Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Experimental Design
2.2. Blood Collection
2.3. Plasma Choline Metabolites
2.4. Plasma Lutein and Zeaxanthin
2.5. Feces Collection
2.6. DNA Extraction, PCR Amplification, and Sequencing of Taxonomic Marker
2.7. Stratification for Microbiota
2.8. Sequence Data Processing and Statistical Analyses
2.9. Statistical Analysis
3. Results
3.1. Plasma Choline Metabolites
3.2. Plasma Lutein and Zeaxanthin
3.3. Gut Microbiota
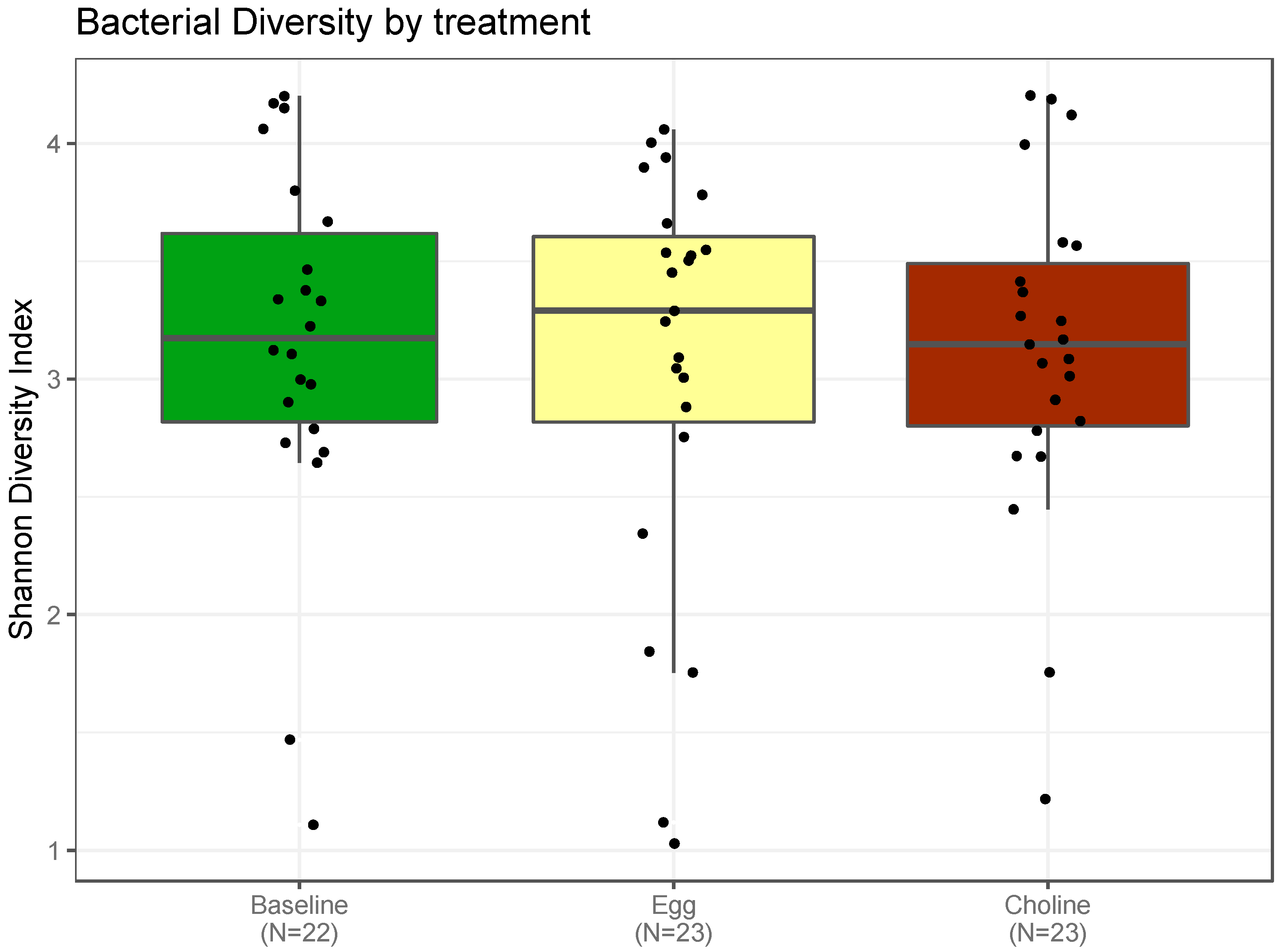
3.3.1. Alpha-Diversity Indexes of the Gut Microbiota
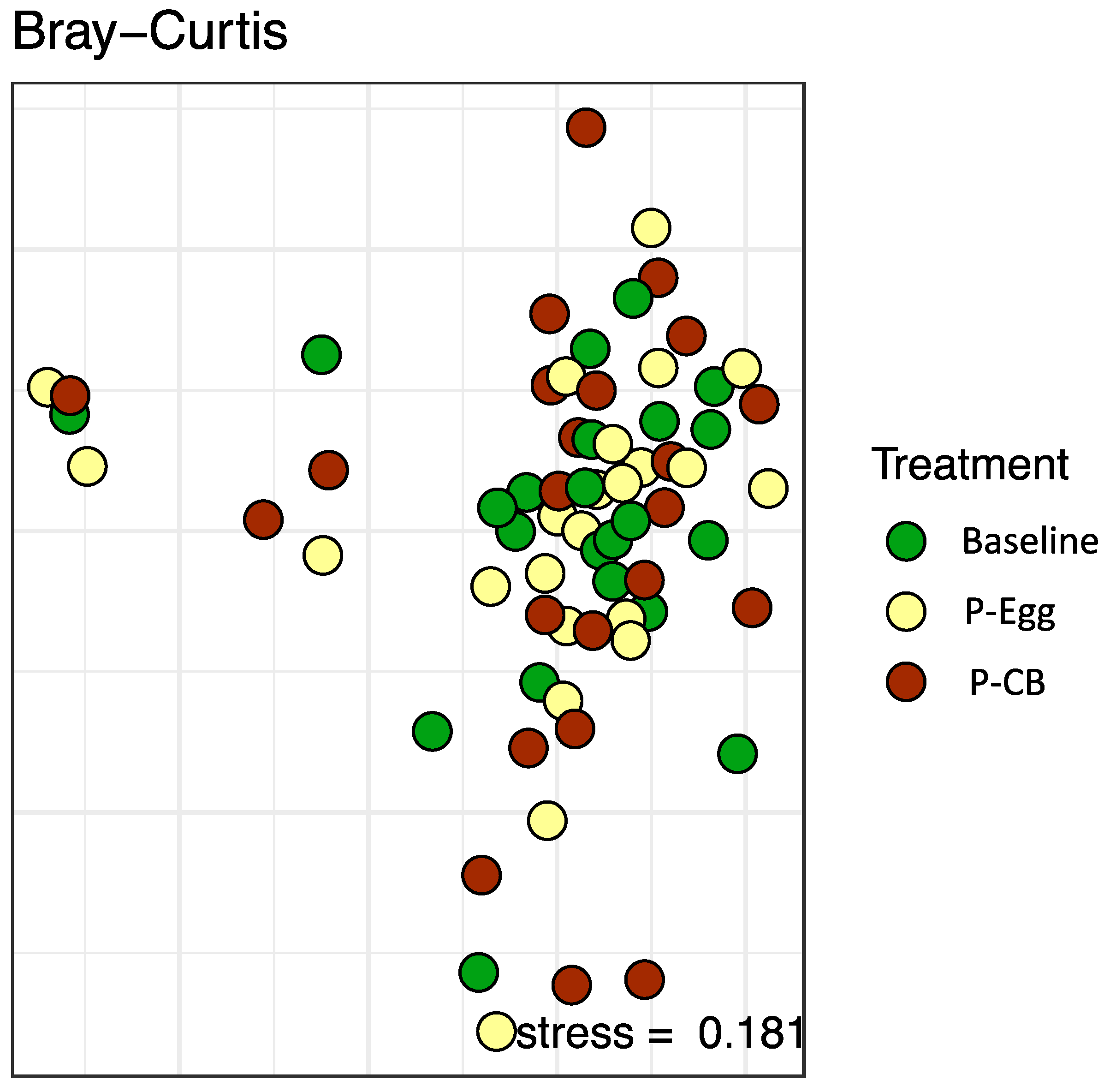
3.3.2. Beta-Diversity Indexes of the Gut Microbiota
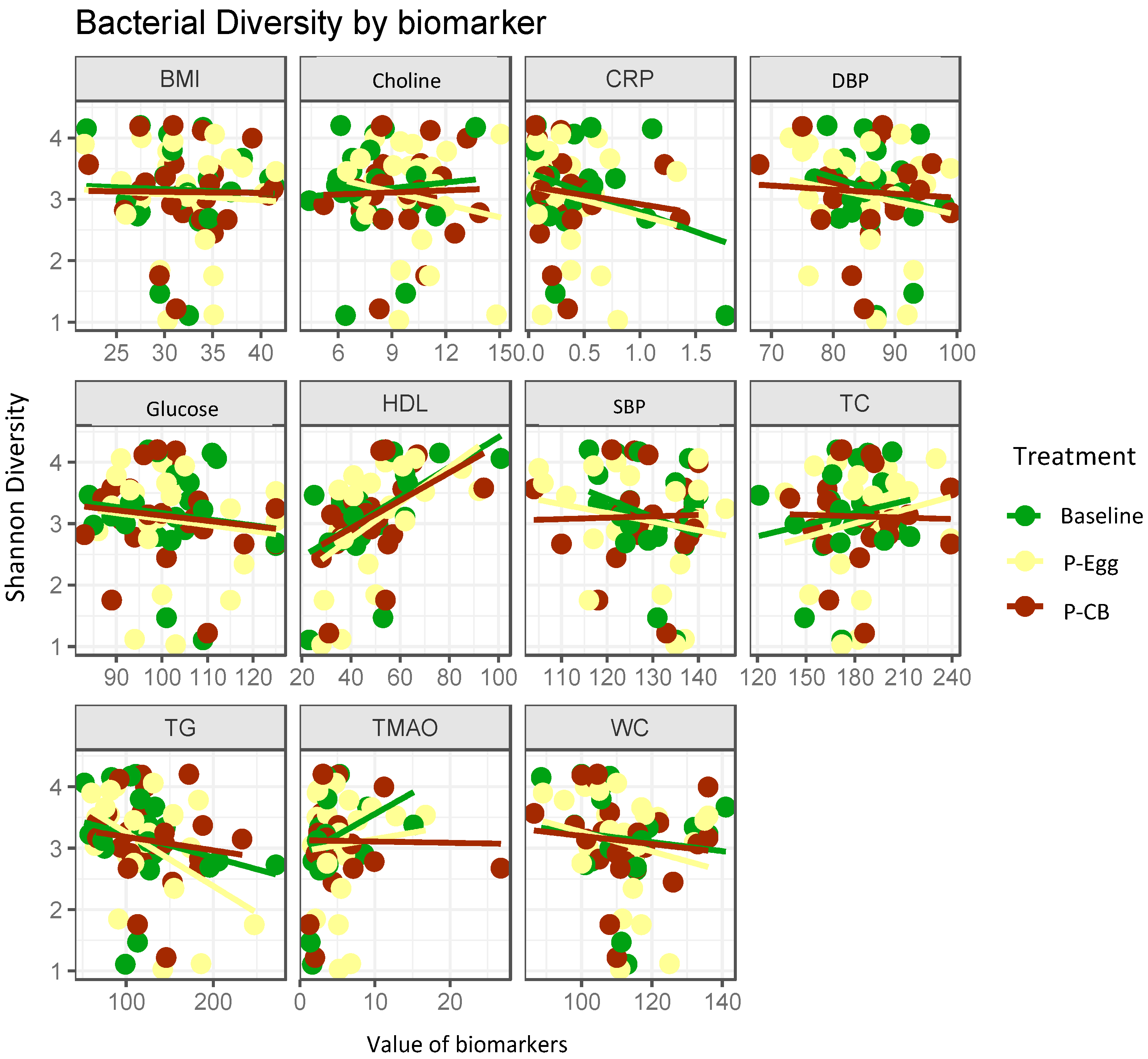
3.3.3. Correlation Analysis between Bacterial Diversity and Metabolic Parameters
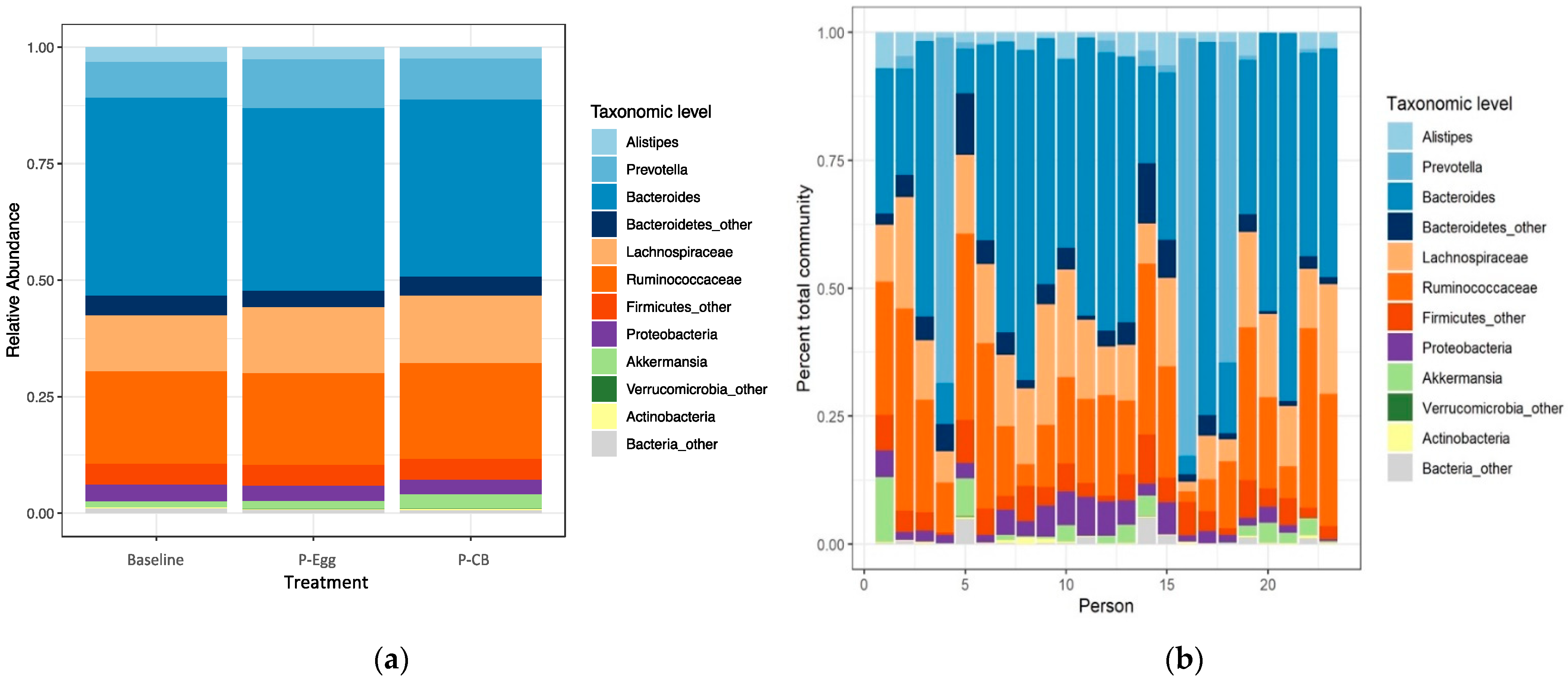
3.3.4. Comparison of Taxonomic Signatures in MetS Patients at Baseline and When Consuming Three Eggs/d Versus CB Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.X.; Wong, C.H.; Kim, J.E. Impact of Whole Egg Intake on Blood Pressure, Lipids and Lipoproteins in Middle-Aged and Older Population: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.S.; Fusco, E.; Kern, M. Egg Consumption and Heart Health: A Review. Nutrition 2017, 37, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Emamat, H.; Totmaj, A.S.; Tangestani, H.; Hekmatdoost, A. The Effect of Egg and Its Derivatives on Vascular Function: A Systematic Review of Interventional Studies. Clin. Nutr. ESPEN 2020, 39, 15–21. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available online: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed on 17 February 2022).
