Beneficial Effects of Saw Palmetto Fruit Extract on Urinary Symptoms in Japanese Female Subjects by a Multicenter, Randomized, Double-Blind, Placebo-Controlled Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Selection Inclusion Criteria
- The ability to provide written consent prior to participation in the study;
- Women with frequent urination, nocturia, and/or urgency for at least 2 months;
- Urinary symptoms that do not require medical treatment based on definitions by physicians;
- The ability to take the test product for the purpose of research during the study period;
- The ability to take daily notes during the study period.
2.3. Exclusion Criteria
- Received treatments for urinary disorders within the past 2 months;
- Unable to have the desire to urinate;
- Have dysuria as the main symptom;
- Unable to communicate;
- Have a lifestyle-related disease;
- Currently enrolled in/will be enrolled in other studies;
- Receiving medications, newly designated quasi-drugs, Kampo medicine, health food products, and/or supplements for urinary disorders;
- Any other conditions considered to be inappropriate by the principal investigator.
2.4. Randomization and Blinding
2.5. Study Design
2.6. Intervention
2.7. Evaluations
2.8. Primary Outcomes
2.9. Secondary Outcomes
2.10. Safety
2.11. Statistical Analysis
3. Results
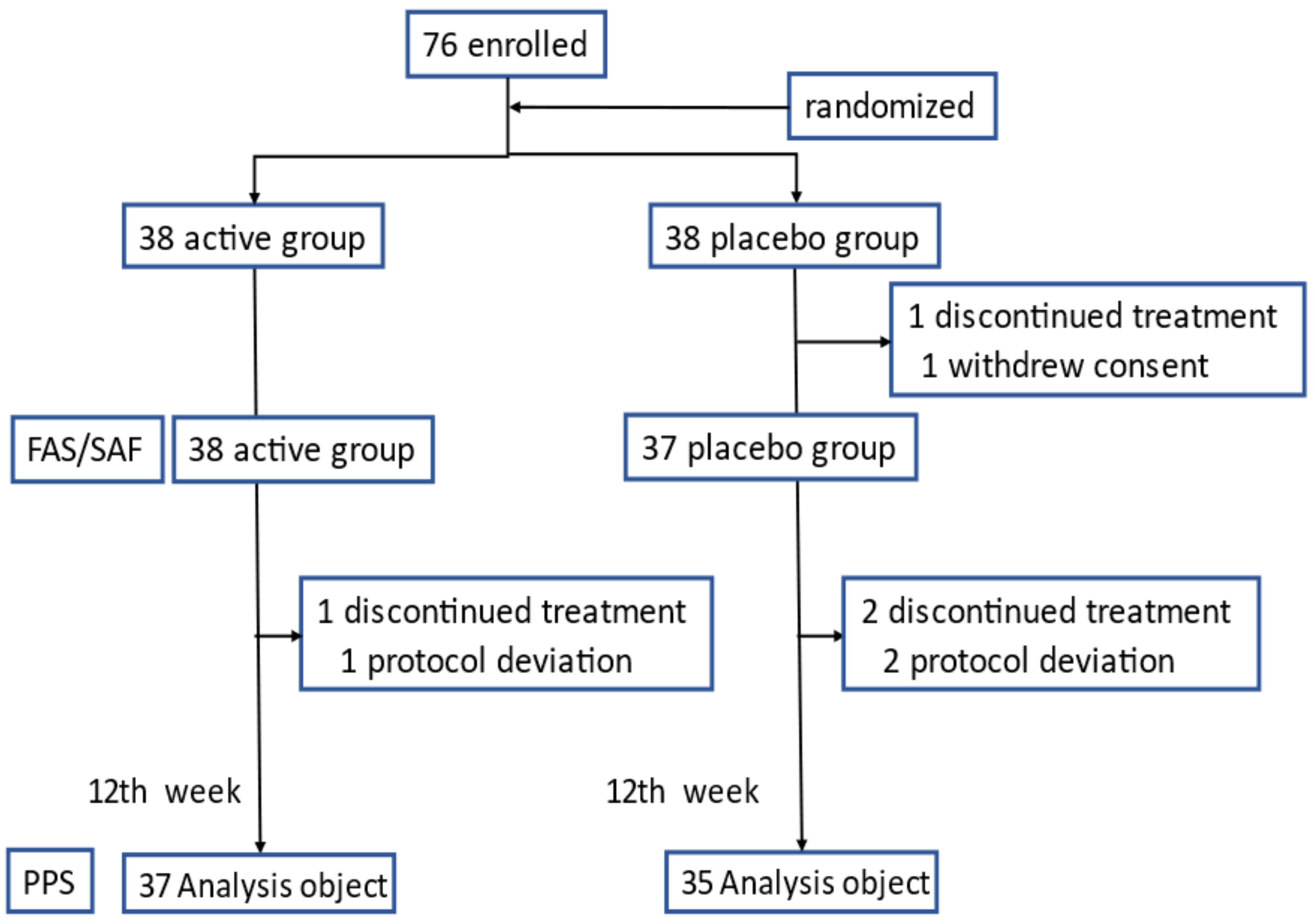
3.1. Study Subjects
3.2. Primary Efficacy Outcomes
3.3. Secondary Efficacy Outcomes
3.4. Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Cazzaniga, W.; Pederzoil, F.; Moretti, D.; Deho, F.; Montanari, E.; Montorsi, F.; Salonia, A. Clinically meaningful improvements in LUTS/BPH severity in men treated with silodosin plus hexanic extract of serenoa repens or silodosin alone. Sci. Rep. 2017, 7, 15179. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.L.; Pak, S.C. Serenoa repens for lower urinary tract symptoms/benign prostatic hyperplasia: Current evidence and its clinical implications in naturopathic medicine. J. Altern. Complement. Med. 2017, 23, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Alagiakrishnan, K.; Wiens, C.A. An approach to drugs induced delirium in the elderly. Postgrad. Med. J. 2004, 80, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Mate, K.E.; Kerr, K.P.; Pond, D.; Williams, E.J.; Marley, J.; Disler, P.; Brodaty, H.; Magin, P.J. Impact of multiple low-level anticholinergic medications on anticholinergic load of community-dwelling elderly with and without dementia. Drugs Aging 2015, 32, 159–167. [Google Scholar] [CrossRef]
