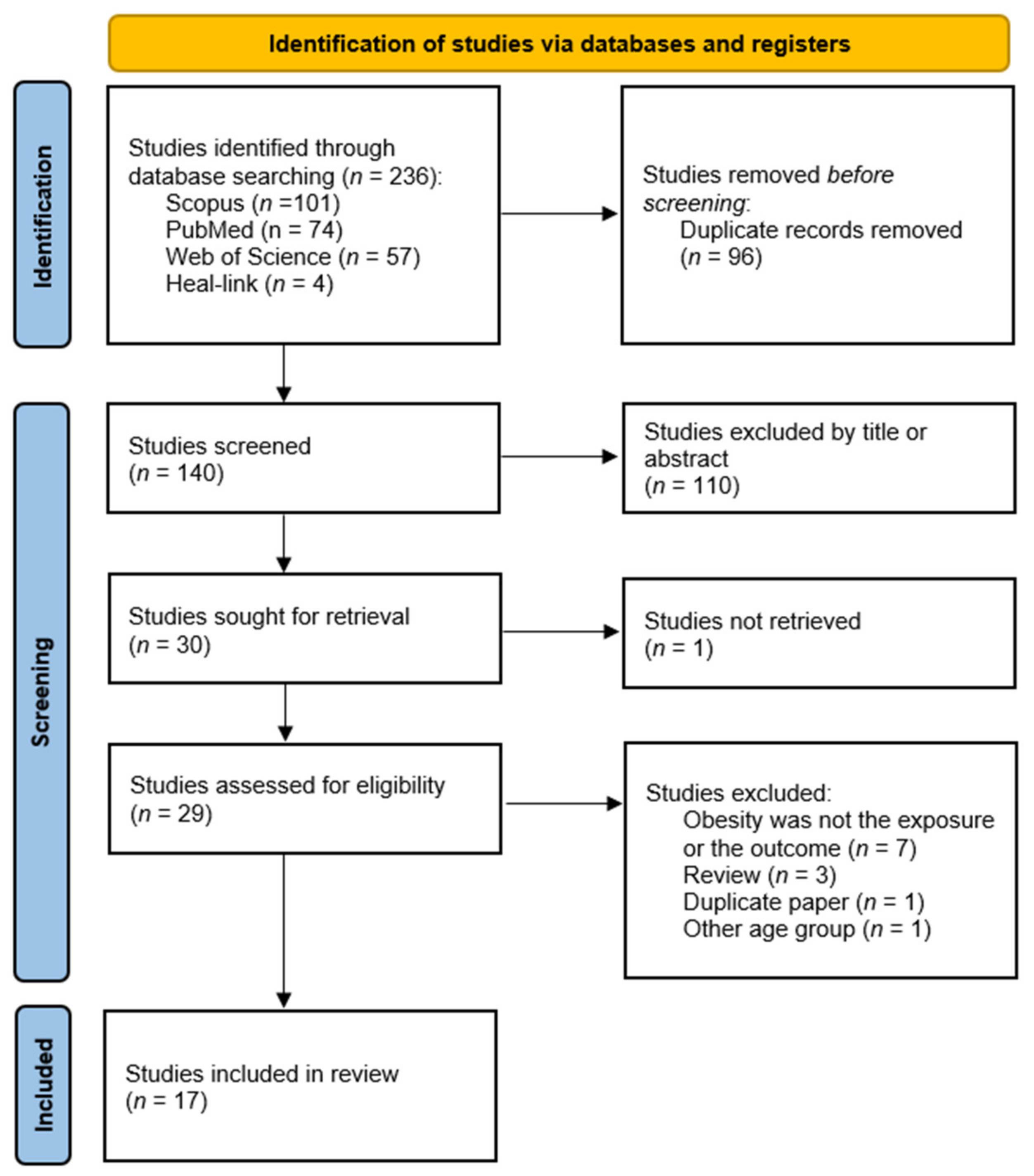
3.1. Literature Search
3.2. Study Characteristics
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.; McGill, N.I.; Lindsey, L.A.; Green, D.K.; Cooke, H.J. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991, 256, 45–48. [Google Scholar] [CrossRef]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef] [PubMed]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985, 43 Pt 1, 405–413. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Cesare, A.J.; Reddel, R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010, 11, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatric Res. 2002, 52, 377–381. [Google Scholar] [CrossRef]
- Friedrich, U.; Schwab, M.; Griese, E.U.; Fritz, P.; Klotz, U. Telomeres in neonates: New insights in fetal hematopoiesis. Pediatric Res. 2001, 49, 252–256. [Google Scholar] [CrossRef]
- Menon, R.; Yu, J.; Basanta-Henry, P.; Brou, L.; Berga, S.L.; Fortunato, S.J.; Taylor, R.N. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS ONE 2012, 7, e31136. [Google Scholar] [CrossRef]
- Vasu, V.; Turner, K.J.; George, S.; Greenall, J.; Slijepcevic, P.; Griffin, D.K. Preterm infants have significantly longer telomeres than their term born counterparts. PLoS ONE 2017, 12, e0180082. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.L.; Newman, A.B. Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Nordfjall, K.; Eliasson, M.; Stegmayr, B.; Melander, O.; Nilsson, P.; Roos, G. Telomere Length Is Associated With Obesity Parameters but With a Gender Difference. Obesity 2008, 16, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Willeit, J.; Brandstatter, A.; Ehrlenbach, S.; Mayr, A.; Gasperi, A.; Weger, S.; Oberhollenzer, F.; Reindl, M.; Kronenberg, F.; et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arter. Thromb. Vasc. Biol. 2010, 30, 1649–1656. [Google Scholar] [CrossRef]
- Friedrich, U.; Griese, E.; Schwab, M.; Fritz, P.; Thon, K.; Klotz, U. Telomere length in different tissues of elderly patients. Mech. Ageing Dev. 2000, 119, 89–99. [Google Scholar] [CrossRef]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.K.; Granick, M.; Aviv, A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Blackburn, E.H.; Shannon, K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. USA 1998, 95, 5607–5610. [Google Scholar] [CrossRef]
- Zeichner, S.L.; Palumbo, P.; Feng, Y.; Xiao, X.; Gee, D.; Sleasman, J.; Goodenow, M.; Biggar, R.; Dimitrov, D. Rapid telomere shortening in children. Blood 1999, 93, 2824–2830. [Google Scholar] [CrossRef]
- Rufer, N.; Brummendorf, T.H.; Kolvraa, S.; Bischoff, C.; Christensen, K.; Wadsworth, L.; Schulzer, M.; Lansdorp, P.M. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 1999, 190, 157–167. [Google Scholar] [CrossRef]
- Takubo, K.; Nakamura, K.; Izumiyama, N.; Furugori, E.; Sawabe, M.; Arai, T.; Esaki, Y.; Mafune, K.; Kammori, M.; Fujiwara, M.; et al. Telomere shortening with aging in human liver. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, B533–B536. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P.M. Telomeres, stem cells, and hematology. Blood 2008, 111, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S. Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009, 169, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Kark, J.D.; Susser, E.; Kimura, M.; Sinnreich, R.; Chen, W.; Steenstrup, T.; Christensen, K.; Herbig, U.; von Bornemann Hjelmborg, J.; et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 2013, 12, 615–621. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M.; Smith, K.R.; O’Brien, E.; Sivatchenko, A.; Kerber, R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003, 361, 393–395. [Google Scholar] [CrossRef]
- Haussmann, M.F.; Vleck, C.M.; Nisbet, I.C. Calibrating the telomere clock in common terns, Sterna hirundo. Exp. Gerontol. 2003, 38, 787–789. [Google Scholar] [CrossRef]
- Heidinger, B.J.; Blount, J.D.; Boner, W.; Griffiths, K.; Metcalfe, N.B.; Monaghan, P. Telomere length in early life predicts lifespan. Proc. Natl. Acad. Sci. USA 2012, 109, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Boonekamp, J.J.; Simons, M.J.P.; Hemerik, L.; Verhulst, S. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 2013, 12, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Boonekamp, J.J.; Mulder, G.A.; Salomons, H.M.; Dijkstra, C.; Verhulst, S. Nestling telomere shortening, but not telomere length, reflects developmental stress and predicts survival in wild birds. Proc. R. Soc. B Biol. Sci. 2014, 281, 3287. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.L.; Burke, T.A.; Hammers, M.; Komdeur, J.; Richardson, D.S. Telomere length and dynamics predict mortality in a wild longitudinal study. Mol. Ecol. 2013, 22, 249–259. [Google Scholar] [CrossRef]
