Review of the Impact Pathways of Biofortified Foods and Food Products
Abstract
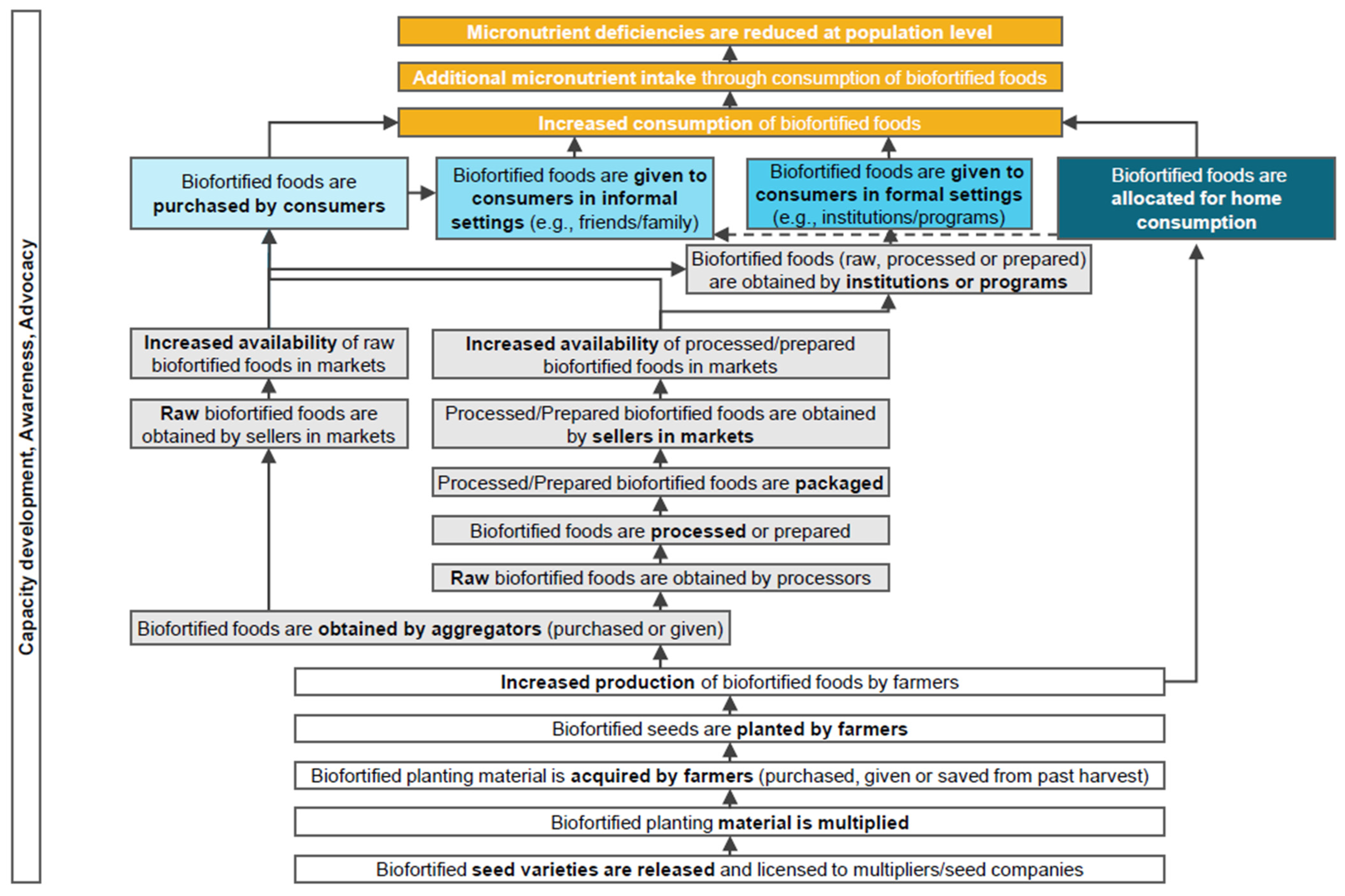
1. Introduction
- purchased directly by consumers;
- given to consumers in an informal setting;
- given to consumers in a formal setting; or
- allocated by farmers for home consumption.
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.1.1. Impact Pathway 1, Direct Purchase and Consumption
- The frequency of consumption or the amount of biofortified crops consumed obtained through direct purchase;
- Changes in micronutrient status or prevalence of deficiency compared to consumption of purchased biofortified crops.
- The proportion and/or frequency of households purchasing biofortified crops for consumption;
- The amount of said crops purchased.
2.1.2. Impact Pathway 2, Indirect Consumption
- The frequency of consumption or the amount of biofortified crops consumed obtained by indirect means;
- Changes in micronutrient status or prevalence of deficiency with respect to consumption of biofortified crops obtained indirectly.
- The proportion and/or frequency of households or household members receiving biofortified crops for consumption as a gift or payment in kind, and the amount of crops received/consumed;
- The proportion of households or household members giving biofortified crops to neighbors, their own children or other family members for consumption, and the amount of crops distributed/consumed.
