Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease
Abstract
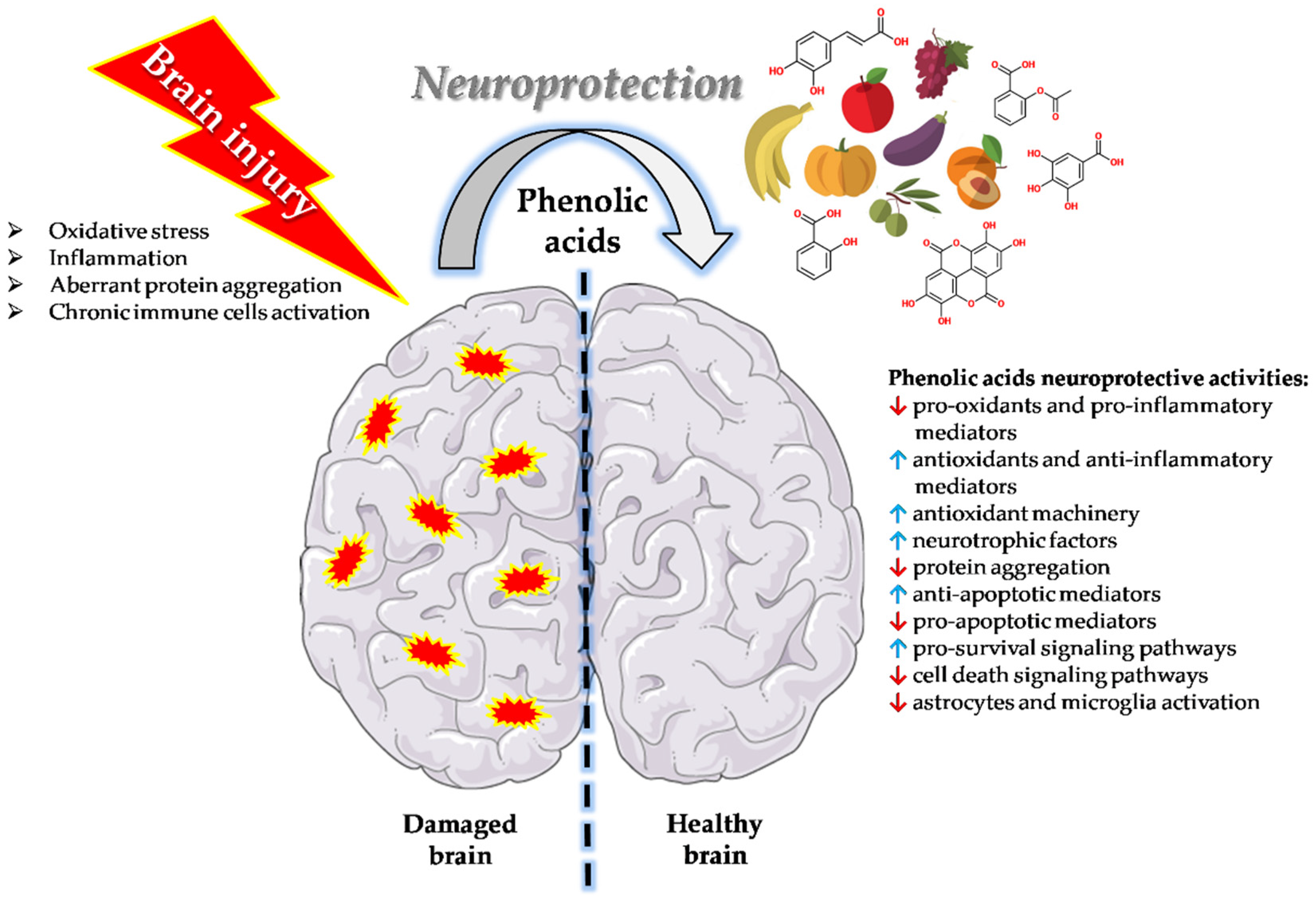
:1. Introduction
2. Chemical Composition, Major Groups, and Dietary Sources of Phenolic Acids
3. Studies Conducted on Humans on Phenolic Acids and Cognitive Status
4. Preclinical Studies on Phenolic Acids and Cognitive Disorders: Molecular Mechanisms and Neuroprotective Activity
4.1. Caffeic Acid and Caffeic Acid Phenethyl Ester
4.2. Chlorogenic Acid
4.3. Ferulic Acid
4.4. Gallic Acid
4.5. Rosmarinic Acid
4.6. Acetylsalicylic Acid
4.7. Tannic Acid
4.8. Protocatechuic Acid
4.9. p-coumaric Acid
4.10. Sinapic Acid
4.11. Ellagic Acid
4.12. Salicylic Acid
4.13. Homovanillic Acid
4.14. Syringic Acid
4.15. Cinnamic Aldehyde
4.16. Phenolic Acids Potential in Drug Discovery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godos, J.; Currenti, W.; Angelino, D.; Mena, P.; Castellano, S.; Caraci, F.; Galvano, F.; Del Rio, D.; Ferri, R.; Grosso, G. Diet and mental health: Review of the recent updates on molecular mechanisms. Antioxidants 2020, 9, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2021, 108013. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.E. The impact of flavonoids on memory: Physiological and molecular considerations. Chem. Soc. Rev. 2009, 38, 1152–1161. [Google Scholar] [CrossRef]
- Zhao, B. Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochem. Res. 2009, 34, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [Green Version]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Abul Khair, S.B.; Kazim, A.S.; Minhas, S.T.; Al-Tel, T.H.; Al-Hayani, A.A.; Haque, M.E.; Eliezer, D.; et al. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014, 6, 197. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Rioux Bilan, A. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural Regen. Res. 2018, 13, 955–961. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The neuroprotective effects of phenolic acids: Molecular mechanism of action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Grasso, M.; Caruso, G.; Godos, J.; Bonaccorso, A.; Carbone, C.; Castellano, S.; Currenti, W.; Grosso, G.; Musumeci, T.; Caraci, F. Improving Cognition with Nutraceuticals Targeting TGF-β1 Signaling. Antioxidants 2021, 10, 1075. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wojcik, E.; Borowiec, K. Phenolic Acids Exert Anticholinesterase and Cognition-Improving Effects. Curr. Alzheimer Res. 2018, 15, 531–543. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Kesse-Guyot, E.; Fezeu, L.; Andreeva, V.A.; Touvier, M.; Scalbert, A.; Hercberg, S.; Galan, P. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J. Nutr. 2012, 142, 76–83. [Google Scholar] [CrossRef]
- Goni, L.; Fernández-Matarrubia, M.; Romanos-Nanclares, A.; Razquin, C.; Ruiz-Canela, M.; Martínez-González, M.Á.; Toledo, E. Polyphenol intake and cognitive decline in the Seguimiento Universidad de Navarra (SUN) Project. Br. J. Nutr. 2021, 126, 43–52. [Google Scholar] [CrossRef]
- Godos, J.; Caraci, F.; Micek, A.; Castellano, S.; D’Amico, E.; Paladino, N.; Ferri, R.; Galvano, F.; Grosso, G. Dietary Phenolic Acids and Their Major Food Sources Are Associated with Cognitive Status in Older Italian Adults. Antioxidants 2021, 10, 700. [Google Scholar] [CrossRef]
- Ran, L.S.; Liu, W.H.; Fang, Y.Y.; Xu, S.B.; Li, J.; Luo, X.; Pan, D.J.; Wang, M.H.; Wang, W. Alcohol, coffee and tea intake and the risk of cognitive deficits: A dose-response meta-analysis. Epidemiol. Psychiatr. Sci. 2021, 30, e13. [Google Scholar] [CrossRef]
- Basu Mallik, S.; Mudgal, J.; Nampoothiri, M.; Hall, S.; Dukie, S.A.; Grant, G.; Rao, C.M.; Arora, D. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016, 632, 218–223. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Grasso, M.; Santangelo, R.; Lazzarino, G.; Lunte, S.M.; Caraci, F. Inflammation as the common biological link between depression and cardiovascular diseases: Can carnosine exert a protective role? Curr. Med. Chem. 2020, 27, 1782–1800. [Google Scholar] [CrossRef]
- Di Pietro, V.; Yakoub, K.M.; Caruso, G.; Lazzarino, G.; Signoretti, S.; Barbey, A.K.; Tavazzi, B.; Lazzarino, G.; Belli, A.; Amorini, A.M. Antioxidant therapies in traumatic brain injury. Antioxidants 2020, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Fazili, N.A.; Naeem, A. Anti-fibrillation potency of caffeic acid against an antidepressant induced fibrillogenesis of human α-synuclein: Implications for Parkinson’s disease. Biochimie 2015, 108, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Coelho, V.R.; Vieira, C.G.; de Souza, L.P.; Moysés, F.; Basso, C.; Papke, D.K.M.; Pires, T.R.; Siqueira, I.R.; Picada, J.N.; Pereira, P. Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice. Life Sci. 2015, 122, 65–71. [Google Scholar] [CrossRef]