- Yamada, S.; Ito, Y.; Nishijima, S.; Kadekawa, K.; Sugaya, K. Basic and clinical aspects of antimuscarinic agents used to treat overactive bladder. Pharmacol. Therap. 2018, 189, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.M.; Powell, G.E.; McFann, K.; Nahin, R.L. Complementary and alternative medicine use among adults: United States, 2002. Adv. Data 2004, 343, 1–20. [Google Scholar] [CrossRef]
- Debruyne, F.; Boyle, P.; Da Silva, F.C.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.-P.; Schulman, C.C. Evaluation of the clinical benefit of permixon and tamsulosin in severe BPH patients-PERMAL study subset analysis. Eur. Urol. 2004, 45, 773–780. [Google Scholar] [CrossRef]
- Avins, A.L.; Bent, S.; Staccone, S.; Badua, E.; Padula, A.; Goldberg, H.; Neuhaus, J.; Hudes, E.; Shinohara, K.; Kane, C.A. Detailed safety assessment of a saw palmetto extract. Complement. Therap. Med. 2008, 16, 147–154. [Google Scholar] [CrossRef][Green Version]
- Suzuki, M.; Ito, Y.; Fujino, T.; Abe, M.; Umegaki, K.; Onoue, S.; Noguchi, H.; Yamada, S. Pharmacological effects of saw palmetto extract in the lower urinary tract. Acta Pharmacol. Sin. 2009, 30, 271–281. [Google Scholar] [CrossRef]
- Pagano, E.; Laudato, M.; Griffo, M.; Capasso, R. Phytotherapy of benign prostatic hyperplasia. A minireview. Phytotherap. Res. 2014, 28, 949–955. [Google Scholar] [CrossRef]
- Vela-Navarrete, R.; Alcaraz, A.; Rodríguez-Antolín, A.; López, B.M.; Fernández-Gómez, J.M.; Angulo, J.C.; Díaz, D.C.; Romero-Otero, J.; Brenes, F.J.; Carballido, J.; et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018, 122, 1049–1065. [Google Scholar] [PubMed]
- Cai, T.; Cui, Y.; Yu, S.; Li, Q.; Zhou, Z.; Gao, Z. Comparison of serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia: A systematic review and meta-analysis. Am. J. Mens Health 2020, 14, 15557988320905407. [Google Scholar] [CrossRef] [PubMed]
- Iehle, C.; Delos, S.; Guirou, O.; Tate, R.; Raynaud, J.P.; Martin, P.M. Human prostatic steroid 5 alpha-reductase isoforms-a comparative study of selective inhibitors. J. Steroid Biochem. Mol. Biol. 1995, 54, 273–279. [Google Scholar] [CrossRef]
- Sultan, C.; Terraza, A.; Devillier, C.; Carilla, E.; Briley, M.; Loire, C.; Descomps, B. Inhibition of androgen metabolism and binding by a liposterolic extract of “Serenoa repens B” in human foreskin fibroblasts. J. Steroid Biochem. 1984, 20, 515–519. [Google Scholar] [CrossRef]