2.1.3. Impact Pathway 3, Formal Consumption
- The frequency of consumption or the amount of biofortified crops consumed in the context of a formal setting such as school meal programs, public distribution systems, hospitals, etc.;
- changes in micronutrient status or deficiency prevalence with respect to consumption of biofortified crops in the context of said formal setting.
- The proportion and/or frequency of school meal programs, public distribution systems, or hospitals, etc., including biofortified crops on their rotation/menu.
2.1.4. Impact Pathway 4, Farmer Household Consumption
- The frequency or amount of biofortified crops consumed by farmer households, as a portion of crops grown by the said household;
- Changes in micronutrient status or prevalence of deficiency with respect to consumption of biofortified crops by farmers’ households, as a portion of crops grown by the said household.
- Reasons why farmers retained a given biofortified crop for consumption;
- Proportion of households retaining biofortified crops for home consumption;
- Farmer household consumption of crops as predictors of other outcomes e.g., diarrhea;
- Potential factors impacting dietary intakes of micronutrients versus biofortified crops.
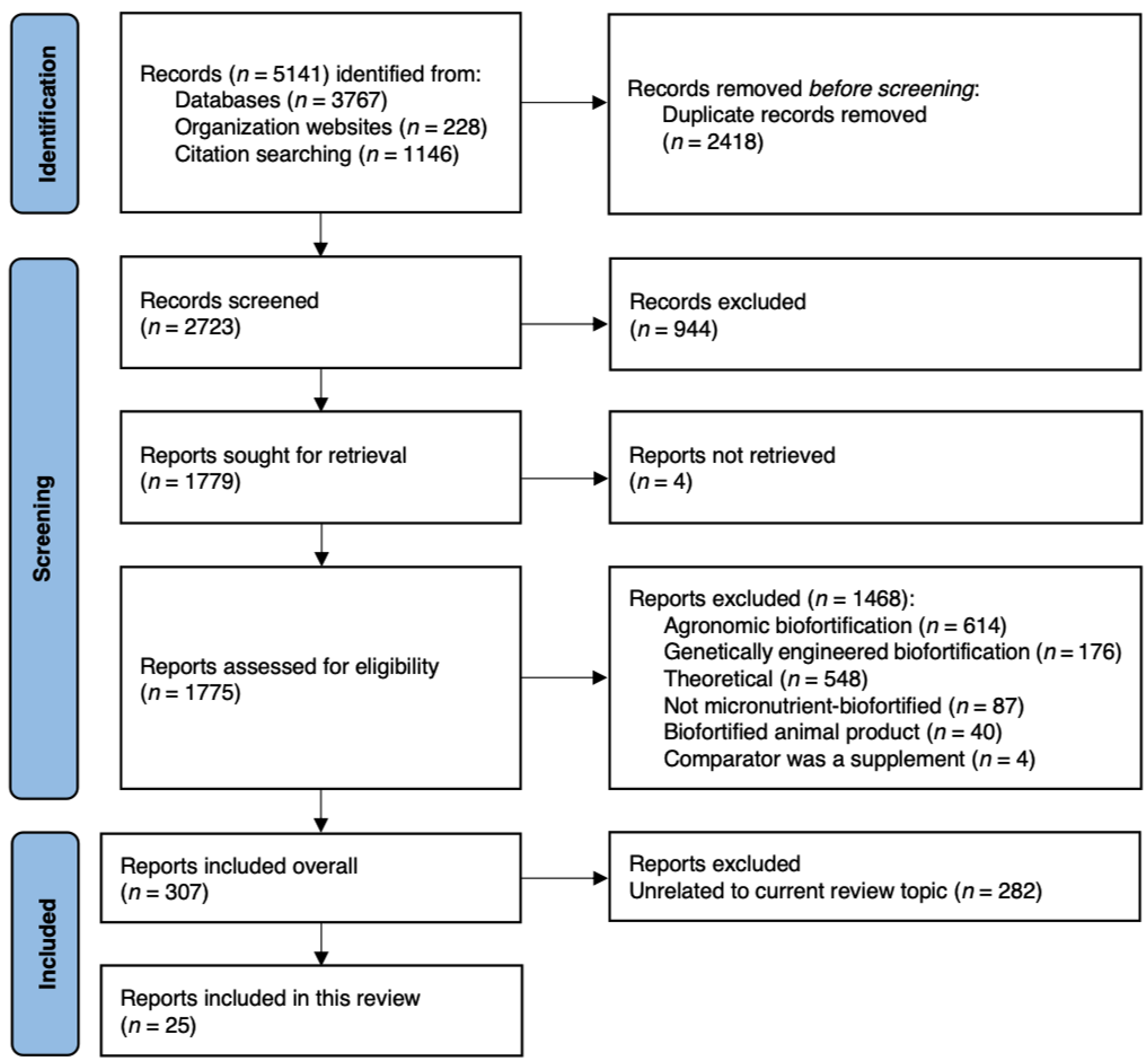
2.2. Literature Search and Methodology
2.3. Data Screening and Extraction
2.4. Data Synthesis and Analysis
3. Results and Discussion
- Impact Pathway 1, Direct Purchase and Consumption: 5
- Impact Pathway 2, Indirect Consumption: 3
- Impact Pathway 3, Formal Consumption: 3
- Impact Pathway 4, Farmer Household Consumption: 21
3.1. Impact Pathway 1, Direct Purchase and Consumption
3.2. Impact Pathway 2, Indirect Consumption
3.3. Impact Pathway 3, Formal Consumption
3.4. Impact Pathway 4, Farmer Household Consumption
3.4.1. Reaching End Users (REU) Project
3.4.2. Rwanda High-Iron Beans Survey
3.4.3. Reaching Agents of Change (RAC) Project
3.4.4. Building Nutritious Food Baskets (BNFB) Project
3.4.5. Sweetpotato Action for Security and Health in Africa (SASHA) Project and Marando Bora Project
3.4.6. Other Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 22–33. [Google Scholar] [CrossRef] [PubMed]
- Beach, R.H.; Sulser, T.B.; Crimmins, A.; Cenacchi, N.; Cole, J.; Fukagawa, N.K.; Mason-D’Croz, D.; Myers, S.; Sarofim, M.C.; Smith, M.; et al. Combining the effects of increased atmospheric carbon dioxide on protein, iron, and zinc availability and projected climate change on global diets: A modelling study. Lancet Planet Health 2019, 3, e307–e317. [Google Scholar] [CrossRef]
- Welch, R.M. Biotechnology, biofortification, and global health. Food Nutr. Bull. 2005, 26, 419–421. [Google Scholar] [CrossRef]
- Council for Agricultural Science and Technology (CAST). Food Biofortification—Reaping the Benefits of Science to Overcome Hidden Hunger—A paper in the series on The Need for Agricultural Innovation to Sustainably Feed the World by 2050. In Issue Paper 69; CAST: Ames, IA, USA, 2020; pp. 2–40. [Google Scholar]
- Strobbe, S.; Van Der Straeten, D. Toward Eradication of B-Vitamin Deficiencies: Considerations for Crop Biofortification. Front. Plant. Sci. 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- HarvestPlus. Climate Change and Biofortification; HarvestPlus/International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2018. [Google Scholar]
- HarvestPlus. HarvestPlus Biofortified Crops Map and Table Updated with 2020 Data. Available online: https://www.harvestplus.org/knowledge-market/in-the-news/harvestplus-biofortified-crops-map-and-table-updated-2020-data (accessed on 24 November 2021).