- Coelho, V.R.; Vieira, C.G.; de Souza, L.P.; da Silva, L.L.; Pflüger, P.; Regner, G.G.; Papke, D.K.M.; Picada, J.N.; Pereira, P. Behavioral and genotoxic evaluation of rosmarinic and caffeic acid in acute seizure models induced by pentylenetetrazole and pilocarpine in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective Effect of Caffeic Acid against Alzheimer’s Disease Pathogenesis via Modulating Cerebral Insulin Signaling, β-Amyloid Accumulation, and Synaptic Plasticity in Hyperinsulinemic Rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Alkis, H.E.; Kuzhan, A.; Dirier, A.; Tarakcioglu, M.; Demir, E.; Saricicek, E.; Demir, T.; Ahlatci, A.; Demirci, A.; Cinar, K. Neuroprotective effects of propolis and caffeic acid phenethyl ester (CAPE) on the radiation-injured brain tissue (Neuroprotective effects of propolis and CAPE). Int. J. Radiat. Res. 2015, 13, 297–303. [Google Scholar]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp. Ther. Med. 2015, 9, 1582–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.-F.; Kuo, Y.-H.; Yeh, W.-L.; Wu, C.Y.-J.; Lin, H.-Y.; Lai, S.-W.; Liu, Y.-S.; Wu, L.-H.; Lu, J.-K.; Lu, D.-Y. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int. J. Mol. Sci. 2015, 16, 5572–5589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginis, Z.; Ozturk, G.; Albayrak, A.; Kurt, S.N.; Albayrak, M.; Fadillioglu, E. Protective effects of caffeic acid phenethyl ester on ifosfamide-induced central neurotoxicity in rats. Toxicol. Ind. Health 2016, 32, 337–343. [Google Scholar] [CrossRef]
- Akyol, S.; Erdemli, H.K.; Armutcu, F.; Akyol, O. In vitro and in vivo neuroprotective effect of caffeic acid phenethyl ester. J. Intercult. Ethnopharmacol. 2015, 4, 192–193. [Google Scholar] [CrossRef]
- Dos Santos, N.A.G.; Martins, N.M.; Silva, R.d.B.; Ferreira, R.S.; Sisti, F.M.; dos Santos, A.C. Caffeic acid phenethyl ester (CAPE) protects PC12 cells from MPP+ toxicity by inducing the expression of neuron-typical proteins. Neurotoxicology 2014, 45, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Bak, J.; Kim, H.J.; Kim, S.Y.; Choi, Y.-S. Neuroprotective effect of caffeic acid phenethyl ester in 3-nitropropionic acid-induced striatal neurotoxicity. Korean J. Physiol. Pharmacol. 2016, 20, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Mikami, Y.; Yamazawa, T. Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci. 2015, 139, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-J.; Luo, T.; Wu, F.; Mei, Y.-W.; Peng, J.; Liu, H.; Li, H.-R.; Zhang, S.-L.; Dong, J.-H.; Fang, Y.; et al. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia. Life Sci. 2015, 127, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, H.; Li, H.; Tang, Y.; Yang, L.; Cao, S.; Qin, D. Antidepressant Potential of Chlorogenic Acid-Enriched Extract from Eucommia ulmoides Oliver Bark with Neuron Protection and Promotion of Serotonin Release through Enhancing Synapsin I Expression. Molecules 2016, 21, 260. [Google Scholar] [CrossRef] [Green Version]
- Gul, Z.; Demircan, C.; Bagdas, D.; Buyukuysal, R.L. Protective Effects of Chlorogenic Acid and its Metabolites on Hydrogen Peroxide-Induced Alterations in Rat Brain Slices: A Comparative Study with Resveratrol. Neurochem. Res. 2016, 41, 2075–2085. [Google Scholar] [CrossRef]
- Aseervatham, G.S.B.; Suryakala, U.; Doulethunisha; Sundaram, S.; Bose, P.C.; Sivasudha, T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed. Pharmacother. 2016, 82, 54–64. [Google Scholar] [CrossRef]
- Fang, S.-Q.; Wang, Y.-T.; Wei, J.-X.; Shu, Y.-H.; Xiao, L.; Lu, X.-M. Beneficial effects of chlorogenic acid on alcohol-induced damage in PC12 cells. Biomed. Pharmacother. 2016, 79, 254–262. [Google Scholar] [CrossRef]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80. [Google Scholar] [CrossRef]
- Chen, J.; Lin, D.; Zhang, C.; Li, G.; Zhang, N.; Ruan, L.; Yan, Q.; Li, J.; Yu, X.; Xie, X.; et al. Antidepressant-like effects of ferulic acid: Involvement of serotonergic and norepinergic systems. Metab. Brain Dis. 2015, 30, 129–136. [Google Scholar] [CrossRef]
- Lenzi, J.; Rodrigues, A.F.; Rós, A.d.S.; de Castro, A.B.; de Lima, D.D.; Magro, D.D.D.; Zeni, A.L.B. Ferulic acid chronic treatment exerts antidepressant-like effect: Role of antioxidant defense system. Metab. Brain Dis. 2015, 30, 1453–1463. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Haque, M.E. Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des. Devel. Ther. 2015, 9, 5499–5510. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Deng, H.-M.; Zhu, M.-M.; Xiao, F.; Yang, L.; Zhang, Z.-J.; Xiao, Y.; Nie, H. Inhibitory effect of ferulic acid on inflammatory response in microglia induced by lipopolysaccharides. Zool. Res. 2011, 32, 311–316. [Google Scholar] [CrossRef]