- Fairlie, J.; Holland, R.; Pilkington, J.G.; Pemberton, J.M.; Harrington, L.; Nussey, D.H. Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell 2016, 15, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Youngren, K.; Jeanclos, E.; Aviv, H.; Kimura, M.; Stock, J.; Hanna, M.; Skurnick, J.; Bardeguez, A.; Aviv, A. Synchrony in telomere length of the human fetus. Hum. Genet. 1998, 102, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Gazitt, Y.; Cao, X.J.; Zhao, X.Y.; Lansdorp, P.M.; Aviv, A. Synchrony of telomere length among hematopoietic cells. Exp. Hematol. 2010, 38, 854–859. [Google Scholar] [CrossRef]
- Wright, D.L.; Jones, E.L.; Mayer, J.F.; Oehninger, S.; Gibbons, W.E.; Lanzendorf, S.E. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol. Hum. Reprod. 2001, 7, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.; Verhulst, S.; Guirguis, G.; Kark, J.D.; Labat, C.; Roche, N.E.; Martimucci, K.; Patel, K.; Heller, D.S.; Kimura, M.; et al. Telomere length dynamics in early life: The blood-and-muscle model. FASEB J. 2018, 32, 529–534. [Google Scholar] [CrossRef]
- Andrew, T.; Aviv, A.; Falchi, M.; Surdulescu, G.L.; Gardner, J.P.; Lu, X.B.; Kimura, M.; Kato, B.S.; Valdes, A.M.; Spector, T.D. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am. J. Hum. Genet 2006, 78, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Atzmon, G.; Cho, M.; Cawthon, R.M.; Budagov, T.; Katz, M.; Yang, X.; Siegel, G.; Bergman, A.; Huffman, D.M.; Schechter, C.B.; et al. Evolution in health and medicine Sackler colloquium: Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. S1), 1710–1717. [Google Scholar] [CrossRef]
- Bakaysa, S.L.; Mucci, L.A.; Slagboom, P.E.; Boomsma, D.I.; McClearn, G.E.; Johansson, B.; Pedersen, N.L. Telomere length predicts survival independent of genetic influences. Aging Cell 2007, 6, 769–774. [Google Scholar] [CrossRef]
- Vasa-Nicotera, M.; Brouilette, S.; Mangino, M.; Thompson, J.R.; Braund, P.; Clemitson, J.R.; Mason, A.; Bodycote, C.L.; Raleigh, S.M.; Louis, E.; et al. Mapping of a major locus that determines telomere length in humans. Am. J. Hum. Genet 2005, 76, 147–151. [Google Scholar] [CrossRef]
- Slagboom, P.E.; Droog, S.; Boomsma, D.I. Genetic determination of telomere size in humans: A twin study of three age groups. Am. J. Hum. Genet 1994, 55, 876–882. [Google Scholar]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.H.; Ott, S.; Garant, M.J.; Blackburn, E.H.; Mitchell, B.D.; Shuldiner, A.R.; Hsueh, W.C. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef]
- Akkad, A.; Hastings, R.; Konje, J.C.; Bell, S.C.; Thurston, H.; Williams, B. Telomere length in small-for-gestational-age babies. BJOG 2006, 113, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Factor-Litvak, P.; Susser, E.; Kezios, K.; McKeague, I.; Kark, J.D.; Hoffman, M.; Kimura, M.; Wapner, R.; Aviv, A. Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics 2016, 137, e20153927. [Google Scholar] [CrossRef]
- Nordfjall, K.; Larefalk, A.; Lindgren, P.; Holmberg, D.; Roos, G. Telomere length and heredity: Indications of paternal inheritance. Proc. Natl. Acad. Sci. USA 2005, 102, 16374–16378. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and telomere length: Systematic review and meta-analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.E.; Hunt, S.C.; Stone, R.C.; Horvath, K.; Herbig, U.; Ranciaro, A.; Hirbo, J.; Beggs, W.; Reiner, A.P.; Wilson, J.G.; et al. Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum. Mol. Genet. 2016, 25, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; Peek, M.K.; Mitra, N.; Ravichandran, K.; Branas, C.; Spangler, E.; Zhou, W.; Paskett, E.D.; Gehlert, S.; DeGraffinreid, C.; et al. Race, Ethnicity, Psychosocial Factors, and Telomere Length in a Multicenter Setting. PLoS ONE 2016, 11, e0146723. [Google Scholar] [CrossRef]