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Global Alliance for Improved Nutrition (GAIN) and HarvestPlus. The Commercialisation of Biofortified Crops Programme: Monitoring Reference Manual; GAIN and HarvestPlus: Geneva, Switzerland; Washington, DC, USA, 2020. [Google Scholar]
- Huey, S.L.; Krisher, J.T.; Friesen, V.; Mbuya, M.; Monterrosa, E.; Mehta, S. Review of efficacy, effectiveness, and impact of biofortified foods and food products. Prospero 2021, CRD42021254461. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021254461 (accessed on 7 February 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Asare-Marfo, D.H.; Herrington, C.; Birachi, E.; Birol, E.; Cook, K.; Diressie, M.T.; Dusenge, L.; Funes, J.; Katsvairo, L.; Katungi, E.; et al. Assessing the Adoption of High-Iron Bean Varieties and Their Impact on Iron Intakes and Other Livelihood Outcomes in Rwanda: Main Survey Report; HarvestPlus: Washington, DC, USA, 2016. [Google Scholar]
- Petry, N.; Wirth, J.P.; Friesen, V.M.; Rohner, F.; Nkundineza, A.; Chanzu, E.; Tadesse, K.G.; Gahutu, J.B.; Neufeld, L.M.; Birol, E.; et al. Assessing the Coverage of Biofortified Foods: Development and Testing of Methods and Indicators in Musanze, Rwanda. Curr. Dev. Nutr. 2020, 4, nzaa107. [Google Scholar] [CrossRef]
- Brouwer, R.; Tedesco, I. Shackled Orange: Biofortified Varieties in the Sweetpotato Commodity Chain in Mozambique. Sustain. Agric. Res. 2019, 8, 55–67. [Google Scholar] [CrossRef]
- Phorbee, O.; Mulongo, G.; Njoku, N.; Maru, J.; Munyua, H. Baseline Report on Biofortification and Thematic Areas of Building Nutritious Food Baskets Project in Nigeria. (Nairobi, Kenya); International Potato Centre (CIP): Lima, Peru, 2017. [Google Scholar]
- Uchitelle-Pierce, B.; Ubomba-Jaswa, P.A. Marketing biofortified crops: Insights from consumer research. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 12051–12062. [Google Scholar] [CrossRef]
- de Brauw, A.; Moursi, M.; Munhaua, A.B. Vitamin A intakes remain higher among intervention participants 3 years after a biofortification intervention in Mozambique. Br. J. Nutr. 2019, 122, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Lagerkvist, C.J.; Carey, E.E.; Okello, J.J.; Kwikiriza, N.; Abidin, P.E.; Adekambi, S. Goal-setting and volitional behavioural change: Results from a school meals intervention with vitamin-A biofortified sweetpotato in Nigeria. Appetite 2018, 129, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.d.O.; Deliza, R.; Nutti, M.R.; de Carvahlo, J.L.V. Biofortificação de alimentos no município de Itaguaí: Melhorando a qualidade nutricional da merenda escolar (Biofortification in Itaguaí municipality: Improving nutritional quality of school meal). Reun. Biofortificação No Bras. 2015, 5, 113–116. [Google Scholar]
- The Global Child Nutrition Foundation. Chapter 6: Health and Nutrition. In The Global Survey of School Meal Programs; The Global Child Nutrition Foundation: Seattle, WA, USA, 2019; pp. 48–52. [Google Scholar]
- Low, J.W.; Arimond, M.; Osman, N.; Cunguara, B.; Zano, F.; Tschirley, D. A food-based approach introducing orange-fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. J. Nutr. 2007, 137, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Arimond, M.; Ball, A.-M.; Bechoff, A.; Bosch, D.; Bouis, H.; Brauw, A.; Coote, C.; Dove, R.; Eozenou, P.; Gilligan, D.; et al. Reaching and Engaging End Users (REU) Orange Fleshed Sweet Potato (OFSP) in East and Southern Africa; HarvestPlus: Washington, DC, USA, 2010. [Google Scholar]