- Paubert-Braquet, M.; Cousse, H.; Raynaud, J.P.; Mencia-Huerta, J.M.; Braquet, P. Effect of the lipidosterolic extract of Serenoa repens (Permixon) and its major components on basic fibroblast growth factor-induced proliferation of cultures of human prostate biopsies. Eur. Urol. 1998, 33, 340–347. [Google Scholar] [CrossRef]
- Goepel, M.; Hecker, U.; Krege, S.; Rubben, H.; Michel, M.C. Saw palmetto extracts potently and noncompetitively inhibit human α1-adrenoceptors in vitro. Prostate 1999, 38, 208–215. [Google Scholar] [CrossRef]
- Koch, E. Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): Viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms. Planta Med. 2001, 67, 489–500. [Google Scholar] [CrossRef]
- Gutierrez, M.; García de Boto, M.J.; Cantabrana, B.; Hidalgo, A. Mechanisms involved in the spasmolytic effect of extracts from Sabal serrulata fruit on smooth muscle. Gen. Pharmacol. 1996, 27, 171–176. [Google Scholar] [CrossRef]
- Oki, T.; Suzuki, M.; Nishioka, Y.; Yasuda, A.; Umegaki, K.; Yamada, S. Effects of saw palmetto extract on micturition reflex of rats and its autonomic receptor binding activity. J. Urol. 2005, 173, 1395–1399. [Google Scholar] [CrossRef]
- Suzuki, M.; Oki, T.; Sugiyama, T.; Umegaki, K.; Uchida, S.; Yamada, S. Muscarinic and alpha 1-adrenergic receptor binding characteristics of saw palmetto extract in rat lower urinary tract. Urology 2007, 69, 1216–1220. [Google Scholar] [CrossRef]
- Nasrin, S.; Masuda, E.; Kugaya, H.; Osano, A.; Ito, Y.; Yamada, S. Effects of saw palmetto extract on urodynamic parameters, bladder muscarinic and purinergic receptors and urinary cytokines in rats with cyclophosphamide-induced cystitis. Low. Urin. Tract Symptoms 2014, 6, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Ito, Y.; Morikawa, K.; Kagota, S.; Shinozuka, K. Effect of saw palmetto extract on urodynamic parameters, bladder contractility and pharmacological receptors in female rats. Acad. J. Med. Plants 2020, 8, 171–178. [Google Scholar]
- Yamada, S.; Kato, Y. Effects of saw palmetto extract on the vanilloid receptor TRPV1. Low. Urin. Tract Symptoms 2022, 14, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Yoshida, M.; Yamanashi, T.; Gotoh, M. Core lower urinary tract symptom score (CLSS) questionnaire: A reliable tool in the overall assessment of lower urinary tract symptoms. Int. J. Urol. 2008, 15, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.; Tan, Z.; Peng, J.; Guo, Y.; Chen, Y.; Zhou, H.; Ouyang, D. The pharmacogenetics of natural products: A pharmacokinetic and pharmacodynamic perspective. Pharmacol. Res. 2019, 146, 104283. [Google Scholar] [CrossRef]