- Kimura, M.; Cherkas, L.F.; Kato, B.S.; Demissie, S.; Hjelmborg, J.B.; Brimacombe, M.; Cupples, A.; Hunkin, J.L.; Gardner, J.P.; Lu, X.; et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008, 4, e37. [Google Scholar] [CrossRef]
- Prescott, J.; Du, M.; Wong, J.Y.Y.; Han, J.; De Vivo, I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum. Reprod. 2012, 27, 3622–3631. [Google Scholar] [CrossRef]
- Muzzinler, A.; Mons, U.; Dieffenbach, A.K.; Butterbach, K.; Saum, K.U.; Schick, M.; Stammer, H.; Boukamp, P.; Holleczek, B.; Stegmaier, C.; et al. Smoking habits and leukocyte telomere length dynamics among older adults: Results from the ESTHER cohort. Exp. Gerontol. 2015, 70, 18–25. [Google Scholar] [CrossRef]
- Valdes, A.M.; Andrew, T.; Gardner, J.P.; Kimura, M.; Oelsner, E.; Cherkas, L.F.; Aviv, A.; Spector, T.D. Obesity, cigarette smoking, and telomere length in women. Lancet 2005, 366, 662–664. [Google Scholar] [CrossRef]
- Sassenroth, D.; Meyer, A.; Salewsky, B.; Kroh, M.; Norman, K.; Steinhagen-Thiessen, E.; Demuth, I. Sports and Exercise at Different Ages and Leukocyte Telomere Length in Later Life—Data from the Berlin Aging Study II (BASE-II). PLoS ONE 2015, 10, e0142131. [Google Scholar] [CrossRef]
- Price, L.H.; Kao, H.T.; Burgers, D.E.; Carpenter, L.L.; Tyrka, A.R. Telomeres and early-life stress: An overview. Biol. Psychiatry 2013, 73, 15–23. [Google Scholar] [CrossRef]
- Coimbra, B.M.; Carvalho, C.M.; Moretti, P.N.; Mello, M.F.; Belangero, S.I. Stress-related telomere length in children: A systematic review. J. Psychiatr. Res. 2017, 92, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, G.W.; Hampson, S.E.; Cote, H.C.; Hill, P.L.; Klest, B. Childhood Personality, Betrayal Trauma, and Leukocyte Telomere Length in Adulthood: A Lifespan Perspective on Conscientiousness and Betrayal Traumas as Predictors of a Biomarker of Cellular Aging. Eur. J. Personal. 2016, 30, 426–437. [Google Scholar] [CrossRef]
- Lindqvist, D.; Epel, E.S.; Mellon, S.H.; Penninx, B.W.; Revesz, D.; Verhoeven, J.E.; Reus, V.I.; Lin, J.; Mahan, L.; Hough, C.M.; et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 2015, 55, 333–364. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhu, W.; Hu, S.; Yu, X.; Yang, Y. Association between oxidative stress and telomere length in Type 1 and Type 2 diabetic patients. J. Endocrinol. Investig. 2013, 36, 1032–1037. [Google Scholar]
- Babizhayev, M.A.; Vishnyakova, K.S.; Yegorov, Y.E. Oxidative damage impact on aging and age-related diseases: Drug targeting of telomere attrition and dynamic telomerase activity flirting with imidazole-containing dipeptides. Recent Pat. Drug Deliv. Formul. 2014, 8, 163–192. [Google Scholar] [CrossRef] [PubMed]
- Entringer, S.; de Punder, K.; Buss, C.; Wadhwa, P.D. The fetal programming of telomere biology hypothesis: An update. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170151. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A. The mitochondrial genome, paternal age and telomere length in humans. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170210. [Google Scholar] [CrossRef]
- De Onis, M.; Blossner, M.; Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010, 92, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Gungor, N.K. Overweight and Obesity in Children and Adolescents. J. Clin. Res. Pediatric Endocrinol. 2014, 6, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.; Martos, R.; Gascon, F.; Canete, R.; Zafra, M.A.; Morales, R. Low-grade systemic inflammation, hypoadiponectinemia and a high concentration of leptin are present in very young obese children, and correlate with metabolic syndrome. Diabetes Metab. 2005, 31, 55–62. [Google Scholar] [CrossRef]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, J.A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef]
- Bjerregaard, L.G.; Jensen, B.W.; Angquist, L.; Osler, M.; Sorensen, T.I.A.; Baker, J.L. Change in Overweight from Childhood to Early Adulthood and Risk of Type 2 Diabetes. N. Engl. J. Med. 2018, 378, 1302–1312. [Google Scholar] [CrossRef]
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfaffle, R.; Kiess, W.; Korner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y.S. Childhood obesity. Lancet 2010, 375, 1737–1748. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight Fact Sheet N°311. January 2015. Retrieved 2 February 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 January 2022).