- HarvestPlus. Disseminating Orange-Fleshed Sweet Potato. Findings from a HarvestPlus Project in Mozambique and Uganda; HarvestPlus: Washington, DC, USA, 2010. [Google Scholar]
- Jones, K.M.; de Brauw, A. Using Agriculture to Improve Child Health: Promoting Orange Sweet Potatoes Reduces Diarrhea. World Dev. 2015, 74, 15–24. [Google Scholar] [CrossRef]
- de Brauw, A.; Eozenou, P.; Gilligan, D.O.; Hotz, C.; Kumar, N.; Meenakshi, J.V. Biofortification, crop adoption and health information: Impact pathways in Mozambique and Uganda. Am. J. Agric. Econ. 2018, 100, 906–930. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Loechl, C.; de Brauw, A.; Eozenou, P.; Gilligan, D.; Moursi, M.; Munhaua, B.; van Jaarsveld, P.; Carriquiry, A.; Meenakshi, J.V. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. Br. J. Nutr. 2012, 108, 163–176. [Google Scholar] [CrossRef]
- Hotz, C.; Loechl, C.; Lubowa, A.; Tumwine, J.K.; Ndeezi, G.; Nandutu Masawi, A.; Baingana, R.; Carriquiry, A.; de Brauw, A.; Meenakshi, J.V.; et al. Introduction of β-carotene-rich orange sweet potato in rural Uganda resulted in increased vitamin A intakes among children and women and improved vitamin A status among children. J. Nutr. 2012, 142, 1871–1880. [Google Scholar] [CrossRef]
- Gilligan, D.O.; Kumar, N.; McNiven, S.; Meenakshi, J.V.; Quisumbing, A. Bargaining power, decision making, and biofortification: The role of gender in adoption of orange sweet potato in Uganda. Food Policy 2020, 95, 101909. [Google Scholar] [CrossRef]
- Gilligan, D.O.; Kumar, N.; McNiven, S.; Meenakshi, J.V.; Quisumbing, A. Bargaining Power and Biofortification: The Role of Gender in Adoption of Orange Sweet Potato in Uganda. International Food Policy Research Institute (IFPRI)—Discussion Paper 01353; IFPRI Headquarters: Washington, DC, USA, 2014; pp. 1–19. [Google Scholar]
- Asare-Marfo, D.; Herrington, C.; Alwang, J.; Birachi, E.; Birol, E.; Diressie, M.T.; Dusenge, L.; Funes, J.E.; Katungi, E.; Labarta, R.; et al. Listing Exercise Report; HarvestPlus: Washington, DC, USA, 2016. [Google Scholar]
- Sellitti, S.; Vaiknoras, K.; Smale, M.; Jamora, N.; Andrade, R.; Wenzl, P.; Labarta, R. The contribution of the CIAT genebank to the development of iron-biofortified bean varieties and well-being of farm households in Rwanda. Food Secur. 2020, 12, 975–991. [Google Scholar] [CrossRef]
- Vaiknoras, K.; Larochelle, C. The impact of iron-biofortified bean adoption on bean productivity, consumption, purchases and sales. World Dev. 2021, 139, 105260. [Google Scholar] [CrossRef] [PubMed]
- Vaiknoras, K.; Larochelle, C. The Impact of Biofortified Iron Bean Adoption on Productivity, and Bean Consumption, Purchases and Sales. In Proceedings of the 2018 Agricultural & Applied Economics Association Annual Meeting, Washington, DC, USA, 5–7 August 2018. [Google Scholar] [CrossRef]
- Mulongo, G.; Munyua, H.; Mbabu, A.; Maru, J. What is required to scale-up and sustain biofortification? Achievements, challenges and lessons from scaling-up Orange-Fleshed Sweetpotato in Sub-Sahara Africa. J. Agric. Food Res. 2021, 4, 100102. [Google Scholar] [CrossRef] [PubMed]
- Girard, A.W.; Grant, F.; Watkinson, M.; Okuku, H.S.; Wanjala, R.; Cole, D.; Levin, C.; Low, J. Promotion of Orange-Fleshed Sweet Potato Increased Vitamin A Intakes and Reduced the Odds of Low Retinol-Binding Protein among Postpartum Kenyan Women. J. Nutr. 2017, 147, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Shikuku, K.M.; Okello, J.J.; Wambugu, S.; Sindi, K.; Low, J.W.; McEwan, M. Nutrition and food security impacts of quality seeds of biofortified orange-fleshed sweetpotato: Quasi-experimental evidence from Tanzania. World Dev. 2019, 124, 104646. [Google Scholar] [CrossRef] [PubMed]