- Dong, G.-C.; Kuan, C.-Y.; Subramaniam, S.; Zhao, J.-Y.; Sivasubramaniam, S.; Chang, H.-Y.; Lin, F.-H. A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel. Mater. Sci. Eng. C 2015, 49, 691–699. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Hu, C.-Y.; Shen, J.-D.; Wu, S.-H.; Li, Y.-C.; Yi, L.-T. Elevation of synaptic protein is associated with the antidepressant-like effects of ferulic acid in a chronic model of depression. Physiol. Behav. 2017, 169, 184–188. [Google Scholar] [CrossRef]
- Huang, H.; Hong, Q.; Tan, H.-L.; Xiao, C.-R.; Gao, Y. Ferulic acid prevents LPS-induced up-regulation of PDE4B and stimulates the cAMP/CREB signaling pathway in PC12 cells. Acta Pharmacol. Sin. 2016, 37, 1543–1554. [Google Scholar] [CrossRef] [Green Version]
- Koh, P.-O. Ferulic acid attenuates the down-regulation of MEK/ERK/p90RSK signaling pathway in focal cerebral ischemic injury. Neurosci. Lett. 2015, 588, 18–23. [Google Scholar] [CrossRef]
- Lin, W.-C.; Peng, Y.-F.; Hou, C.-W. Ferulic acid protects PC12 neurons against hypoxia by inhibiting the p-MAPKs and COX-2 pathways. Iran. J. Basic Med. Sci. 2015, 18, 478–484. [Google Scholar]
- Kim, M.-J.; Seong, A.-R.; Yoo, J.-Y.; Jin, C.-H.; Lee, Y.-H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.-G. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808. [Google Scholar] [CrossRef]
- Mirshekar, M.A.; Sarkaki, A.; Farbood, Y.; Gharib Naseri, M.K.; Badavi, M.; Mansouri, M.T.; Haghparast, A. Neuroprotective effects of gallic acid in a rat model of traumatic brain injury: Behavioral, electrophysiological, and molecular studies. Iran. J. Basic Med. Sci. 2018, 21, 1056–1063. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Mattson, M.P.; Cheng, B.; Culwell, A.R.; Esch, F.S.; Lieberburg, I.; Rydel, R.E. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 1993, 10, 243–254. [Google Scholar] [CrossRef]
- Ishida, A.; Furukawa, K.; Keller, J.N.; Mattson, M.P. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport 1997, 8, 2133–2137. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Zhuo, Z.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; et al. Gallic acid disruption of Aβ1-42 aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2019, 124, 67–80. [Google Scholar] [CrossRef]
- Gerzson, M.F.B.; Bona, N.P.; Soares, M.S.P.; Teixeira, F.C.; Rahmeier, F.L.; Carvalho, F.B.; da Cruz Fernandes, M.; Onzi, G.; Lenz, G.; Gonçales, R.A.; et al. Tannic Acid Ameliorates STZ-Induced Alzheimer’s Disease-Like Impairment of Memory, Neuroinflammation, Neuronal Death and Modulates Akt Expression. Neurotox. Res. 2020, 37, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, B.; Mansouri, M.T. Protective Effects of Gallic Acid against Streptozotocin-induced Oxidative Damage in Rat Striatum. Drug Res. 2015, 65, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Oyagbemi, A.A.; Omobowale, T.O.; Saba, A.B.; Olowu, E.R.; Dada, R.O.; Akinrinde, A.S. Gallic Acid Ameliorates Cyclophosphamide-Induced Neurotoxicity in Wistar Rats Through Free Radical Scavenging Activity and Improvement in Antioxidant Defense System. J. Diet. Suppl. 2016, 13, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Mombeini, M.A.; Fatemi, I.; Aminzadeh, A.; Kalantari, H.; Nesari, A.; Najafzadehvarzi, H.; Mehrzadi, S. Neuroprotective effects of Ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol. Res. 2019, 41, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, B.; Adelakun, S.A.; Agie, J.A. Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug Chem. Toxicol. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Eramo, S.L.M.; Mancuso, C.; Troiani, D.; Paludetti, G. Rosmarinic acid up-regulates the noise-activated Nrf2/HO-1 pathway and protects against noise-induced injury in rat cochlea. Free Radic. Biol. Med. 2015, 85, 269–281. [Google Scholar] [CrossRef]
- Lee, A.Y.; Wu, T.T.; Hwang, B.R.; Lee, J.; Lee, M.-H.; Lee, S.; Cho, E.J. The Neuro-Protective Effect of the Methanolic Extract of Perilla frutescens var. japonicaand Rosmarinic Acid against H₂O₂-Induced Oxidative Stress in C6 Glial Cells. Biomol. Ther. 2016, 24, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mello Andrade, J.M.; Dos Santos Passos, C.; Kieling Rubio, M.A.; Mendonça, J.N.; Lopes, N.P.; Henriques, A.T. Combining in vitro and in silico approaches to evaluate the multifunctional profile of rosmarinic acid from Blechnum brasiliense on targets related to neurodegeneration. Chem. Biol. Interact. 2016, 254, 135–145. [Google Scholar] [CrossRef]