- Das, U.N. Is obesity an inflammatory condition? Nutrition 2001, 17, 953–966. [Google Scholar] [CrossRef]
- Pogodzinski, D.; Ostrowska, L.; Smarkusz-Zarzecka, J.; Zysk, B. Secretome of Adipose Tissue as the Key to Understanding the Endocrine Function of Adipose Tissue. Int. J. Mol. Sci. 2022, 23, 2309. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Metabolic syndrome X: An inflammatory condition? Curr. Hypertens. Rep. 2004, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Carulli, L.; Anzivino, C.; Baldelli, E.; Zenobii, M.F.; Rocchi, M.B.; Bertolotti, M. Telomere length elongation after weight loss intervention in obese adults. Mol. Genet. Metab. 2016, 118, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, G.; Schoina, M.; Valsamakis, G.; Salakos, N.; Avloniti, A.; Chatzinikolaou, A.; Margeli, A.; Skevaki, C.; Papagianni, M.; Kanaka-Gantenbein, C.; et al. Interrelations among the adipocytokines leptin and adiponectin, oxidative stress and aseptic inflammation markers in pre- and early-pubertal normal-weight and obese boys. Endocrine 2017, 55, 925–933. [Google Scholar] [CrossRef]
- Mundstock, E.; Sarria, E.E.; Zatti, H.; Mattos Louzada, F.; Kich Grun, L.; Herbert Jones, M.; Guma, F.T.; Mazzola In Memoriam, J.; Epifanio, M.; Stein, R.T.; et al. Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity 2015, 23, 2165–2174. [Google Scholar] [CrossRef]
- Wang, Q.; Zhan, Y.; Pedersen, N.L.; Fang, F.; Hagg, S. Telomere Length and All-Cause Mortality: A Meta-analysis. Ageing Res. Rev. 2018, 48, 11–20. [Google Scholar] [CrossRef]
- O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.C.; Satterfield, S.; et al. Cumulative Inflammatory Load Is Associated with Short Leukocyte Telomere Length in the Health, Aging and Body Composition Study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef]
- Slattery, K.; Bentley, D.; Coutts, A.J. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: Implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 2015, 45, 453–471. [Google Scholar] [CrossRef]
- Shin, Y.A. How Does Obesity and Physical Activity Affect Aging?: Focused on Telomere as a Biomarker of Aging. J. Obes. Metab. Syndr. 2019, 28, 92–104. [Google Scholar] [CrossRef]
- Crocker, M.K.; Yanovski, J.A. Pediatric Obesity: Etiology and Treatment. Pediatric Clin. 2011, 58, 1217. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Phillips, M.; Fouty, A.; Babu, J.R.; Geetha, T. Telomere Length as a Biomarker for Race-Related Health Disparities. Genes 2021, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Shi, Q.; Fan, X.; Qi, K. Association of telomere length and telomerase methylation with n-3 fatty acids in preschool children with obesity. BMC Pediatrics 2021, 21, 24. [Google Scholar] [CrossRef]
- Ooi, D.S.Q.; Dorajoo, R.; Gurung, R.L.; Dehghan, R.; Lim, Y.Y.; Ho, C.W.L.; Tay, V.; Karuppiah, V.; Loke, K.Y.; Lim, S.C.; et al. Association of leukocyte telomere length with obesity-related traits in Asian children with early-onset obesity. Pediatric Obes. 2021, 16, e12771. [Google Scholar] [CrossRef] [PubMed]
- Licea-Cejudo, R.C.; Arenas-Sandoval, L.K.; Salazar-Leon, J.; Martinez-Martinez, M.V.; Carreon-Rodriguez, A.; Pedraza-Alva, G.; Perez-Martinez, L. A dysfunctional family environment and a high body fat percentage negatively affect telomere length in Mexican boys aged 8-10 years. Acta Paediatr. 2020, 109, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Clemente, D.B.P.; Maitre, L.; Bustamante, M.; Chatzi, L.; Roumeliotaki, T.; Fossati, S.; Grazuleviciene, R.; Gutzkow, K.B.; Lepeule, J.; Martens, D.S.; et al. Obesity is associated with shorter telomeres in 8 year-old children. Sci. Rep. 2019, 9, 18739. [Google Scholar] [CrossRef]
- Lamprokostopoulou, A.; Moschonis, G.; Manios, Y.; Critselis, E.; Nicolaides, N.C.; Stefa, A.; Koniari, E.; Gagos, S.; Charmandari, E. Childhood obesity and leucocyte telomere length. Eur. J. Clin. Investig. 2019, 49, e13178. [Google Scholar] [CrossRef] [PubMed]
- Theall, K.P.; Chaparro, M.P.; Denstel, K.; Bilfield, A.; Drury, S.S. Childhood obesity and the associated roles of neighborhood and biologic stress. Prev. Med. Rep. 2019, 14, 100849. [Google Scholar] [CrossRef]
- Zhu, H.; Bhagatwala, J.; Pollock, N.K.; Parikh, S.; Gutin, B.; Stallmann-Jorgensen, I.; Thomas, J.; Harshfield, G.A.; Dong, Y. High sodium intake is associated with short leukocyte telomere length in overweight and obese adolescents. Int. J. Obesity 2015, 39, 1249–1253. [Google Scholar] [CrossRef]
- Buxton, J.L.; Walters, R.G.; Visvikis-Siest, S.; Meyre, D.; Froguel, P.; Blakemore, A.I. Childhood obesity is associated with shorter leukocyte telomere length. J. Clin. Endocrinol. Metab. 2011, 96, 1500–1505. [Google Scholar] [CrossRef]