- Shikuku, K.M.; Okello, J.J.; Sindi, K.; Low, J.W.; McEwan, M. Effect of farmers’ multidimensional beliefs on adoption of biofortified crops: Evidence from sweetpotato farmers in Tanzania. J. Dev. Stud. 2019, 55, 227–242. [Google Scholar] [CrossRef]
- Ogutu, S.O.; Fongar, A.; Godecke, T.; Jackering, L.; Mwololo, H.; Njuguna, M.; Wollni, M.; Qaim, M. How to Make Farming and Agricultural Extension More Nutrition-Sensitive: Evidence from a Randomized Controlled Trial in Kenya. In Proceedings of the 30th International Conference of Agricultural Economists, Vancouver, BC, Canada, 28 July–2 August 2018. [Google Scholar]
- Ogutu, S.O.; Fongar, A.; Godecke, T.; Jackering, L.; Mwololo, H.; Njuguna, M.; Wollni, M.; Qaim, M. How to make farming and agricultural extension more nutrition-sensitive: Evidence from a randomised controlled trial in Kenya. Eur. Rev. Agric. Econ. 2020, 47, 95–118. [Google Scholar] [CrossRef]
- Prakash, P.; Avinash, K.; Devesh, R.; Debdutt, B.; Sheela, I. Biofortification for reducing hidden hunger: A value chain analysis of sweet potato in Odisha, India. Agric. Econ. Res. Rev. 2017, 30, 201–211. [Google Scholar] [CrossRef][Green Version]
- Reyes, B.; Mazariegos, M.; Maruyama, E.; Birol, E.; Boy, E.; Scollard, P.; Khor, L.Y.; Pérez, S.; Zeller, M.; González, C.; et al. Socieconomic and Nutrition Impact of a Biofortified Bean Intervention in Adolescent Girls in Eastern Guatemala. PowerPoint Presentation; HarvestPlus: Washington, DC, USA, 2020. [Google Scholar]
- Sweetpotato Knowledge Portal. HarvestPlus Reaching End Users Orange Fleshed Sweetpotato Project. Available online: https://www.sweetpotatoknowledge.org/project/harvestplus-reaching-end-users-orange-fleshed-sweetpotato-project/ (accessed on 12 December 2021).
- Institute of Medicine. Dietary Reference Intakes; Institute of Medicine: Washington, DC, USA, 2006; pp. 1–529. [Google Scholar]
- Vaiknoras, K.; Larochelle, C.; Birol, E.; Asare-Marfo, D.; Herrington, C. Promoting rapid and sustained adoption of biofortified crops: What we learned from iron-biofortified bean delivery approaches in Rwanda. Food Policy 2019, 83, 271–284. [Google Scholar] [CrossRef]
- Maziya-Dixon, B.B.; Akinyele, I.O.; Sanusi, R.A.; Oguntona, T.E.; Nokoe, S.K.; Harris, E.W. Vitamin A deficiency is prevalent in children less than 5 y of age in Nigeria. J. Nutr. 2006, 136, 2255–2261. [Google Scholar] [CrossRef]
- Cole, D.C.; Levin, C.; Loechl, C.; Thiele, G.; Grant, F.; Girard, A.W.; Sindi, K.; Low, J. Planning an integrated agriculture and health program and designing its evaluation: Experience from Western Kenya. Eval. Program. Plann. 2016, 56, 11–22. [Google Scholar] [CrossRef]
- McEwan, M.A.; Lusheshanija, D.; Shikuku, K.M.; Sindi, K. Specialised Sweetpotato Vine Multiplication in Lake Zone, Tanzania: What “Sticks” and What Changes? Open Agric. 2017, 2, 64–69. [Google Scholar] [CrossRef]
- Walton, J. Improving nutrition through biofortification: From strategy to implementation. Cereal Foods World 2019, 64, 2. [Google Scholar] [CrossRef]
- Walton, J. Paving the Way to Commercialization: The Role of Standards for Biofortified Products. Available online: https://a4nh.cgiar.org/2021/12/22/paving-the-way-to-commercialization-the-role-of-standards-for-biofortified-products/ (accessed on 7 February 2022).