- Shang, A.-J.; Yang, Y.; Wang, H.-Y.; Tao, B.-Z.; Wang, J.; Wang, Z.-F.; Zhou, D.-B. Spinal cord injury effectively ameliorated by neuroprotective effects of rosmarinic acid. Nutr. Neurosci. 2017, 20, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Wang, D.-D.; Xu, Y.-X.; Wang, C.; Cao, L.; Liu, Y.-S.; Zhu, C.-Q. Aging as a Precipitating Factor in Chronic Restraint Stress-Induced Tau Aggregation Pathology, and the Protective Effects of Rosmarinic Acid. J. Alzheimers Dis. 2016, 49, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-S.; Kim, H.-B.; Choi, G.-Y.; Lee, S.; Lee, S.-O.; Kim, S.; Park, J.-H. Acute rosmarinic acid treatment enhances long-term potentiation, BDNF and GluR-2 protein expression, and cell survival rate against scopolamine challenge in rat organotypic hippocampal slice cultures. Biochem. Biophys. Res. Commun. 2016, 475, 44–50. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017, 86, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Rosińczuk, J.; Dymarek, R.; Całkosiński, I. Histopathological, ultrastructural, and immunohistochemical assessment of hippocampus structures of rats exposed to TCDD and high doses of tocopherol and acetylsalicylic acid. Biomed. Res. Int. 2015, 2015, 645603. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, H.C.; Taha, A.Y.; Rapoport, S.I.; Yuan, Z.-X. Low-dose aspirin (acetylsalicylate) prevents increases in brain PGE2, 15-epi-lipoxin A4 and 8-isoprostane concentrations in 9 month-old HIV-1 transgenic rats, a model for HIV-1 associated neurocognitive disorders. Prostaglandins Leukot. Essent. Fatty Acids 2015, 96, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, X.; Han, Z.; Wang, C.; Zhou, Q.; Lin, J. Statin and Aspirin Pretreatment Are Associated with Lower Neurological Deterioration and Platelet Activity in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Shamsara, A.; Sheibani, V.; Asadi-Shekaari, M.; Nematollahi-Mahani, S.N. Neural like cells and acetyl-salicylic acid alter rat brain structure and function following transient middle cerebral artery occlusion. Biomol. Concepts 2018, 9, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Roehrich, M.-E.; Wyss, J.-C.; Kumar, R.; Pascual, M.; Golshayan, D.; Vassalli, G. Additive effects of rapamycin and aspirin on dendritic cell allostimulatory capacity. Immunopharmacol. Immunotoxicol. 2015, 37, 434–441. [Google Scholar] [CrossRef]
- Xu, Y.X.; Du, F.; Jiang, L.R.; Gong, J.; Zhou, Y.-F.; Luo, Q.Q.; Qian, Z.M.; Ke, Y. Effects of aspirin on expression of iron transport and storage proteins in BV-2 microglial cells. Neurochem. Int. 2015, 91, 72–77. [Google Scholar] [CrossRef]
- Tüzmen, M.N.; Yücel, N.C.; Kalburcu, T.; Demiryas, N. Effects of curcumin and tannic acid on the aluminum- and lead-induced oxidative neurotoxicity and alterations in NMDA receptors. Toxicol. Mech. Methods 2015, 25, 120–127. [Google Scholar] [CrossRef]
- Ashafaq, M.; Tabassum, H.; Vishnoi, S.; Salman, M.; Raisuddin, S.; Parvez, S. Tannic acid alleviates lead acetate-induced neurochemical perturbations in rat brain. Neurosci. Lett. 2016, 617, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Sen, H.M.; Ozkan, A.; Guven, M.; Akman, T.; Aras, A.B.; Sehitoglu, I.; Alacam, H.; Silan, C.; Cosar, M.; Ozisik Karaman, H.I. Effects of tannic acid on the ischemic brain tissue of rats. Inflammation 2015, 38, 1624–1630. [Google Scholar] [CrossRef]
- Ashafaq, M.; Tabassum, H.; Parvez, S. Modulation of behavioral deficits and neurodegeneration by tannic acid in experimental stroke challenged wistar rats. Mol. Neurobiol. 2017, 54, 5941–5951. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Bao, Y.-M.; Jiang, B.; An, L.-J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Jiang, B.; Bao, Y.M.; An, L.J. Protocatechuic acid suppresses MPP+ -induced mitochondrial dysfunction and apoptotic cell death in PC12 cells. Food Chem. Toxicol. 2006, 44, 1659–1666. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Jiang, B.; Bao, Y.-M.; An, L.-J. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol. In Vitro 2008, 22, 430–437. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, G.; Szeto, S.S.W.; Chong, C.M.; Quan, Q.; Huang, C.; Cui, W.; Guo, B.; Wang, Y.; Han, Y.; et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015, 84, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Wang, H.; Wang, J.-H.; Wang, Q.; Ma, Q.-F.; Chen, Y.-Y. Protocatechuic Acid Inhibits Inflammatory Responses in LPS-Stimulated BV2 Microglia via NF-κB and MAPKs Signaling Pathways. Neurochem. Res. 2015, 40, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Sripetchwandee, J.; Sa-Nguanmoo, P.; Pintana, H.; Pannangpetch, P.; Chattipakorn, N.; Chattipakorn, S.C. Protocatechuic acid protects brain mitochondrial function in streptozotocin-induced diabetic rats. Appl. Physiol. Nutr. Metab. 2015, 40, 1078–1081. [Google Scholar] [CrossRef]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Liu, Y.; Wang, F.; Gao, Z.; Elhefny, M.A.; Habotta, O.A.; Abdel Moneim, A.E.; Kassab, R.B. Neuroprotective effects of protocatechuic acid on sodium arsenate induced toxicity in mice: Role of oxidative stress, inflammation, and apoptosis. Chem. Biol. Interact. 2021, 337, 109392. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.A.; Garcia-Parrilla, M.A.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Yuksel, Y.; Sehitoglu, M.H.; Tokmak, M.; Aras, A.B.; Akman, T.; Golge, U.H.; Goksel, F.; Karavelioglu, E.; Cosar, M. The Effect of Coumaric Acid on Ischemia-Reperfusion Injury of Sciatic Nerve in Rats. Inflammation 2015, 38, 2124–2132. [Google Scholar] [CrossRef]