- Al-Attas, O.S.; Al-Daghri, N.; Bamakhramah, A.; Shaun Sabico, S.; McTernan, P.; Huang, T.T. Telomere length in relation to insulin resistance, inflammation and obesity among Arab youth. Acta Paediatr. 2010, 99, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Zannolli, R.; Mohn, A.; Buoni, S.; Pietrobelli, A.; Messina, M.; Chiarelli, F.; Miracco, C. Telomere length and obesity. Acta Paediatr. 2008, 97, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, G.; Raftopoulou, C.; Nicolaides, N.C.; Genitsaridi, S.M.; Karampatsou, S.I.; Papadopoulou, M.; Kassari, P.; Charmandari, E. A Comprehensive, Multidisciplinary, Personalized, Lifestyle Intervention Program Is Associated with Increased Leukocyte Telomere Length in Children and Adolescents with Overweight and Obesity. Nutrients 2021, 13, 2682. [Google Scholar] [CrossRef]
- Ojeda-Rodriguez, A.; Morell-Azanza, L.; Martin-Calvo, N.; Zalba, G.; Chueca, M.; Azcona-Sanjulian, M.C.; Marti, A. Association between favourable changes in objectively measured physical activity and telomere length after a lifestyle intervention in pediatric patients with abdominal obesity. Appl. Physiol. Nutr. Metab. 2021, 46, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Morell-Azanza, L.; Ojeda-Rodriguez, A.; Azcona-SanJulian, M.C.; Zalba, G.; Marti, A. Associations of telomere length with anthropometric and glucose changes after a lifestyle intervention in abdominal obese children. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 694–700. [Google Scholar] [CrossRef]
- Ojeda-Rodriguez, A.; Morell-Azanza, L.; Zalba, G.; Zazpe, I.; Azcona-Sanjulian, M.C.; Marti, A. Associations of telomere length with two dietary quality indices after a lifestyle intervention in children with abdominal obesity: A randomized controlled trial. Pediatric Obes. 2020, 15, e12661. [Google Scholar] [CrossRef]
- Garcia-Calzon, S.; Moleres, A.; Marcos, A.; Campoy, C.; Moreno, L.A.; Azcona-Sanjulian, M.C.; Martinez-Gonzalez, M.A.; Martinez, J.A.; Zalba, G.; Marti, A.; et al. Telomere Length as a Biomarker for Adiposity Changes after a Multidisciplinary Intervention in Overweight/Obese Adolescents: The EVASYON Study. PLoS ONE 2014, 9, e89828. [Google Scholar] [CrossRef]
- Oliveira, G.J.; Barbiero, S.M.; Cesa, C.C.; Pellanda, L.C. Comparison of NCHS, CDC, and WHO curves in children with cardiovascular risk. Rev. Assoc. Med. Bras. 2013, 59, 375–380. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Casanova, I.; Sarmiento, O.L.; Gazmararian, J.A.; Cunningham, S.A.; Martorell, R.; Pratt, M.; Stein, A.D. Comparing three body mass index classification systems to assess overweight and obesity in children and adolescents. Rev. Panam. Salud. Publica. 2013, 33, 349–355. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Vasquez, E.; Germain, C.M.; Emeny, R.T.; Rippberger, P.; Lopez-Jimenez, F.; Bartels, S.J. Association of adiposity, telomere length and mortality: Data from the NHANES 1999–2002. Int. J. Obes. 2018, 42, 198–204. [Google Scholar] [CrossRef]
- Chen, S.F.; Yeh, F.; Lin, J.; Matsuguchi, T.; Blackburn, E.; Lee, E.T.; Howard, B.V.; Zhao, J.Y. Short leukocyte telomere length is associated with obesity in American Indians: The strong heart family study. Aging 2014, 6, 380–389. [Google Scholar] [CrossRef]
- Lee, M.; Martin, H.; Firpo, M.A.; Demerath, E.W. Inverse Association between Adiposity and Telomere Length: The Fels Longitudinal Study. Am. J. Hum. Biol. 2011, 23, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, A.L.; Rodriguez, S.; Gaunt, T.R.; Fraser, A.; Anderson, E.L. Early life adiposity and telomere length across the life course: A systematic review and meta-analysis. Wellcome Open Res. 2017, 2, 118. [Google Scholar] [CrossRef] [PubMed]
- Rode, L.; Nordestgaard, B.G.; Weischer, M.; Bojesen, S.E. Increased body mass index, elevated C-reactive protein, and short telomere length. J. Clin. Endocrinol. Metab. 2014, 99, E1671–E1675. [Google Scholar] [CrossRef]
- Ping, F.; Li, Z.Y.; Lv, K.; Zhou, M.C.; Dong, Y.X.; Sun, Q.; Li, Y.X. Deoxyribonucleic acid telomere length shortening can predict the incidence of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. J. Diabetes Investig. 2017, 8, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Brouilette, S.W.; Moore, J.S.; McMahon, A.D.; Thompson, J.R.; Ford, I.; Shepherd, J.; Packard, C.J.; Samani, N.J.; West of Scotland Coronary Prevention Study, G. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet 2007, 369, 107–114. [Google Scholar] [CrossRef]