- Taguchi, M.; Inoue, T.; Muguruma, K.; Murota, T.; Kinoshita, H.; Matsuda, T. Impact of loop-tail urethral stents on ureteral stent-related symptoms immediately after ureteroscopic lithotripsy: Comparison with pigtail ureteral stents. Investig. Clin. Urol. 2017, 58, 440–446. [Google Scholar] [CrossRef]
- Fujimura, T.; Kume, H.; Tsurumaki, Y.; Yoshimura, Y.; Hosoda, C.; Suzuki, M.; Fukuhara, H.; Enomoto, Y.; Nishimatsu, H.; Homma, Y. Core lower urinary tract symptom score (CLSS) for the assessment of female lower urinary tract symptoms: A comparative study. Int. J. Urol. 2011, 18, 778–784. [Google Scholar] [CrossRef]
- Fujimura, T.; Kume, H.; Nishimatsu, H.; Sugihara, T.; Nomiya, A.; Tsurumaki, Y.; Miyazaki, H.; Suzuki, M.; Fukuhara, H.; Enomoto, Y.; et al. Assessment of lower urinary tract symptoms in men by international prostate symptom score and core lower urinary tract symptom score. BJU Int. 2012, 109, 1512–1517. [Google Scholar] [CrossRef]
- Birder, L.A.; Nakamura, Y.; Kiss, S.; Nealen, M.L.; Barrick, S.; Kanai, A.J.; Wang, E.; Ruiz, G.; Caterina, M.J. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat. Neurosci. 2002, 5, 856–860. [Google Scholar] [CrossRef]
- Fong, Y.K.; Milani, S.; Djavan, B. Role of phytotherapy in men with lower urinary tract symptoms. Curr. Opin. Urol. 2005, 15, 45–48. [Google Scholar] [CrossRef]
- Bent, S.; Kane, C.; Shinohara, K.; Neuhaus, J.; Hudes, E.S.; Goldberg, H.; Avins, A.L. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006, 354, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Scandura, C.; Di Mauro, M.; Cacciamani, G.; Albersen, M.; Hatzichristodoulou, G.; Fode, M.; Capogrosso, P.; Cimino, S.; Marcelissen, T.; et al. Clinical efficacy of Serenoa repens versus placebo versus alpha-blockers for the treatment of lower urinary tract symptoms/benign prostatic enlargement: A systematic review and network meta-analysis of randomized placebo-controlled clinical trials. Eur. Urol. Focus 2021, 7, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.K.; Wyllie, M.G. Not all brands are created equal: A comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer Prostatic Dis. 2004, 7, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Penugonda, K.; Lindshield, B.L. Fatty acid and phytosterol content of commercial saw palmetto supplements. Nutrients 2013, 5, 3617–3633. [Google Scholar] [CrossRef]