- Guven, M.; Sehitoglu, M.H.; Yuksel, Y.; Tokmak, M.; Aras, A.B.; Akman, T.; Golge, U.H.; Karavelioglu, E.; Bal, E.; Cosar, M. The neuroprotective effect of coumaric acid on spinal cord ischemia/reperfusion injury in rats. Inflammation 2015, 38, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Aras, A.B.; Akman, T.; Sen, H.M.; Ozkan, A.; Salis, O.; Sehitoglu, I.; Kalkan, Y.; Silan, C.; Deniz, M.; et al. Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran. J. Basic Med. Sci. 2015, 18, 356–363. [Google Scholar]
- Sakamula, R.; Thong-Asa, W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis. 2018, 33, 765–773. [Google Scholar] [CrossRef]
- Daroi, P.A.; Dhage, S.N.; Juvekar, A.R. p-Coumaric acid mitigates lipopolysaccharide induced brain damage via alleviating oxidative stress, inflammation and apoptosis. J. Pharm. Pharmacol. 2021, 20, rgab077. [Google Scholar] [CrossRef]
- Oh, D.-R.; Kim, M.-J.; Choi, E.-J.; Kim, Y.; Lee, H.-S.; Bae, D.; Choi, C. Protective Effects of p-Coumaric Acid Isolated from Vaccinium bracteatum Thunb. Leaf Extract on Corticosterone-Induced Neurotoxicity in SH-SY5Y Cells and Primary Rat Cortical Neurons. Processes 2021, 9, 869. [Google Scholar] [CrossRef]
- Shin, D.-S.; Kim, K.W.; Chung, H.Y.; Yoon, S.; Moon, J.-O. Effect of sinapic acid against carbon tetrachloride-induced acute hepatic injury in rats. Arch. Pharm. Res. 2013, 36, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Kim, D.H.; Park, S.J.; Kim, J.M.; Lee, Y.W.; Jung, J.M.; Lee, C.H.; Hong, J.G.; Liu, X.; Cai, M.; et al. Neuroprotective effect of sinapic acid in a mouse model of amyloid β(1-42) protein-induced Alzheimer’s disease. Pharmacol. Biochem. Behav. 2012, 103, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zare, K.; Eidi, A.; Roghani, M.; Rohani, A.H. The neuroprotective potential of sinapic acid in the 6-hydroxydopamine-induced hemi-parkinsonian rat. Metab. Brain Dis. 2015, 30, 205–213. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Lee, S.-W.; Oh, M.-S.; Lee, H.J. Effects of sinapic Acid of 4 vessel occlusion model-induced ischemia and cognitive impairments in the rat. Clin. Psychopharmacol. Neurosci. 2011, 9, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Yoon, B.H.; Jung, W.Y.; Kim, J.M.; Park, S.J.; Park, D.H.; Huh, Y.; Park, C.; Cheong, J.H.; Lee, K.-T.; et al. Sinapic acid attenuates kainic acid-induced hippocampal neuronal damage in mice. Neuropharmacology 2010, 59, 20–30. [Google Scholar] [CrossRef]
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Farbood, Y.; Naghizadeh, B.; Shabani, S.; Mirshekar, M.A.; Sarkaki, A. Beneficial effects of ellagic acid against animal models of scopolamine- and diazepam-induced cognitive impairments. Pharm. Biol. 2016, 54, 1947–1953. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Li, S.-R.; Li, K.; Li, X.; Yin, X.; Pang, Z. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/β-catenin signaling in vivo and in vitro. Mol. Nutr. Food Res. 2017, 61, 1600587. [Google Scholar] [CrossRef]
- Farbood, Y.; Sarkaki, A.; Dolatshahi, M.; Taqhi Mansouri, S.M.; Khodadadi, A. Ellagic Acid Protects the Brain Against 6-Hydroxydopamine Induced Neuroinflammation in a Rat Model of Parkinson’s Disease. Basic Clin. Neurosci. 2015, 6, 83–89. [Google Scholar]
- Chen, S.-Y.; Zheng, K.; Wang, Z. Neuroprotective effects of Ellagic acid on Neonatal Hypoxic Brain Injury via Inhibition of Inflammatory Mediators and Down-regulation of JNK/p38 MAPK Activation. Trop. J. Pharm. Res. 2016, 15, 241. [Google Scholar] [CrossRef] [Green Version]
- Thrash-Williams, B.; Karuppagounder, S.S.; Bhattacharya, D.; Ahuja, M.; Suppiramaniam, V.; Dhanasekaran, M. Methamphetamine-induced dopaminergic toxicity prevented owing to the neuroprotective effects of salicylic acid. Life Sci. 2016, 154, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Cetin, D.; Hacımuftuoglu, A.; Tatar, A.; Turkez, H.; Togar, B. The in vitro protective effect of salicylic acid against paclitaxel and cisplatin-induced neurotoxicity. Cytotechnology 2016, 68, 1361–1367. [Google Scholar] [CrossRef]
- Lambert, G.W.; Eisenhofer, G.; Jennings, G.L.; Esler, M.D. Regional homovanillic acid production in humans. Life Sci. 1993, 53, 63–75. [Google Scholar] [CrossRef]
- Ibero-Baraibar, I.; Perez-Cornago, A.; Ramirez, M.J.; Martínez, J.A.; Zulet, M.A. An Increase in Plasma Homovanillic Acid with Cocoa Extract Consumption Is Associated with the Alleviation of Depressive Symptoms in Overweight or Obese Adults on an Energy Restricted Diet in a Randomized Controlled Trial. J. Nutr. 2015, 146, 897S–904S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Kerkhof, N.W.A.; Fekkes, D.; van der Heijden, F.M.M.A.; Egger, J.I.M.; Verhoeven, W.M.A. Relationship between plasma homovanillic acid and outcome in patients with psychosis spectrum disorders. Neuropsychobiology 2015, 71, 212–217. [Google Scholar] [CrossRef]