- Houben, J.M.; Moonen, H.J.; van Schooten, F.J.; Hageman, G.J. Telomere length assessment: Biomarker of chronic oxidative stress? Free Radic. Biol. Med. 2008, 44, 235–246. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Dhabhar, F.S.; Su, Y.; Reus, V.I.; Rosser, R.; Burke, H.M.; Kupferman, E.; et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS ONE 2011, 6, e17837. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Lin, J.; Matsuguchi, T.; Blackburn, E.; Zhang, Y.; Cole, S.A.; Best, L.G.; Lee, E.T.; Howard, B.V. Short leukocyte telomere length predicts risk of diabetes in american indians: The strong heart family study. Diabetes 2014, 63, 354–362. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, G.; Avloniti, A.; Chatzinikolaou, A.; Stefanaki, C.; Papagianni, M.; Papassotiriou, I.; Fatouros, I.G.; Chrousos, G.P.; Kanaka-Gantenbein, C.; Mastorakos, G. In early pubertal boys, testosterone and LH are associated with improved anti-oxidation during an aerobic exercise bout. Endocrine 2019, 66, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Alleman, R.J.; Katunga, L.A.; Nelson, M.A.; Brown, D.A.; Anderson, E.J. The “Goldilocks Zone” from a redox perspective-Adaptive vs. deleterious responses to oxidative stress in striated muscle. Front. Physiol. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Wellman, K.; Bloomer, R.J. Acute exercise and oxidative stress: A 30 year history. Dyn. Med 2009, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, G.; Fatouros, I.G.; Valsamakis, G.; Schoina, M.; Avloniti, A.; Chatzinikolaou, A.; Kambas, A.; Draganidis, D.; Mantzou, A.; Papagianni, M.; et al. Antioxidation improves in puberty in normal weight and obese boys, in positive association with exercise-stimulated growth hormone secretion. Pediatric Res. 2015, 78, 158–164. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Demissie, S.; Levy, D.; Benjamin, E.J.; Cupples, L.A.; Gardner, J.P.; Herbert, A.; Kimura, M.; Larson, M.G.; Meigs, J.B.; Keaney, J.F.; et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 2006, 5, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Qin, K.; Chen, D.Z.; Lu, C.Y.; Chen, W.Q.; Guo, V.Y. Systematic review and meta-analysis of the association between paediatric obesity and telomere length. Acta Paediatr. 2021, 110, 2695–2703. [Google Scholar] [CrossRef]
- Salpea, K.D.; Talmud, P.J.; Cooper, J.A.; Maubaret, C.G.; Stephens, J.W.; Abelak, K.; Humphries, S.E. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 2010, 209, 42–50. [Google Scholar] [CrossRef]
- Dusserre, E.; Moulin, P.; Vidal, H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim. Biophys. Acta 2000, 1500, 88–96. [Google Scholar] [CrossRef]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediat. Inflamm. 2013, 2013, 136584. [Google Scholar] [CrossRef] [PubMed]
- Korner, A.; Kratzsch, J.; Gausche, R.; Schaab, M.; Erbs, S.; Kiess, W. New predictors of the metabolic syndrome in children--role of adipocytokines. Pediatric Res. 2007, 61, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2015, 16, 378–400. [Google Scholar] [CrossRef]
- Soares, A.F.; Guichardant, M.; Cozzone, D.; Bernoud-Hubac, N.; Bouzaidi-Tiali, N.; Lagarde, M.; Geloen, A. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic. Biol. Med. 2005, 38, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Rahmat, A.; Patimah, I.; Abed, Y. Obesity can predict and promote systemic inflammation in healthy adults. Int. J. Cardiol. 2016, 215, 318–324. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H.; et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Palmai-Pallag, T.; Bachrati, C.Z. Inflammation-induced DNA damage and damage-induced inflammation: A vicious cycle. Microbes. Infect. 2014, 16, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Erol, A. Systemic DNA Damage Response and Metabolic Syndrome as a Premalignant State. Curr. Mol. Med. 2010, 10, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Yoshida, Y.; Suda, M.; Minamino, T. DNA Damage Response and Metabolic Disease. Cell Metab. 2014, 20, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Caldon, C.E. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front. Oncol. 2014, 4, 106. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.L.; Kronmal, R.A.; Gardner, J.P.; Psaty, B.M.; Jenny, N.S.; Tracy, R.P.; Walston, J.; Kimura, M.; Aviv, A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am. J. Epidemiol. 2007, 165, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Muezzinler, A.; Zaineddin, A.K.; Brenner, H. Body mass index and leukocyte telomere length in adults: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 192–201. [Google Scholar] [CrossRef]