- Murillo, A.G.; Fernandez, M.L. Potential of Dietary Non-Provitamin A Carotenoids in the Prevention and Treatment of Diabetic Microvascular Complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Mutungi, G.; Waters, D.; Ratliff, J.; Puglisi, M.; Clark, R.M.; Volek, J.S.; Fernandez, M.L. Eggs Distinctly Modulate Plasma Carotenoid and Lipoprotein Subclasses in Adult Men Following a Carbohydrate-Restricted Diet. J. Nutr. Biochem. 2010, 21, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Blesso, C.N.; Andersen, C.J.; Bolling, B.W.; Fernandez, M.L. Egg Intake Improves Carotenoid Status by Increasing Plasma HDL Cholesterol in Adults with Metabolic Syndrome. Food Funct. 2013, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A. Population Studies of TMAO and Its Precursors May Help Elucidate Mechanisms. Am. J. Clin. Nutr. 2020, 111, 1115–1116. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A Small Molecule of Great Expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Phosphatidylcholine and Choline Homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, J.; Skye, S.M.; Graham, B.; Zabell, A.; Li, X.S.; Li, L.; Shelkay, S.; Fu, X.; Neale, S.; O’Laughlin, C.; et al. Dietary Choline Supplements, but Not Eggs, Raise Fasting TMAO Levels in Participants with Normal Renal Function: A Randomized Clinical Trial. Am. J. Med. 2021, 134, 1160–1169.e3. [Google Scholar] [CrossRef]
- Hazen, S.L.; Brown, J.M. Eggs as a Dietary Source for Gut Microbial Production of Trimethylamine-N-Oxide. Am. J. Clin. Nutr. 2014, 100, 741–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, B.J.; Vallim, T.Q.D.A.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Buffa, J.A.; Wang, Z.; Warrier, M.; Schugar, R.; Shih, D.M.; Gupta, N.; Gregory, J.C.; Org, E.; Fu, X.; et al. Flavin Monooxygenase 3, the Host Hepatic Enzyme in the Metaorganismal Trimethylamine N-Oxide-Generating Pathway, Modulates Platelet Responsiveness and Thrombosis Risk. J. Thromb. Haemost. 2018, 16, 1857–1872. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Wang, Z.; Tang, W.H.W.; Hazen, S.L. Gut Microbe-Generated Trimethylamine N-Oxide from Dietary Choline Is Prothrombotic in Subjects. Circulation 2017, 135, 1671–1673. [Google Scholar] [CrossRef] [Green Version]
- Lemos, B.S.; Medina-Vera, I.; Malysheva, O.V.; Caudill, M.A.; Fernandez, M.L. Effects of Egg Consumption and Choline Supplementation on Plasma Choline and Trimethylamine-N-Oxide in a Young Population. J. Am. Coll. Nutr. 2018, 37, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Holm, P.I.; Hustad, S. Betaine: A Key Modulator of One-Carbon Metabolism and Homocysteine Status. Clin. Chem. Lab. Med. (CCLM) 2005, 43, 1069–1075. [Google Scholar] [CrossRef]
- Holm, P.I.; Hustad, S.; Ueland, P.M.; Vollset, S.E.; Grotmol, T.; Schneede, J. Modulation of the Homocysteine-Betaine Relationship by Methylenetetrahydrofolate Reductase 677 C->T Genotypes and B-Vitamin Status in a Large-Scale Epidemiological Study. J. Clin. Endocrinol. Metab. 2007, 92, 1535–1541. [Google Scholar] [CrossRef]
- Holm, P.I.; Bleie, Ø.; Ueland, P.M.; Lien, E.A.; Refsum, H.; Nordrehaug, J.E.; Nygård, O. Betaine as a Determinant of Postmethionine Load Total Plasma Homocysteine Before and After B-Vitamin Supplementation. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.; Verhoef, P. Effects of Betaine Intake on Plasma Homocysteine Concentrations and Consequences for Health. Curr. Drug Metab. 2005, 6, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut Microbiota and Metabolic Syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Dibella, M.; Thomas, M.S.; Alyousef, H.; Millar, C.; Blesso, C.; Malysheva, O.; Caudill, M.A.; Fernandez, M.L. Choline Intake as Supplement or as a Component of Eggs Increases Plasma Choline and Reduces Interleukin-6 without Modifying Plasma Cholesterol in Participants with Metabolic Syndrome. Nutrients 2020, 12, 3120. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Lemos, B.S.; Medina-Vera, I.; Blesso, C.N.; Fernandez, M.L. Intake of 3 Eggs per Day When Compared to a Choline Bitartrate Supplement, Downregulates Cholesterol Synthesis without Changing the LDL/HDL Ratio. Nutrients 2018, 10, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holm, P.I.; Ueland, P.M.; Kvalheim, G.; Lien, E.A. Determination of Choline, Betaine, and Dimethylglycine in Plasma by a High-Throughput Method Based on Normal-Phase Chromatography-Tandem Mass Spectrometry. Clin. Chem. 2003, 49, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Craft, N.E.; Wise, S.A.; Soares, J.H. Individual Carotenoid Content of SRM 1548 Total Diet and Influence of Storage Temperature, Lyophilization, and Irradiation on Dietary Carotenoids. J. Agric. Food Chem. 2002, 41, 208–213. [Google Scholar] [CrossRef]
- Biddle, M.J.; Lennie, T.A.; Bricker, G.V.; Kopec, R.E.; Schwartz, S.J.; Moser, D.K. Lycopene Dietary Intervention: A Pilot Study in Patients with Heart Failure. J. Cardiovasc. Nurs. 2015, 30, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced Bioavailability of Lycopene When Consumed as Cis-Isomers from Tangerine Compared to Red Tomato Juice, a Randomized, Cross-over Clinical Trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef] [Green Version]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the Miseq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, R.P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package, Version 2.3-01; 2015. [Google Scholar]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tveitevåg Svingen, G.F.; Ueland, P.M.; Pedersen, E.K.R.; Schartum-Hansen, H.; Seifert, R.; Ebbing, M.; Løland, K.H.; Tell, G.S.; Nygård, O. Plasma Dimethylglycine and Risk of Incident Acute Myocardial Infarction in Patients With Stable Angina Pectoris. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2041–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, U.; Alfthan, G.; Aro, A.; Uusitupa, M. Long-Term Effect of Betaine on Risk Factors Associated with the Metabolic Syndrome in Healthy Subjects. Eur. J. Clin. Nutr. 2011, 65, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiattarella, G.G.; Trimarco, B. Microbial Metabolites as Predictive Biomarkers: A Paradigm Shift for Cardiovascular Risk Stratification. Eur. Heart J. 2019, 40, 2710–2712. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.A.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating Trimethylamine N-Oxide and the Risk of Cardiovascular Diseases: A Systematic Review and Meta-Analysis of 11 Prospective Cohort Studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with Early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Sawrey-Kubicek, L.; Bardagjy, A.S.; Houts, H.; Tang, X.; Sacchi, R.; Randolph, J.M.; Steinberg, F.M.; Zivkovic, A.M. Whole Egg Consumption Increases Plasma Choline and Betaine without Affecting TMAO Levels or Gut Microbiome in Overweight Postmenopausal Women. Nutr. Res. 2020, 78, 36–41. [Google Scholar] [CrossRef]
- Meyer, K.A.; Benton, T.Z.; Bennett, B.J.; Jacobs, D.R.; Lloyd-Jones, D.M.; Gross, M.D.; Carr, J.J.; Gordon-Larsen, P.; Zeisel, S.H. Microbiota-Dependent Metabolite Trimethylamine n-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J. Am. Heart Assoc. 2016, 5, e003970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; Chalcraft, K.; Ak, H.; Davis, A.D.; Lonn, E.; Miller, R.; Potter, M.A.; Yusuf, S.; Anand, S.S.; McQueen, M.J. The Relationship Between Trimethylamine-N-Oxide and Prevalent Cardiovascular Disease in a Multiethnic Population Living in Canada. Can. J. Cardiol. 