- Neider, D.; Lindström, L.H.; Bodén, R. Risk factors for suicide among patients with schizophrenia: A cohort study focused on cerebrospinal fluid levels of homovanillic acid and 5-hydroxyindoleacetic acid. Neuropsychiatr. Dis. Treat. 2016, 12, 1711–1714. [Google Scholar] [CrossRef] [Green Version]
- Rob, M.M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Activity and Identification of Phytotoxic Substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Güven, M.; Aras, A.B.; Topaloğlu, N.; Özkan, A.; Şen, H.M.; Kalkan, Y.; Okuyucu, A.; Akbal, A.; Gökmen, F.; Coşar, M. The protective effect of syringic acid on ischemia injury in rat brain. Turk. J. Med. Sci. 2015, 45, 233–240. [Google Scholar] [CrossRef]
- Tokmak, M.; Yuksel, Y.; Sehitoglu, M.H.; Guven, M.; Akman, T.; Aras, A.B.; Cosar, M.; Abbed, K.M. The neuroprotective effect of syringic acid on spinal cord ischemia/reperfusion injury in rats. Inflammation 2015, 38, 1969–1978. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, L.; Sun, S.; Yi, Z.; Jiang, X.; Jia, D. Neuroprotective effects of syringic acid against OGD/R-induced injury in cultured hippocampal neuronal cells. Int. J. Mol. Med. 2016, 38, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Rashedinia, M.; Alimohammadi, M.; Shalfroushan, N.; Khoshnoud, M.J.; Mansourian, M.; Azarpira, N.; Sabahi, Z. Neuroprotective effect of syringic acid by modulation of oxidative stress and mitochondrial mass in diabetic rats. BioMed Res. Int. 2020, 2020, 8297984. [Google Scholar] [CrossRef]
- Ogut, E.; Akcay, G.; Yildirim, F.B.; Derin, N.; Aslan, M. The influence of syringic acid treatment on total dopamine levels of the hippocampus and on cognitive behavioral skills. Int. J. Neurosci. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dang, M.; Zhang, W.; Lei, Y.; Ramesh, T.; Priya Veeraraghavan, V.; Hou, X. Neuroprotective effects of Syringic acid against aluminium chloride induced oxidative stress mediated neuroinflammation in rat model of Alzheimer’s disease. J. Funct. Foods 2020, 71, 104009. [Google Scholar] [CrossRef]
- Song, M.; Du, Z.; Lu, G.; Li, P.; Wang, L. Syringic acid protects retinal ganglion cells against H2O2-induced apoptosis through the activation of PI3K/Akt signaling pathway. Cell. Mol. Biol. 2016, 62, 50–54. [Google Scholar] [PubMed]
- Vargas-Tah, A.; Gosset, G. Production of Cinnamic and p-Hydroxycinnamic Acids in Engineered Microbes. Front. Bioeng. Biotechnol. 2015, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Bae, W.-Y.; Choi, J.-S.; Jeong, J.-W. The neuroprotective effects of cinnamic aldehyde in an MPTP mouse model of parkinson’s disease. Int. J. Mol. Sci. 2018, 19, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Zhang, X.; Dong, L.; Wen, Y.; Zheng, X.; Zhang, C.; Chen, R.; Zhang, Y.; Li, Y.; He, T.; et al. Cinnamaldehyde inhibits inflammation and brain damage in a mouse model of permanent cerebral ischaemia. Br. J. Pharmacol. 2015, 172, 5009–5023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gürer, B.; Kertmen, H.; Kuru Bektaşoğlu, P.; Öztürk, Ö.Ç.; Bozkurt, H.; Karakoç, A.; Arıkök, A.T.; Çelikoğlu, E. The effects of Cinnamaldehyde on early brain injury and cerebral vasospasm following experimental subarachnoid hemorrhage in rabbits. Metab. Brain Dis. 2019, 34, 1737–1746. [Google Scholar] [CrossRef]
- Emamghoreishi, M.; Farrokhi, M.R.; Amiri, A.; Keshavarz, M. The neuroprotective mechanism of cinnamaldehyde against amyloid-β in neuronal SHSY5Y cell line: The role of N-methyl-D-aspartate, ryanodine, and adenosine receptors and glycogen synthase kinase-3β. Avicenna J. Phytomed. 2019, 9, 271–280. [Google Scholar]
- Rashidi, R.; Moallem, S.A.; Moshiri, M.; Hadizadeh, F.; Etemad, L. Protective Effect of Cinnamaldehyde on METH-induced Neurotoxicity in PC12 Cells via Inhibition of Apoptotic Response and Oxidative Stress. Iran. J. Pharm. Res. 2021, 20, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; He, X.; Zhou, X.; Li, X.; Rong, B.; Wang, F. Combination of ellagic acid and trans-cinnamaldehyde alleviates aging-induced cognitive impairment via modulation of mitochondrial function and inflammatory and apoptotic mediators in the prefrontal cortex of aged rats. Chin. J. Physiol. 2020, 63, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Kuru Bektaşoğlu, P.; Koyuncuoğlu, T.; Demir, D.; Sucu, G.; Akakın, D.; Peker Eyüboğlu, İ.; Yüksel, M.; Çelikoğlu, E.; Yeğen, B.Ç.; Gürer, B. Neuroprotective effect of cinnamaldehyde on secondary brain injury after traumatic brain injury in a rat model. World Neurosurg. 2021, 153, e392–e402. [Google Scholar] [CrossRef] [PubMed]