- Admii, R.E.; Shukri, A.S.A. Extract of Serenoa repens in the treatment of benign prostatic hyperplasia and lower urinary tract symptoms. Urologiia 2018, 2, 114–120. [Google Scholar]
- Kwon, Y. Use of saw palmetto (Serenoa repens) extract for benign prostatic hyperplasia. Food Sci. Biotechnol. 2019, 28, 1599–1606. [Google Scholar] [CrossRef]
- Abe, M.; Ito, Y.; Oyunzul, L.; Oki-Fujino, T.; Yamada, S. Pharmacologically relevant receptor binding characteristics and 5a-reductase inhibitory activity of free fatty acids contained in saw palmetto extract. Biol. Pharm. Bull. 2009, 32, 646–650. [Google Scholar] [CrossRef]
- Abe, M.; Ito, Y.; Suzuki, A.; Onoue, S.; Noguchi, H.; Yamada, S. Isolation and pharmacological characterization of fatty acids from saw palmetto extract. Anal. Sci. 2009, 25, 553–557. [Google Scholar] [CrossRef]
- Kushima, M.; Okamoto, K.; Kizawa, Y.; Sekikawa, T.; Yanmei, L.; Takara, T. A verification study on the improvement of urination issues with ingestion of saw palmetto fruit extract: A randomized, double-blind, parallel-group, placebo-controlled study. Pharmacometrics 2018, 95, 101–111. [Google Scholar]
- Sekikawa, T.; Kizawa, Y.; Li, Y.; Miura, N. A clinical study for evaluating the safety of excessive consumption of saw palmetto extract. Jpn. Pharmacol. Ther. 2019, 47, 445–452. [Google Scholar]
- Avins, A.L.; Lee, J.Y.; Meyers, C.M.; Barry, M.J.; For the Complementary and Alternative Medicine for Urologic Symptoms (CAMUS) Study Group. Safety and toxicity of saw palmetto in the complementary and alternative medicine for urological symptoms (CAMUS) Trial. J. Urol. 2013, 189, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
| Variable | Active Group (n = 38) | Placebo Group (n = 37) | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 69.3 | 7.0 | 69.6 | 6.4 | 0.84 |
| Blood pressure (mmHg) | |||||
| Systolic | 135.4 | 21.0 | 134.7 | 21.8 | 0.88 |
| Diastolic | 74.7 | 13.4 | 71.5 | 12.1 | 0.29 |
| CLSS: | |||||
| Daytime frequency | 1.1 | 0.8 | 1.0 | 0.7 | 0.47 |
| Nocturia | 1.2 | 0.6 | 1.0 | 0.7 | 0.30 |
| Urgency | 0.4 | 0.6 | 0.5 | 0.6 | 0.76 |
| Urgency incontinence | 0.3 | 0.6 | 0.2 | 0.4 | 0.54 |
| Stress incontinence | 0.8 | 0.7 | 0.7 | 0.6 | 0.56 |
| Slow stream | 0.6 | 0.7 | 0.6 | 0.8 | 0.58 |
| Straining | 0.1 | 0.4 | 0.4 | 0.7 | 0.11 |
| Incomplete emptying | 0.2 | 0.5 | 0.2 | 0.5 | 0.96 |
| Bladder pain | 0.0 | 0.0 | 0.1 | 0.4 | 0.18 |
| Urethral pain | 0.0 | 0.0 | 0.1 | 0.4 | 0.18 |