- Masi, S.; Nightingale, C.M.; Day, I.N.; Guthrie, P.; Rumley, A.; Lowe, G.D.; von Zglinicki, T.; D’Aiuto, F.; Taddei, S.; Klein, N.; et al. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13- to 16-year-old adolescents. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2029–2034. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Arikawa, A.Y.; Thomas, W.; Gross, M.; Smith, A.; Phipps, W.R.; Kurzer, M.S.; Schmitz, K.H. Aerobic training reduces systemic oxidative stress in young women with elevated levels of F2-isoprostanes. Contemp. Clin. Trials. 2013, 34, 212–217. [Google Scholar] [CrossRef][Green Version]
- Campbell, P.T.; Gross, M.D.; Potter, J.D.; Schmitz, K.H.; Duggan, C.; Mctiernan, A.; Ulrich, C.M. Effect of Exercise on Oxidative Stress: A 12-Month Randomized, Controlled Trial. Med. Sci. Sport Exerc. 2010, 42, 1448–1453. [Google Scholar] [CrossRef]
- Laufs, U.; Wassmann, S.; Czech, T.; Munzel, T.; Eisenhauer, M.; Bohm, M.; Nickenig, G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscl. Throm. Vas. 2005, 25, 809–814. [Google Scholar] [CrossRef]
- Sarifakioglu, B.; Guzelant, A.Y.; Guzel, E.C.; Guzel, S.; Kiziler, A.R. Effects of 12-week combined exercise therapy on oxidative stress in female fibromyalgia patients. Rheumatol. Int. 2014, 34, 1361–1367. [Google Scholar] [CrossRef]
- Himbert, C.; Thompson, H.; Ulrich, C.M. Effects of Intentional Weight Loss on Markers of Oxidative Stress, DNA Repair and Telomere Length—a Systematic Review. Obes. Facts 2017, 10, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Galie, S.; Canudas, S.; Muralidharan, J.; Garcia-Gavilan, J.; Bullo, M.; Salas-Salvado, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576–601. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.; Fung, T.T.; McEvoy, C.T.; Lin, J.; Epel, E.S. Diet Quality Indices and Leukocyte Telomere Length Among Healthy US Adults: Data From the National Health and Nutrition Examination Survey, 1999–2002. Am. J. Epidemiol. 2018, 187, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, M.F.; Hernandez-Alonso, P.; Canudas, S.; Marti, A.; Fito, M.; Razquin, C.; Salas-Salvado, J. Modulation of Telomere Length by Mediterranean Diet, Caloric Restriction, and Exercise: Results from PREDIMED-Plus Study. Antioxidants 2021, 10, 1596. [Google Scholar]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. a-Biol. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Spyridopoulos, I.; Erben, Y.; Brummendorf, T.H.; Haendeler, J.; Dietz, K.; Seeger, F.; Kissel, C.K.; Martin, H.; Hoffmann, J.; Assmus, B.; et al. Telomere gap between granulocytes and lymphocytes is a determinant for hematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscl. Throm. Vas. 2008, 28, 968–974. [Google Scholar] [CrossRef][Green Version]
- Starkweather, A.R.; Alhaeeri, A.A.; Montpetit, A.; Brumelle, J.; Filler, K.; Montpetit, M.; Mohanraj, L.; Lyon, D.E.; Jackson-Cook, C.K. An Integrative Review of Factors Associated with Telomere Length and Implications for Biobehavioral Research. Nurs. Res. 2014, 63, 36–50. [Google Scholar] [CrossRef]
- Turner, K.J.; Vasu, V.; Griffin, D.K. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef]
- Lin, J.; Cheon, J.; Brown, R.; Coccia, M.; Puterman, E.; Aschbacher, K.; Sinclair, E.; Epel, E.; Blackburn, E.H. Systematic and Cell Type-Specific Telomere Length Changes in Subsets of Lymphocytes. J. Immunol. Res. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Epel, E. How “Reversible” Is Telomeric Aging? Cancer Prev. Res. 2012, 5, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Biegler, K.A.; Anderson, A.K.L.; Wenzel, L.B.; Osann, K.; Nelson, E.L. Longitudinal Change in Telomere Length and the Chronic Stress Response in a Randomized Pilot Biobehavioral Clinical Study: Implications for Cancer Prevention. Cancer Prev. Res. 2012, 5, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, J.H.; Lee, D.C.; Lim, I.; Bang, H. Habitual physical exercise has beneficial effects on telomere length in postmenopausal women. Menopause 2012, 19, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Lowder, T.W.; Spielmann, G.; Bigley, A.B.; LaVoy, E.C.; Kunz, H. Exercise and the aging immune system. Ageing Res. Rev. 2012, 11, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Nussey, D.H.; Baird, D.; Barrett, E.; Boner, W.; Fairlie, J.; Gemmell, N.; Hartmann, N.; Horn, T.; Haussmann, M.; Olsson, M.; et al. Measuring telomere length and telomere dynamics in evolutionary biology and ecology. Methods Ecol. Evol. 2014, 5, 299–310. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
|
| |
Primary objective
| Secondary objective
| |
| Primary Objective: Cross-Sectional Studies | ||||
| Author/Year | Selection | Comparability | Outcome | Overall Score |