2015, 31, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota with Reduced Trimethylamine-n-Oxide Level in Patients with Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4, e002699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andraos, S.; Jones, B.; Lange, K.; Clifford, S.A.; Thorstensen, E.B.; Kerr, J.A.; Wake, M.; Saffery, R.; Burgner, D.P.; O’sullivan, J.M. Trimethylamine N-Oxide (TMAO) Is Not Associated with Cardiometabolic Phenotypes and Inflammatory Markers in Children and Adults. Curr. Dev. Nutr. 2020, 4, nzaa179. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; McCarty, M.; Okeefe, J. Association of Moderately Elevated Trimethylamine N-Oxide with Cardiovascular Risk: Is TMAO Serving as a Marker for Hepatic Insulin Resistance. Open Heart 2019, 6, e000890. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic Syndrome: Pathophysiology, Management, and Modulation by Natural Compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Niemann, B.; Rohrbach, S.; Miller, M.R.; Newby, D.E.; Fuster, V.; Kovacic, J.C. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 230–251. [Google Scholar] [CrossRef]
- Francisqueti, F.V.; Chiaverini, L.C.T.; dos Santos, K.C.; Minatel, I.O.; Ronchi, C.B.; Ferron, A.J.T.; Ferreira, A.L.A.; Corrêa, C.R. The Role of Oxidative Stress on the Pathophysiology of Metabolic Syndrome. Rev. Assoc. Med. Bras. 2017, 63, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The Metabolic Syndrome and Antioxidant Concentrations Findings From the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shi, W.Q.; Cao, Y.; He, L.P.; Guan, K.; Ling, W.H.; Chen, Y.M. Higher Serum Carotenoid Concentrations Associated with a Lower Prevalence of the Metabolic Syndrome in Middle-Aged and Elderly Chinese Adults. Br. J. Nutr. 2014, 112, 2041–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; McClintock, C.S.; Shaw, J.E. Metabolic Syndrome and Serum Carotenoids: Findings of a Cross-Sectional Study in Queensland, Australia. Br. J. Nutr. 2009, 102, 1668–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Agócs, A.; Deli, J. Lutein Exerts Antioxidant and Anti-Inflammatory Effects and Influences Iron Utilization of Bv-2 Microglia. Antioxidants 2021, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Sluji, I.; Beulens, J.W.; Grobbee, D.E.; van der Schouw, Y.T. Dietary carotenoid is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 199, 987–992. [Google Scholar]
- Beydoun, M.A.; Atilio Canas, J.; Beydoun, H.A.; Chen, X.; Shroff, M.R.; Zonderman, A.B. Serum Antioxidant Concentrations and Metabolic Syndrome Are Associated among U.S. Adolescents in Recent National Surveys. J. Nutr. 2012, 142, 1693–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handelman, G.J.; Nightingale, Z.D.; Lichtenstein, A.H.; Schaefer, E.J.; Blumberg, J.B. Lutein and Zeaxanthin Concentrations in Plasma after Dietary Supplementation with Egg Yolk. Am. J. Clin. Nutr. 1999, 70, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Renzi, L.M. Carotenoids. Adv. Nutr. 2013, 4, 474–476. [Google Scholar] [CrossRef] [Green Version]
- Goodrow, E.F.; Wilson, T.A.; Houde, S.C.; Vishwanathan, R.; Scollin, P.A.; Handelman, G.; Nicolosi, R.J. Consumption of One Egg per Day Increases Serum Lutein and Zeaxanthin Concentrations in Older Adults without Altering Serum Lipid and Lipoprotein Cholesterol Concentrations. J. Nutr. 2006, 136, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- DiMarco, D.M.; Norris, G.H.; Millar, C.L.; Blesso, C.N.; Fernandez, M.L. Intake of up to 3 Eggs per Day Is Associated with Changes in HDL Function and Increased Plasma Antioxidants in Healthy, Young Adults. J. Nutr. 2017, 147, 323–329. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors Affecting the Composition of the Gut Microbiota, and Its Modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, M.S.; Blesso, C.; Calle, M.; Chun, O.; Puglisi, M.; Fernandez, M.L. Dietary Influences on Gut Microbiota with a Focus on Metabolic Syndrome. Metab. Syndr. Relat. Disorders, 2022; in press. [Google Scholar]