| Database Name | Final Search String | Date of Search | Records (#) |
|---|---|---|---|
| MEDLINE | Biofortification(MeSH) OR biofortif*(tiab) OR “bio-fortif*“(tiab) | 9 March 2021 | 1434 |
| AgEcon | All of the words (biofortif*) in All Fields OR All of the words (bio-fortif*) in All fields | 7 April 2021 | 73 |
| AGRICOLA | TX (biofortif* OR bio-fortif*) AND TX (Adopt* OR Farmer* OR Household* OR Accept* OR Sensory OR DALY OR “disability adjusted life year*” OR Market* OR School meal program* OR Retention OR Mill* OR Process* OR Stor* OR Cook* OR Polish* OR Bioavailab* OR Cost-effectiveness OR Bioaccessib* OR Bioactiv* OR Efficacy) | 7 April 2021 | 722 |
| CAB Abstracts | TS = biofortif* OR TS = bio-fortif* AND TS = (Adopt* OR Farmer* OR Household* OR Accept* OR Sensory OR DALY OR “disability adjusted life year*” OR Market* OR School meal program* OR Retention OR Mill* OR Process* OR Stor* OR Cook* OR Polish* OR Bioavailab* OR Cost-effectiveness OR Bioaccessib* OR Bioactiv* OR Efficacy) | 7 April 2021 | 1538 |
| TOTAL | 3767 | ||
| Organization Website | Studies Identified on 7 April 2021 |
|---|---|
| HarvestPlus | 75 (manual) |
| CIMMYT Publications Repository | 0 (captured in other databases) |
| IITA | 2 (manual) |
| CIAT | 0 (captured in other databases) |
| IRRI | 0 (captured in other databases) |
| ICRISAT | 151 = ”biofortif*” |
| ICARDA | 0 (irrelevant) |
| TOTAL | 228 |
| Country, Setting | Study Design | Population, n | Biofortified Crops Involved, Food Product or Processing | Outcomes Reported | Ref. |
|---|---|---|---|---|---|
| Rwanda, rural | Impact assessment study, including a listing survey and a detailed household survey in 2015 to examine the impact of releasing high-iron beans in 2010. | Farmer households from listing survey, n = 19,575 households in 120 randomly selected villages in beginning of season B in 2015; plus 1397 bean-farming households; Farmer households; n = 422 | Iron bush beans released in 2010: RWR2445 RWR2154 MAC44 RWV1129 Year released: 2012 RWV3006 RWV3316 RWV2887 RWV3317 CAB2 MAC42 | Frequency of consumption Consumption per capita | [12] |
| Rwanda, rural, peri-urban | Household surveys in 2019 | Households, n = 250 | Iron beans: cultivar NR PVA OSP: cultivar NR | Current or ever consumption Sources to purchase from | [13] |
| Mozambique, urban | Analysis using several data sources including government statistical data, primary data on prices of sweet potato roots, semi-structured interviews, and a survey among Maputo City residents regarding production and consumption between 2014–2015. | Maputo City residents, n = 656 In the latter survey | PVA OSP: cultivar NR | Frequency of consumption Annual meals with OSP Annual total urban consumption (tons) | [14] |
| Nigeria, rural | Building Nutritious Food Baskets (BNFB) project, a situation analysis in two phases: first, a desk review and content analysis; second, field visits and consultations with relevant stakeholders from 2015–2018 | Farmers and consumers, n = 420 farmers and n = 735 consumers | PVA cassava: cultivar NR, released in 2011; PVA OSP: cultivar NR, released in 2012; PVA maize: cultivar NR, released in 2014 | Current or ever consumption Frequency of consumption | [15] |
| Uganda, urban, per-urban | Consumer research, including study, design, testing, and implementation of behavioral interventions | Uganda: consumers, n = 122 interviews in 3 markets | Uganda: PVA OSP: cultivar NR | Ever consumption Frequency of purchase | [16] |
| Country, Setting | Study Design | Population, n | Biofortified Crops Involved, Food Product or Processing | Outcomes Reported | Ref. |
|---|---|---|---|---|---|
| Mozambique, rural | Program evaluation in 2012, from 2006–2009 REU project | Women of reproductive age (n = 346) and children under 6 years of age (n = 178) in 36 villages | PVA OSP, cultivar: NR | Proportion households giving crop to neighbors by intervention group | [17] |
| Rwanda, rural | Impact assessment study, including a listing survey and a detailed household survey in 2015 | Farmer households from the listing survey, n = 19,575 households in 120 randomly selected villages at the beginning of season B in 2015; plus 1397 bean-farming households | Iron bush beans released in 2010: RWR2445 RWR2154 MAC44 RWV1129 Year released: 2012 RWV3006 RWV3316 RWV2887 RWV3317 CAB2 MAC42 | Current or ever consumption from gift or in-kind payment | [12] |
| Uganda, urban, per-urban | Consumer research, including study, design, testing, and implementation of behavioral interventions | Consumers, n = 122 interviews in 3 markets | PVA OSP: cultivar NR | Frequency of serving BF crops to children, spouses, other children, other adults | [16] |
| Country, Setting | Study Design | Population, n | Biofortified Crops Involved, Food Product or Processing | Outcomes Reported | Ref. |
|---|---|---|---|---|---|