| Selvaraju 2021 [84] | ** ** | ** | *** | 9 |
| Liu 2021 [85] | ** ** | * | *** | 8 |
| Ooi 2021 [86] | ** ** | * | *** | 8 |
| Licea-Cejudo 2020 [87] | ** ** | * | *** | 8 |
| Clemente 2019 [88] | ** ** | ** | *** | 9 |
| Lamprokostopoulou 2019 [89] | ***** | * | *** | 9 |
| Theall 2019 [90] | ** ** | * | *** | 8 |
| Zhu 2015 [91] | ** ** | ** | *** | 9 |
| Buxton 2011 [92] | ** ** | * | *** | 8 |
| Al-Attas 2010 [93] | ** ** | * | *** | 8 |
| Zannolli 2008 [94] | * ** | * | *** | 7 |
| Secondary Objective: Interventional Studies | ||||
| Author/Year | Selection | Comparability | Outcome | Overall Score |
| Paltoglou 2021 [95] | ** ** | * | *** | 8 |
| Ojeda-Rodríguez 2021 [96] | ***** | * | *** | 9 |
| Morell-Azanza 2020 [97] | ** ** | * | *** | 8 |
| Ojeda-Rodríguez 2020 [98] | ** ** | * | *** | 8 |
| García-Calzón 2014 [99] | ***** | * | *** | 9 |
| Author Year | Nationality | Study Design | Sample Size | Obesity Definition | Age (Years) Mean ± SD or Range | Male (%) | Method/Tissue | TL Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Total | OB & OW (%) | Reference Grouping | |||||||
| Selvaraju 2021 [84] | European American, African American | Cross-sectional | 127 | NA | CDC [NW]-([OW] & [OB]) | 6–10 | NA | qPCR Saliva |
|
| Liu 2021 [85] | Chinese | Cross-sectional | 92 | 50% OB | WHO [NW]-[OB] | 3–4 | NA | qPCR Leukocytes |
|
| Ooi 2021 [86] | Singaporean | Cross-sectional | 394 | 94.2% OB | WHO + 2SD [NW]-[OB] | 14.0 ± 3.02 OB 13.1 ± 3.40 NW | 67.7 | qPCR Leukocytes |
|
| Licea-Cejudo 2020 [87] | Mexican | Cross-sectional | 134 | 65.7% OB | CDC [NW]-([OW] & [OB]) | 8–10 | 52 | qPCR Saliva |
|
| Clemente 2019 [88] | European | Cross-sectional | 1.396 | 6% OB 15.4% OW | WHO [NW]-([OW]&[OB]) | 6–11 8.0 ± 1.5 | 53.9 | qPCR Leukocytes |
|
| Lamprokostopoulou 2019 [89] | Greek | Cross-sectional | 919 | 13.5% OB 30.0% OW | Greek Ref. Chart IOTF [NW]-[OW]-[OB] | 9–13 | 50.2 | qPCR Leukocytes |
|
| Theall 2019 [90] | American | Cross-sectional | 90 | 32.2% OW&OB | CDC [NW]-([OW] & [OB]) | 5–16 | 46% | qPCR Buccal swab |
|
| Zhu 2015 [91] | Caucasian and Afro-American | Cross-sectional | 766 | 24.9% OW&OB | CDC [NW]-([OW] & [OB]) | 14–18 16.1 ± 0.0 | 49.7 | qPCR Leukocytes |
|
| Buxton 2011 [92] | French | Case-control | 793 | 59.4% OB | French Ref. Chart [NW]-[OB] | 2–17 | 53.9 | qPCR Leukocytes |
|
| Al-Attas 2010 [93] | Saudi Arabian | Cross-sectional | 148 | 35.1% OB | IOTF [NW]-[OB] | 5–12 8.5 ± 2.1 boys 9.7 ± 2.6 girls | 46.6 | qPCR Leukocytes |
|
| Zannolli 2008 [94] | Italian | Cross-sectional | 53 | 22.6% OB | Italian Ref. Chart [NW]-[OB] | 8.2 ± 3.5 | NA | TRF PBMC |
|
| Author Year | Nationality | Dietary and Physical Activity Interventions | Sample Size | Obesity Definition | Age (Years) Mean ± SD or Range | Male (%) | Method/ Tissue | TL Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Period | Telomere Measure | Total | OB & OW (%) | Reference Grouping | ||||||
| Paltoglou 2021 [95] | Greek | 12 months | Baseline 12 months | 508 | 52.6% OB 34.2% OW | Greek Ref. Chart [NW][OW] [OB] | 10.14 ± 0.13 | 47 | qPCR Leukocytes |
|
| Ojeda-Rodríguez 2021 [96] | Spanish | 2 months | Baseline 2, 12 months | 102 OW&OB | 75 Intervent. 27 usual care | Spanish Ref. Chart [OW&OB] | 7–16 | 36 | qPCR Leukocytes |
|
| Morell-Azanza 2020 [97] | Spanish | 2 months | Baseline 2 months | 106 OW&OB | Spanish Ref. Chart [OW&OB] | 7–16 11.30 ± 2.49 | 37 | qPCR Leukocytes |
| |
| Ojeda-Rodríguez 2020 [98] | Spanish | 2 months | Baseline 2, 12 months | 87 OW&OB | 64 intervent. 23 usual care | Spanish Ref. Chart [OW&OB] | 7–16 | 39 | qPCR Leukocytes |
|
| García-Calzón 2014 [99] | Spanish | 2 months | Baseline 2 months Follow-up 6 months | 74 OW&OB | 96% OB 4% OW | Spanish Ref. Chart [OW&OB] | 12–16 | 48.6 | qPCR Leukocytes |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raftopoulou, C.; Paltoglou, G.; Charmandari, E. Association between Telomere Length and Pediatric Obesity: A Systematic Review. Nutrients 2022, 14, 1244. https://doi.org/10.3390/nu14061244
Raftopoulou C, Paltoglou G, Charmandari E. Association between Telomere Length and Pediatric Obesity: A Systematic Review. Nutrients. 2022; 14(6):1244. https://doi.org/10.3390/nu14061244
Chicago/Turabian StyleRaftopoulou, Christina, George Paltoglou, and Evangelia Charmandari. 2022. "Association between Telomere Length and Pediatric Obesity: A Systematic Review" Nutrients 14, no. 6: 1244. https://doi.org/10.3390/nu14061244
APA StyleRaftopoulou, C., Paltoglou, G., & Charmandari, E. (2022). Association between Telomere Length and Pediatric Obesity: A Systematic Review. Nutrients, 14(6), 1244. https://doi.org/10.3390/nu14061244