- Sekirov, I.; Russell, S.L.; Caetano, M.; Antunes, L.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.W.F.; Kersten, S. The Role of the Gut Microbiota in Metabolic Health. FASEB J. 2015, 29, 3111–3123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vourakis, M.; Mayer, G.; Rousseau, G. The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef] [PubMed]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial Impact on Cholesterol and Bile Acid Metabolism: Current Status and Future Prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-Free C57BL/6J Mice Are Resistant to High-Fat-Diet-Induced Insulin Resistance and Have Altered Cholesterol Metabolism. The FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef] [Green Version]
- le Roy, T.; Lécuyer, E.; Chassaing, B.; Rhimi, M.; Lhomme, M.; Boudebbouze, S.; Ichou, F.; Haro Barceló, J.; Huby, T.; Guerin, M.; et al. The Intestinal Microbiota Regulates Host Cholesterol Homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar] [CrossRef] [Green Version]
- Schoeler, M.; Caesar, R. Dietary Lipids, Gut Microbiota and Lipid Metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.M.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Wutthi-in, M.; Cheevadhanarak, S.; Yasom, S.; Kerdphoo, S.; Thiennimitr, P.; Phrommintikul, A.; Chattipakorn, N.; Kittichotirat, W.; Chattipakorn, S. Gut Microbiota Profiles of Treated Metabolic Syndrome Patients and Their Relationship with Metabolic Health. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef]
- Furet, J.P.; Kong, L.C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery-Induced Weight Loss: Links with Metabolic and Low-Grade Inflammation Markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.M.; Louis, P.; Flint, H.J. Human Colonic Microbiota Associated with Diet, Obesity and Weight Loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Belzer, C.; de Vos, W.M. Microbes Inside from Diversity to Function: The Case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- Zhou, Q.; Pang, G.; Zhang, Z.; Yuan, H.; Chen, C.; Zhang, N.; Yang, Z.; Sun, L. Association between Gut Akkermansia and Metabolic Syndrome Is Dose-Dependent and Affected by Microbial Interactions: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2177–2188. [Google Scholar] [CrossRef]


| Parameter | Baseline | P-Egg | P-CB |
|---|---|---|---|
| TMAO (nmol/mL) | 4.16 ± 3.05 | 5.14 ± 2.5 | 5.15 ± 5.3 |
| Methionine (nmol/mL) | 29.4 ± 4.0 | 29.8 ± 4.0 | 29.9 ± 5.2 |
| Betaine (nmol/mL) 2 | 37.6 ± 14.8 a | 43.0 ± 14.7 b | 43.5 ± 18.7 b |
| DGM (nmol/mL) | 2.4 ± 0.6 a | 2.8 ± 0.7 b | 2.8 ± 0.6 b |
| Parameter | Baseline | P-Egg | P-CB |
|---|---|---|---|
| Plasma lutein (nmol/L) 2 | 495.1 ± 235 a | 681.6 ± 351 b | 527.7 ± 283 a |
| Plasma zeaxanthin (nmol/L) | 64.8 ± 29 a | 113.8 ± 46 b | 67.3 ± 27 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.S.; DiBella, M.; Blesso, C.N.; Malysheva, O.; Caudill, M.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome. Nutrients 2022, 14, 1179. https://doi.org/10.3390/nu14061179
Thomas MS, DiBella M, Blesso CN, Malysheva O, Caudill M, Sholola M, Cooperstone JL, Fernandez ML. Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome. Nutrients. 2022; 14(6):1179. https://doi.org/10.3390/nu14061179
Chicago/Turabian StyleThomas, Minu S., Marissa DiBella, Christopher N. Blesso, Olga Malysheva, Marie Caudill, Maria Sholola, Jessica L. Cooperstone, and Maria Luz Fernandez. 2022. "Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome" Nutrients 14, no. 6: 1179. https://doi.org/10.3390/nu14061179
APA StyleThomas, M. S., DiBella, M., Blesso, C. N., Malysheva, O., Caudill, M., Sholola, M., Cooperstone, J. L., & Fernandez, M. L. (2022). Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome. Nutrients, 14(6), 1179. https://doi.org/10.3390/nu14061179