| Total score | 4.7 | 2.0 | 4.7 | 3.1 | 0.91 |
| OABSS: | |||||
| Daytime frequency | 0.7 | 0.5 | 0.7 | 0.5 | 0.94 |
| Nocturia | 1.2 | 0.7 | 1.1 | 0.7 | 0.36 |
| Urgency | 0.4 | 0.5 | 0.5 | 0.5 | 0.74 |
| Urgency incontinence | 0.3 | 0.6 | 0.2 | 0.5 | 0.71 |
| Total score | 2.6 | 1.3 | 2.5 | 1.4 | 0.58 |
| Total protein (g/dL) | 7.0 | 0.3 | 6.9 | 0.4 | 0.58 |
| Total bilirubin(mg/dL) | 0.7 | 0.3 | 0.7 | 0.3 | 0.84 |
| AST (U/L) | 19.7 | 4.9 | 22.6 | 7.4 | 0.05 |
| ALT (U/L) | 16.3 | 6.3 | 18.6 | 8.8 | 0.20 |
| γ-GTP (IU/L) | 24.6 | 20.7 | 25.1 | 16.8 | 0.91 |
| Total cholesterol (mg/dL) | 210.3 | 37.3 | 216.7 | 34.8 | 0.44 |
| Triglyceride(mg/dL) | 151.7 | 78.7 | 152.8 | 95.0 | 0.96 |
| eGFR (mL/min/1.73 m2) | 66.8 | 11.6 | 68.4 | 15.1 | 0.61 |
| CPK (U/L) | 112.7 | 56.1 | 100.7 | 45.5 | 0.31 |
| Uric acid (mg/dL) | 4.8 | 1.1 | 5.0 | 1.0 | 0.52 |
| Blood urea nitrogen(mg/dL) | 15.8 | 4.0 | 17.0 | 4.1 | 0.18 |
| Creatinine(mg/dL) | 0.7 | 0.1 | 0.7 | 0.1 | 0.74 |
| Variable | Active Group (n = 37) | Placebo Group (n = 35) | p Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Daytime frequency | 4 week | −0.4 | 0.9 | −0.2 | 1.0 | |
| 8 week | −0.4 | 1.1 | −0.3 | 0.8 | ||
| 12 week | −0.8 | 0.9 | −0.4 | 0.8 | 0.04 * | |
| Nocturia | 4 week | −0.1 | 0.7 | −0.1 | 0.8 | |
| 8 week | −0.1 | 0.7 | −0.2 | 0.6 | ||
| 12 week | −0.6 | 0.7 | −0.4 | 0.6 | 0.22 | |
| Urgency | 4 week | 0.0 | 0.6 | 0.0 | 0.7 | |
| 8 week | −0.1 | 0.7 | 0.0 | 0.6 | ||
| 12 week | −0.1 | 0.7 | 0.0 | 0.7 | 0.50 | |
| Urgency | 4 week | −0.1 | 0.7 | −0.1 | 0.5 | |
| incontinence | 8 week | −0.2 | 0.6 | −0.1 | 0.5 | |
| 12 week | −0.2 | 0.6 | −0.1 | 0.5 | 0.71 | |
| Stress incontinence | 4 week | −0.1 | 0.7 | −0.3 | 0.7 | |
| 8 week | −0.4 | 0.9 | −0.3 | 0.6 | ||
| 12 week | −0.2 | 0.8 | −0.4 | 0.6 | 0.17 | |
| Slow stream | 4 week | 0.0 | 0.8 | 0.1 | 0.8 | |
| 8 week | 0.0 | 0.7 | 0.1 | 1.0 | ||
| 12 week | 0.1 | 0.8 | −0.1 | 0.8 | 0.57 | |
| Straining | 4 week | 0.1 | 0.4 | 0.1 | 0.7 | |
| 8 week | 0.0 | 0.4 | 0.1 | 0.6 | ||
| 12 week | 0.1 | 0.4 | 0.1 | 0.8 | 0.83 | |
| Incomplete emptying | 4 week | 0.0 | 0.5 | 0.0 | 0.5 | |
| 8 week | 0.0 | 0.4 | 0.1 | 0.7 | ||
| 12 week | −0.1 | 0.5 | 0.1 | 0.7 | 0.23 | |
| Bladder pain | 4 week | 0.0 | 0.0 | −0.1 | 0.4 | |
| 8 week | 0.0 | 0.0 | −0.1 | 0.4 | ||
| 12 week | 0.0 | 0.0 | −0.1 | 0.4 | 0.18 | |
| Urethral pain | 4 week | 0.0 | 0.0 | −0.1 | 0.4 | |
| 8 week | 0.0 | 0.0 | −0.1 | 0.4 | ||
| 12 week | 0.0 | 0.0 | −0.1 | 0.4 | 0.15 | |