| Nigeria, rural | Intervention study in primary schools and follow-up surveys in 2016 | Schoolchildren (7–12 y), n = 556 across 12 primary schools | PVA OSP, cultivar: NR | Average proportion of OSP in school meals | [18] |
| Brazil | Cross-sectional study in 3 rural public schools | Schoolchildren (5–12 y), n = 327 | PVA OSP, cultivar: NR PVA cassava, cultivar: NR FeZn beans, cultivar: NR PVA maize, cultivar: NR | Amount (g) of each biofortified crop portion served to students as part of the school meal | [19] |
| 6 regions including 85 countries, rural | Global Survey of School Meal Programs Report in 2019 * | Children (all ages), in 85 countries, n = 297.3 million receiving food through school meal programs | Any biofortified crops | Proportion of school lunch programs serving BF crops | [20] |
| Country, Setting | Study Design | Population, n | Biofortified Crops Involved, Food Product or Processing | Outcomes Reported | Ref. |
|---|---|---|---|---|---|
| Mozambique, rural | Quasi-experimental study comparing 2-year intervention integrating agriculture and nutrition vs. control from 2003–2005 (informed the REU project, below) | Households, n = 741 from 3 districts | PVA OSP: Kandee, Japan, Lo, Taimung 64, Jonathan, CN, Resisto, Caromex, Cordner | Daily OSP consumption Prevalence of vitamin A deficiency (serum retinol < 0.7 µmol/L) Mean serum retinol Change in serum retinol | [21] |
| Reaching End Users (REU) project | |||||
| Mozambique, Uganda; rural | Technical report of the REU project, a clustered randomized trial using 2 dissemination strategies to increase OSP use, from 2006–2009 | Uganda: Farmer households, n = 10,000 Mozambique: Farmer households, n = 12,000 | PVA OSP: SPK 004 (Kakamega), Ejumula (Ejumula), SPK 004/6 (VITA), SPK 004/6/6 (Kabode), Cordner, Gabagaba, Jonathan, 0 5023 419, LO 323, MGCL 01, Resisto | Mean vitamin A intake OSP consumption Vitamin A intake from OSP | [22,23] |
| Mozambique, rural | Impact evaluation of the REU project from 2007–2009 | Children (0–5 y), n = 781 | OSP consumption as predictor of diarrhea incidence and severity | [24] | |
| Mozambique, rural | Program evaluation of 2006–2009 REU project, 3 years after endline | Women of reproductive age (n = 346) and children under 6 years of age (n = 178) in 36 villages | Long-term: Mean vitamin A intake OSP consumption Vitamin A intake from OSP | [17] | |
| Mozambique, Uganda; rural | Program evaluation of 2006–2009 REU project | Mozambique: Families with resident children between the ages of 6 and 35 months at the start of the study, n = 379 Uganda: Families with resident children between the ages of 36 and 71 months at baseline, n = 446 | Variables mediating impact on vitamin A intakes by country | [25] | |
| Mozambique, rural | Cluster-randomized controlled effectiveness study comparing two large-scale >2-y intervention programs (intensive inputs vs. reduced inputs) from 2006–2009 | Children (6 mo to 5.5 y), n = 441 in 36 clusters; women, n = 441 in 36 clusters | Mean vitamin A intake OSP consumption Vitamin A intake from OSP | [26] | |
| Uganda, rural | Follow-up analysis of the impact of the REU project on serum retinol | Children (6–35 mo), n = 264; children (3–5 y), n = 544; women, n = 539 | Mean vitamin A intake OSP consumption Vitamin A intake from OSP Prevalence of vitamin A deficiency (serum retinol < 0.7 µmol/L) Prevalence of vitamin A (serum retinol < 1.05 µmol/L) | [27] | |
| Uganda, rural | Impact evaluation, including gender roles and intra-household bargaining, from REU project from 2007–2009 | Households, n = 775 | Women’s bargaining power as a predictor of child’s OSP intake | [28,29] | |
| Rwanda high iron beans project | |||||
| Rwanda, rural | Impact assessment study, including a listing survey and a detailed household survey in 2015 to examine impact of releasing high iron beans in 2010 | Farmer households from listing survey, n = 19,575 households in 120 randomly selected villages in beginning of season B in 2015; plus 1397 bean-farming households; Farmer households; n = 422 | Iron bush beans released in 2010: RWR2445 RWR2154 MAC44 RWV1129 Year released: 2012 RWV3006 RWV3316 RWV2887 RWV3317 CAB2 MAC42 | Proportion of households that retain beans for their own consumption Frequency of consumption Consumption per capita | [12,30] |
| Survey data from the 2015 impact assessment survey to investigate adoption | Farmer households: non-adopters, n = 971 adopters, n = 219 | Fe common bean: CAB2, RWV3316, RWV3317, RWV3006, RWV2887, MAC44, MAC42 | Effect of adoption on the duration of consumption from own production Effect of adoption on duration of market purchases | [31] | |