- Etaee, F.; Komaki, A.; Faraji, N.; Rezvani-Kamran, A.; Komaki, S.; Hasanein, P.; Taheri, M.; Omidi, G. The effects of cinnamaldehyde on acute or chronic stress-induced anxiety-related behavior and locomotion in male mice. Stress 2019, 22, 358–365. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Musso, N.; Grasso, M.; Costantino, A.; Lazzarino, G.; Tascedda, F.; Gulisano, M.; Lunte, S.M.; Caraci, F. Microfluidics as a novel tool for biological and toxicological assays in drug discovery processes: Focus on microchip electrophoresis. Micromachines 2020, 11, 593. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
| Phenolic Acids | Basic Description | Disease Models | Mode of Action | Ref. | Chemical Structure |
|---|---|---|---|---|---|
| Caffeic acid | Organic compound classified as a hydroxycinnamic acid. It consists of both phenolic and acrylic functional groups. Since it represents an intermediate in the biosynthesis of lignin (one of the principal components of woody plant biomass and its residues), caffeic acid can be found in all plants. | Mice treated with LPS |
| [18] | 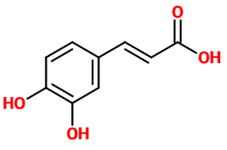 |
| Human α-syn aggregation |
| [21] | |||
| Mouse model of epilepsy |
| [22] | |||
| Mouse model of acute seizure (diazepam and aspilocarpine-induced) |
| [23] | |||
| Rat model of hyperinsulinemia |
| [24] | |||
| Caffeic acid phenethyl ester | Ester of caffeic acid and phenethyl alcohol. | Rats exposed to ionizing radiation |
| [25] |  |
| Rats treated with IFOS |
| [28,29] | |||
| Mouse model of HD (3-nitropropionic acid-induced) |
| [31] | |||
| BV-2 cells treated with LPS |
| [27] | |||
| PC12 cells treated with (MPP+) |
| [30] | |||
| Rat cerebellar granule neurons treated with SNP or glutamate/glycine or H2O2 |
| [38] | |||
| Chlorogenic acid | Ester of caffeic acid and (−)-quinic acid. It belongs to the polyphenol family of esters, including hydroxycinnamic acids (caffeic acid, ferulic acid and p-coumaric acid) with quinic acid. | Cortical mouse neurons treated with L-glutamic acid |
| [32] | 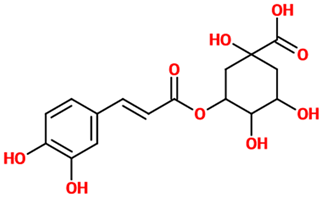 |
| Microglia infected with herpes simplex virus |
| [33] | |||
| Rats treated with H2O2 |
| [35] | |||
| Mouse model of epilepsy (pilocarpine-induced) |
| [36] | |||
| PC12 cells treated with ethanol |
| [37] | |||
| Rat cerebellar granule neurons treated with SNP |
| [38] | |||
| Ferulic acid | Natural phenylpropanoid found in Euphorbia hylonoma herbs. It is a substituted derivative of trans-cinnamic acid. | N/A (untreated mice) |
| [39] | 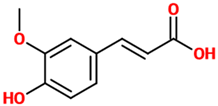 |
| N/A (untreated mice) |
| [40] | |||
| Rat model of PD (ROT-induced) |
| [41] | |||
| Microglial cells treated with LPS |
| [42] | |||
| Neuro-2a cells treated with H2O2 |
| [43] | |||
| Mouse model of chronic unpredictable mild stress |
| [44] | |||
| PC12 cells treated with LPS |
| [45] | |||
| Rat model of focal cerebral ischemic injury |
| [46] | |||
| Hypoxia-stressed PC12 cells |
| [47] | |||
| Gallic acid | Phenolic acid classified as trihydroxybenzoic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and in several other plants. | Mice, Neuro-2A, and primary microglial cells treated with Aβ |
| [48] |  |
| Rat model of TBI |
| [49] | |||
| Transgenic mice model of AD (APP/PS1) |
| [50] | |||
| Transgenic mice model of AD (APP/PS1) |
| [53] | |||
| Rat model of TBI |
| [54] | |||
| Mouse model of diabetes (STZ-induced) |
| [55] | |||
| Rats treated with cyclophosphamide |
| [56] | |||
| Rats treated with sodium arsenite |
| [57] | |||
| Rat model of AD (AlCl3-induced) |
| [58] | |||
| Rosmarinic acid | A polyphenol constituent of many culinary herbs such as rosemary, mint, and basil. From the chemical point of view, it represents a caffeic acid ester, with tyrosine giving another phenolic ring via dihydroxyphenyl-lactic acid. | Kindling mouse model (PTZ-induced) |
| [22] |  |
| Kindling mouse model (PTZ and pilocarpine-induced) |
| [23] | |||
| Rats exposed to noise |
| [59] | |||
| C6 glial cells treated with H2O2 |
| [60,61] | |||
| Rat model of SCI |
| [62] | |||
| Mouse model of a chronic restraint stress |
| [63] | |||
| Rat organotypic hippocampal slice cultures treated with scopolamine |
| [64] | |||
| Rat model of neuropathic pain |
| [65] | |||
| Acetylsalicylic acid | A weakly acidic substance widely used as a medication to reduce pain, fever, as well as inflammatory processes. Chemically, it represents an acetyl derivative of salicylic acid. | Rats treated with tetrachlorodibenzo-p-dioxin |
| [66] |  |
| HIV-1 transgenic rat |
| [67] | |||
| Rat model of ischemia |
| [69] | |||
| Mouse bone marrow-derived immature dendritic cells treated with LPS |
| [70] | |||
| BV-2 cells treated with LPS |
| [71] | |||
| Tannic acid | A specific form of tannins, a class of astringent, polyphenolic biomolecules, characterized by a very efficient metal chelating activity. | N/A (untreated rats) |
| [72] | 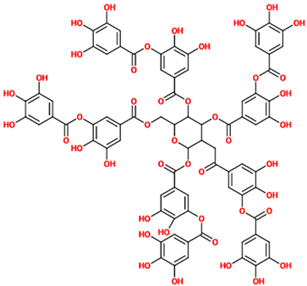 |
| Rats treated with lead acetate |
| [73] | |||