| Total score | 4 week | −0.6 | 2.7 | −0.7 | 3.5 | |
| 8 week | −1.2 | 2.3 | −0.9 | 3.6 | ||
| 12 week | −1.8 | 2.6 | −1.3 | 3.1 | 0.49 | |
| Variable | Active Group (n = 23) | Placebo Group (n = 22) | p Value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Daytime frequency | 4 week | −0.6 | 0.9 | −0.2 | 1.0 | |
| 8 week | −0.6 | 1.0 | −0.4 | 0.9 | ||
| 12 week | −1.0 | 0.9 | −0.3 | 0.9 | 0.03 * | |
| Nocturia | 4 week | −0.1 | 0.7 | 0.0 | 0.7 | |
| 8 week | −0.2 | 0.7 | −0.1 | 0.6 | ||
| 12 week | −0.7 | 0.7 | −0.3 | 0.5 | 0.04 * | |
| Urgency | 4 week | 0.0 | 0.6 | −0.2 | 0.7 | |
| 8 week | −0.1 | 0.7 | 0.0 | 0.7 | ||
| 12 week | −0.1 | 0.6 | 0.0 | 0.8 | 0.53 | |
| Urgency incontinence | 4 week | −0.1 | 0.7 | −0.2 | 0.6 | |
| 8 week | −0.1 | 0.6 | −0.2 | 0.6 | ||
| 12 week | −0.2 | 0.5 | −0.1 | 0.6 | 0.64 | |
| Stress incontinence | 4 week | −0.4 | 0.5 | −0.5 | 0.7 | |
| 8 week | −0.9 | 0.7 | −0.6 | 0.6 | ||
| 12 week | −0.5 | 0.7 | −0.7 | 0.6 | 0.39 | |
| Slow stream | 4 week | 0.0 | 0.6 | 0.0 | 0.9 | |
| 8 week | 0.1 | 0.8 | 0.0 | 1.0 | ||
| 12 week | 0.1 | 0.7 | 0.0 | 0.9 | 0.87 | |
| Straining | 4 week | 0.1 | 0.4 | 0.0 | 0.7 | |
| 8 week | 0.0 | 0.3 | 0.1 | 0.8 | ||
| 12 week | 0.1 | 0.5 | 0.1 | 0.9 | 0.98 | |
| Incomplete emptying | 4 week | 0.1 | 0.5 | 0.0 | 0.6 | |
| 8 week | 0.1 | 0.5 | 0.1 | 0.8 | ||
| 12 week | 0.0 | 0.4 | 0.1 | 0.8 | 0.47 | |
| Bladder pain | 4 week | 0.0 | 0.0 | −0.1 | 0.5 | |
| 8 week | 0.0 | 0.0 | −0.1 | 0.5 | ||
| 12 week | 0.0 | 0.0 | −0.1 | 0.5 | 0.19 | |
| Urethral pain | 4 week | 0.0 | 0.0 | −0.1 | 0.5 | |
| 8 week | 0.0 | 0.0 | −0.1 | 0.5 | ||
| 12 week | 0.0 | 0.0 | −0.1 | 0.5 | 0.19 | |
| Total score | 4 week | −1.0 | 2.3 | −1.3 | 3.8 | |
| 8 week | −1.6 | 2.1 | −1.3 | 4.2 | ||
| 12 week | −2.3 | 2.3 | −1.4 | 3.6 | 0.32 | |
| Variable | Active Group (n = 37) | Placebo Group (n = 35) | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Daytime frequency | −0.4 | 0.6 | −0.1 | 0.7 | 0.04 * |
| Nocturia | −0.6 | 0.9 | −0.4 | 0.7 | 0.68 |
| Urgency | 0.0 | 0.8 | 0.1 | 0.7 | 0.46 |
| Urgency incontinence | −0.1 | 0.8 | −0.1 | 0.5 | 0.56 |
| Total score | −0.6 | 2.3 | −0.6 | 1.3 | 0.76 |
| Variable | Baseline | 12-Week Treatment | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Weight (kg) | Active | 53.5 | 7.4 | 53.8 | 7.2 |
| Placebo | 55.2 | 9.7 | 55.5 | 9.9 | |
| Blood pressure (mmHg) | |||||
| Systolic | Active | 135.6 | 21.3 | 135.1 | 21.7 |
| Placebo | 134.7 | 21.8 | 133.0 | 19.8 | |