| Impact assessment study, including a listing survey and a detailed household survey in 2015 to examine the impact of releasing high-iron beans in 2010. | Farmer households from listing survey, n = 19,575 households in 120 randomly selected villages in beginning of season B in 2015; plus 1397 bean-farming households; Farmer households; n = 422 | Bush beans: RWR2245 | Average amount of beans consumed from own production per adult male equivalent Effect of growing RWR2245 on the length of time beans were consumed from own production | [32,33] | |
| Reaching Agents of Change (RAC) project | |||||
| Tanzania, Mozambique, Nigeria, Ghana, Burkina Faso | RAC project, an ex-post survey from 2011–2015 | Households, n = 309,974 (on track to benefit ≥600,000) | PVA OSP, cultivar: NR | Qualitative, frequency of OSP consumption, and amount of land devoted OSP cultivation | [34] |
| Building Nutritious Food Baskets (BNFB) project | |||||
| Nigeria, rural | BNFB project, a situation analysis in two phases: first, a desk review and content analysis; second, field visits and consultations with relevant stakeholders from 2015–2018 | Farmers and consumers, n = 420 farmers and n = 735 consumers | PVA cassava: cultivar NR, released in 2011; PVA OSP: cultivar NR, released in 2012; PVA maize: cultivar NR, released in 2014 | Reasons for cultivating BF crops Current or ever consumption | [15] |
| Sweetpotato Action for Security and Health in Africa project (SASHA); Marando Bora project | |||||
| Kenya, rural | Mama SASHA project, a cluster-randomized proof-of-concept from 2013–2018 | Pregnant and lactating women followed through 9 months postpartum, n = 206 | PVA OSP: Kabode, Vita | Frequency of consumption Contribution of OSP to vitamin A and beta-carotene intakes Odds of vitamin A deficiency (RBP < 1.17 µmol/L) | [35] |
| Tanzania, rural | Quasi-experimental field experiment, Marando Bora project, and survey data from 2010 and 2013 | Farmer households, n = 434 | PVA OSP: Kabode, Ejumula, Jewel | Proportion of OSP out of total sweet potato production | [36] |
| Quasi-experimental field experiment, Marando Bora project, and survey data from 2010 and 2013 | Farmer households, n = 919 | PVA OSP: Kabode, Ejumula, Jewel | Qualitative, primary reason for growing OSP | [37] | |
| Other projects | |||||
| Mozambique, urban | Analysis using several data sources including government statistical data, primary data on prices of sweet potato roots, semi-structured interviews, and a survey among Maputo City residents regarding production and consumption between 2014–2015 | Maputo City residents, n = 656 In the latter survey | PVA OSP: cultivar NR | Frequency of consumption Annual meals with OSP Annual total urban consumption (tons) | [14] |
| Kenya, rural | Randomized controlled trial comparing agricultural training alone or in combination with nutrition and marketing training from 2015–2016 | 48 farmer groups, with 20–50 active members each | Fe/Zn Black beans: KK15 | Qualitative, primary reason for growing BF beans | [38,39] |
| India, rural | Survey to investigate the value chain in 2016 | 4 villages, n = 310 farmers and consumers | PVA OSP, cultivar: NR | Proportion of farmers growing OSP Reasons when/why OSP is usually eaten | [40] |
| Guatemala, rural | Cluster-randomized trial comparing the distribution of BF or control bean seed, agronomic training, and nutrition information vs. control between 2015 and 2019. | Households (specifically adolescent girls) with bean production and high bean consumption, high prevalence of malnutrition and anemia, low presence of food aid programs, n = 1764 in 120 communities in 7 municipalities of 3 departments of eastern Guatemala | Fe/Zn black beans, cultivar: ICTA Chorti | Quantity of beans saved for consumption Quantity of beans consumed by girls in the last 24 h Amount of iron consumed per day from beans | [41] * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huey, S.L.; Krisher, J.T.; Bhargava, A.; Friesen, V.M.; Konieczynski, E.M.; Mbuya, M.N.N.; Mehta, N.H.; Monterrosa, E.; Nyangaresi, A.M.; Mehta, S. Review of the Impact Pathways of Biofortified Foods and Food Products. Nutrients 2022, 14, 1200. https://doi.org/10.3390/nu14061200
Huey SL, Krisher JT, Bhargava A, Friesen VM, Konieczynski EM, Mbuya MNN, Mehta NH, Monterrosa E, Nyangaresi AM, Mehta S. Review of the Impact Pathways of Biofortified Foods and Food Products. Nutrients. 2022; 14(6):1200. https://doi.org/10.3390/nu14061200
Chicago/Turabian StyleHuey, Samantha L., Jesse T. Krisher, Arini Bhargava, Valerie M. Friesen, Elsa M. Konieczynski, Mduduzi N. N. Mbuya, Neel H. Mehta, Eva Monterrosa, Annette M. Nyangaresi, and Saurabh Mehta. 2022. "Review of the Impact Pathways of Biofortified Foods and Food Products" Nutrients 14, no. 6: 1200. https://doi.org/10.3390/nu14061200
APA StyleHuey, S. L., Krisher, J. T., Bhargava, A., Friesen, V. M., Konieczynski, E. M., Mbuya, M. N. N., Mehta, N. H., Monterrosa, E., Nyangaresi, A. M., & Mehta, S. (2022). Review of the Impact Pathways of Biofortified Foods and Food Products. Nutrients, 14(6), 1200. https://doi.org/10.3390/nu14061200