| Rat model of ischemia/reperfusion injury |
| [74] | |||
| Rat model of MCAO |
| [75] | |||
| Protocatechuic acid | A dihydroxybenzoic acid representing a major metabolite of antioxidant polyphenols found in green tea. It also possesses anti-inflammatory properties. | PC12 cells treated with H2O2 |
| [76] |  |
| PC12 cells treated with MPP+ |
| [77] | |||
| PC12 cells treated ROT |
| [78] | |||
| Zebrafish, mice, and PC12 treated with 6-OHDA |
| [79] | |||
| BV2 cells treated with LPS |
| [80] | |||
| Rat model of diabetes (STZ-induced) |
| [81] | |||
| Cerebellar granule neurons treated with H2O2 and BV2 cells treated with LPS |
| [82] | |||
| Mice treated with sodium arsenate |
| [83] | |||
| PC12 cells treated with Aβ and α-Syn |
| [84] | |||
| p-coumaric acid | A hydroxycinnamic acid representing the hydroxy derivative of cinnamic acid. Among the three isomers of coumaric acid (o-, m-, and p-coumaric acid), p-coumaric acid represents the most abundant isomer that can found in nature. | Rat model of SNI |
| [86] | 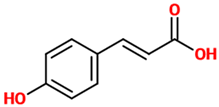 |
| Rat model of SCI |
| [87] | |||
| Rat model of embolic cerebral ischemia |
| [88] | |||
| Rat model of ischemia/reperfusion injury |
| [89] | |||
| Mice treated with LPS |
| [90] | |||
| SH-SY5Y cells and primary rat cortical neurons treated with corticosterone |
| [91] | |||
| Sinapic acid | A small naturally occurring hydroxycinnamic acid belonging to the phenylpropanoid family. Due to its well-known ability to absorb laser radiation and donate protons to the analyte of interest, it is frequently used as a matrix in MALDI mass spectrometry experiments. | Mouse model of AD (Aβ-induced) |
| [93] | 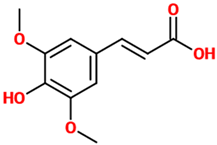 |
| Rat model of early PD (6-OHDA-induced) |
| [94] | |||
| Rat model of global cerebral ischemia |
| [95] | |||
| Mice treated with kainic acid |
| [96] | |||
| Ellagic acid | An organic heterotetracyclic compound found in different fruits and vegetables. From the chemical point of view, it represents the dilactone of hexahydroxydiphenic acid. | Rat model of sporadic AD (STZ-induced) |
| [97] |  |
| Rat models of scopolamine- and diazepam-induced cognitive impairments |
| [98] | |||
| Rat model of nerve injury (photothrombosis-induced) and OGD/R model in neural stem cells |
| [99] | |||
| Rat model of PD (6-OHDA-induced) |
| [100] | |||
| Rat model of neonatal hypoxic-ischemic brain injury |
| [101] | |||
| Salicylic acid | A plant hormone representing a precursor to and a metabolite of acetylsalicylic acid (commonly known as aspirin). | Mice treated with METH |
| [102] | 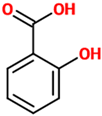 |
| Primary cortex neurons treated with paclitaxel and cisplatin |
| [103] | |||
| Syringic acid | A dimethoxybenzene that is 3,5-dimethyl ether derivative of gallic acid having a role as a plant metabolite. It can be found in several plants such as Ardisia elliptica. | Rat model of brain ischemia injury |
| [109] | 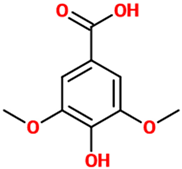 |
| Rat model of SCI |
| [110] | |||
| Hippocampal neurons subjected to OGD/R |
| [111] | |||
| Rat model of diabetes (STZ-induced) |
| [112] | |||
| Rats treated with deltamethrin |
| [113] | |||
| Rat model of AD (AlCl3-induced) |
| [114] | |||
| Retinal ganglion cells treated with H2O2 |
| [115] | |||
| Cinnamic aldehyde | A phenylpropanoid synthesized by the shikimate pathway giving to cinnamon its characteristic flavor and odor. It can be found in the bark of cinnamon trees as well as other species of the genus Cinnamomum. | Mouse model of PD (MPTP-induced) and BE(2)-M17 cells treated with MPTP |
| [117] |  |
| Mouse model of permanent cerebral ischemia |
| [118] | |||
| Rabbit model of early brain injury (subarachnoid hemorrhage-induced) |
| [119] | |||
| SH-SY5Y cells treated with Aβ |
| [120] | |||
| PC-12 cells treated with METH |
| [121] | |||
| Aged rats treated with METH |
| [122] | |||
| Rat model TBI |
| [123] | |||
| Mice subjected to acute or chronic stress |
| [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. https://doi.org/10.3390/nu14040819
Caruso G, Godos J, Privitera A, Lanza G, Castellano S, Chillemi A, Bruni O, Ferri R, Caraci F, Grosso G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients. 2022; 14(4):819. https://doi.org/10.3390/nu14040819
Chicago/Turabian StyleCaruso, Giuseppe, Justyna Godos, Anna Privitera, Giuseppe Lanza, Sabrina Castellano, Alessio Chillemi, Oliviero Bruni, Raffaele Ferri, Filippo Caraci, and Giuseppe Grosso. 2022. "Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease" Nutrients 14, no. 4: 819. https://doi.org/10.3390/nu14040819
APA StyleCaruso, G., Godos, J., Privitera, A., Lanza, G., Castellano, S., Chillemi, A., Bruni, O., Ferri, R., Caraci, F., & Grosso, G. (2022). Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients, 14(4), 819. https://doi.org/10.3390/nu14040819