| Diastolic | Active | 74.8 | 13.6 | 75.2 | 12.4 |
| Placebo | 71.5 | 12.1 | 73.0 | 9.2 | |
| Pulse | Active | 70.6 | 8.8 | 73.5 | 11.7 |
| Placebo | 70.2 | 11.4 | 70.6 | 11.5 | |
| Total protein (g/dL) | Active | 7.0 | 0.3 | 7.1 | 0.3 |
| Placebo | 6.9 | 0.4 | 7.0 | 0.3 | |
| Total bilirubin(mg/dL) | Active | 0.7 | 0.3 | 0.6 | 0.2 |
| Placebo | 0.7 | 0.3 | 0.6 | 0.3 | |
| AST (U/L) | Active | 19.5 | 4.8 | 21.2 | 6.6 |
| Placebo | 22.6 | 7.4 | 22.8 | 5.8 | |
| ALT (U/L) | Active | 16.1 | 6.3 | 18.4 | 10.2 |
| Placebo | 18.6 | 8.8 | 19.7 | 9.9 | |
| γ-GTP (IU/L) | Active | 24.1 | 20.7 | 23.0 | 20.2 |
| Placebo | 25.1 | 16.8 | 23.0 | 12.1 | |
| Total cholesterol (mg/dL) | Active | 209.4 | 37.4 | 218.9 | 35.9 |
| Placebo | 216.7 | 34.8 | 219.3 | 36.0 | |
| Triglyceride(mg/dL) | Active | 149.7 | 78.8 | 145.5 | 91.0 |
| Placebo | 152.8 | 95.0 | 128.9 | 83.8 | |
| eGFR (mL/min/1.73 m2) | Active | 66.1 | 10.8 | 68.6 | 12.1 |
| Placebo | 68.4 | 15.1 | 70.1 | 14.5 | |
| CPK (U/L) | Active | 114.6 | 55.6 | 120.4 | 96.9 |
| Placebo | 100.7 | 45.5 | 104.6 | 45.5 | |
| Uric acid (mg/dL) | Active | 4.8 | 1.1 | 4.9 | 1.3 |
| Placebo | 5.0 | 1.0 | 4.9 | 1.1 | |
| Blood urea nitrogen(mg/dL) | Active | 15.7 | 4.0 | 15.7 | 3.1 |
| Placebo | 17.0 | 4.1 | 15.6 | 4.2 | |
| Creatinine(mg/dL) | Active | 0.7 | 0.1 | 0.7 | 0.1 |
| Placebo | 0.7 | 0.1 | 0.7 | 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Shirai, M.; Ono, K.; Kageyama, S. Beneficial Effects of Saw Palmetto Fruit Extract on Urinary Symptoms in Japanese Female Subjects by a Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 1190. https://doi.org/10.3390/nu14061190
Yamada S, Shirai M, Ono K, Kageyama S. Beneficial Effects of Saw Palmetto Fruit Extract on Urinary Symptoms in Japanese Female Subjects by a Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2022; 14(6):1190. https://doi.org/10.3390/nu14061190
Chicago/Turabian StyleYamada, Shizuo, Michiyo Shirai, Ken Ono, and Shinji Kageyama. 2022. "Beneficial Effects of Saw Palmetto Fruit Extract on Urinary Symptoms in Japanese Female Subjects by a Multicenter, Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 14, no. 6: 1190. https://doi.org/10.3390/nu14061190
APA StyleYamada, S., Shirai, M., Ono, K., & Kageyama, S. (2022). Beneficial Effects of Saw Palmetto Fruit Extract on Urinary Symptoms in Japanese Female Subjects by a Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Nutrients, 14(6), 1190. https://doi.org/10.3390/nu14061190







