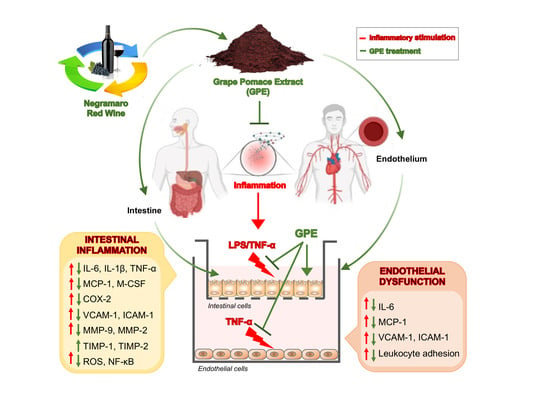
Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Grape Pomace Polyphenolic Extract
2.3. High-Performance Liquid Chromatography (HPLC) Characterization of Polyphenols
2.4. Folin–Ciocalteu Assay
2.5. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.6. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. Cell Cultures
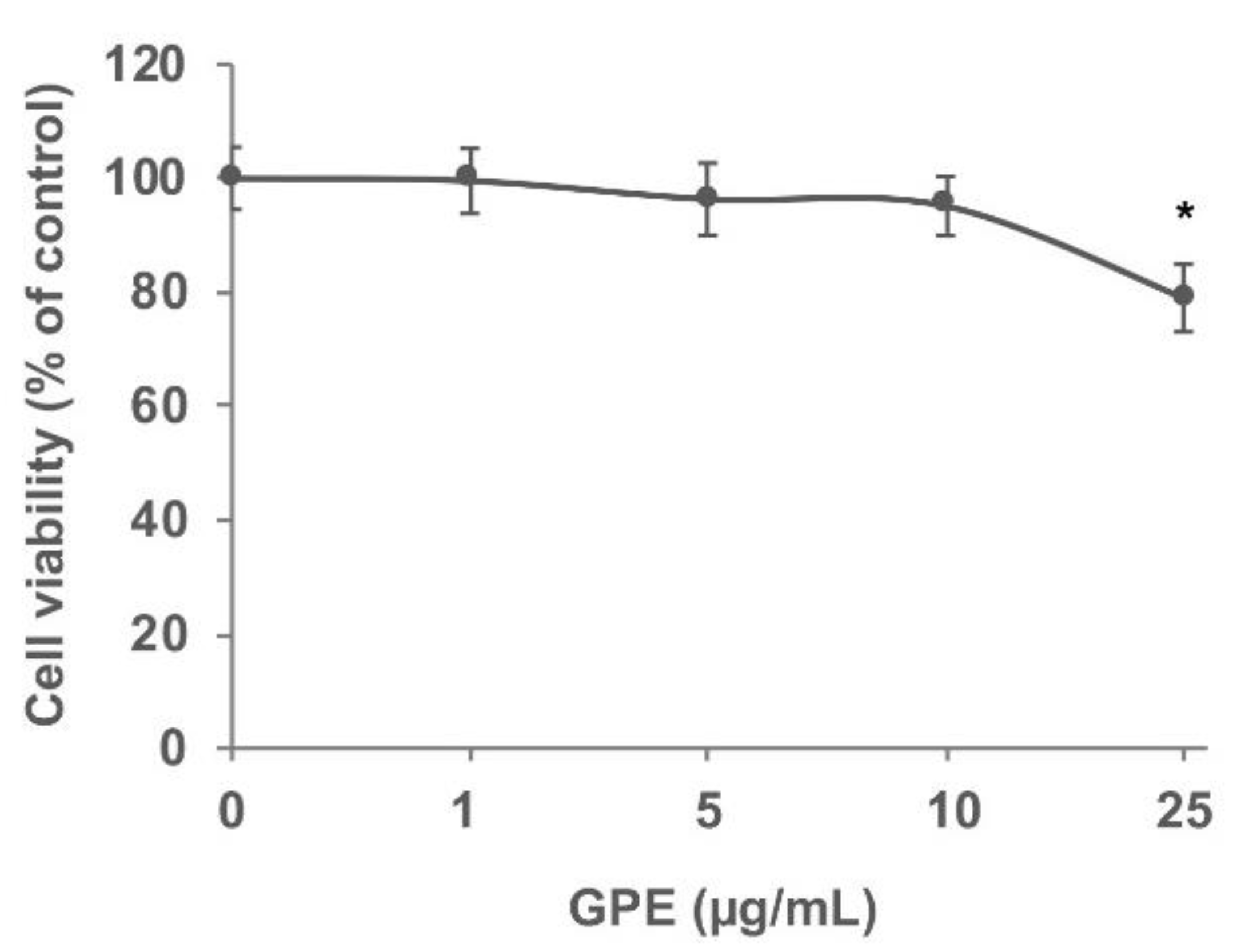
2.8. Cell Viability
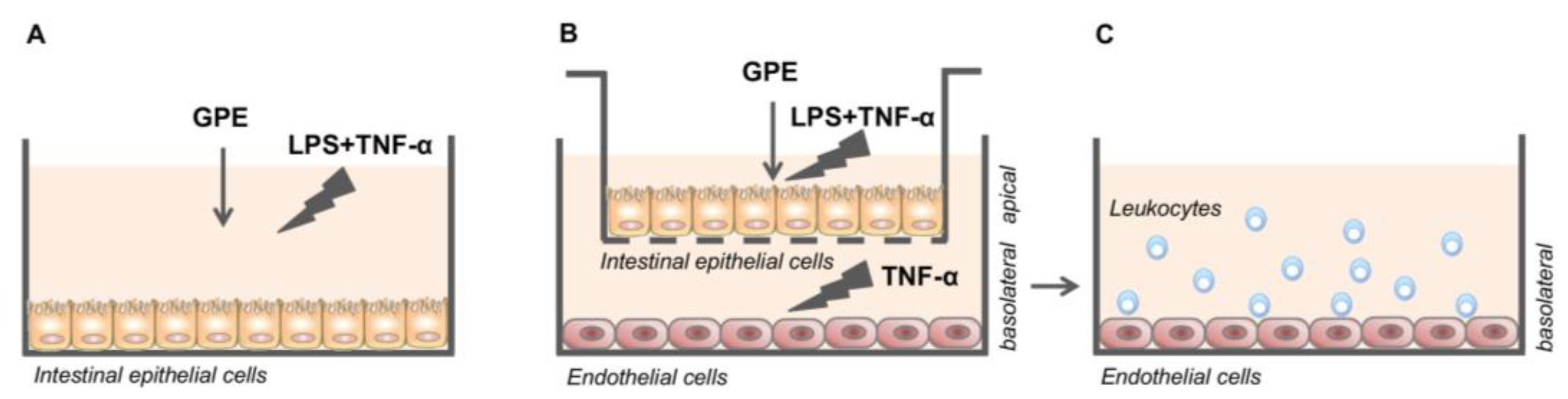
2.9. Treatments of Caco-2 Cell Monolayers
2.10. Caco-2/HMEC-1 Co-Culture System and Treatments
2.11. Leukocyte Adhesion Assay
2.12. Detection of Endothelial Cell Surface Molecules
2.13. Measure of Inflammation Marker Release
2.14. RNA Isolation and Real-Time Quantitative PCR
2.15. Preparation of Nuclear Protein Extracts and NF-κB Activation Assay
2.16. Detection of Intracellular ROS Production
2.17. Statistical Analysis
3. Results
3.1. Total Phenolic Content and Antioxidant Potential of GPE
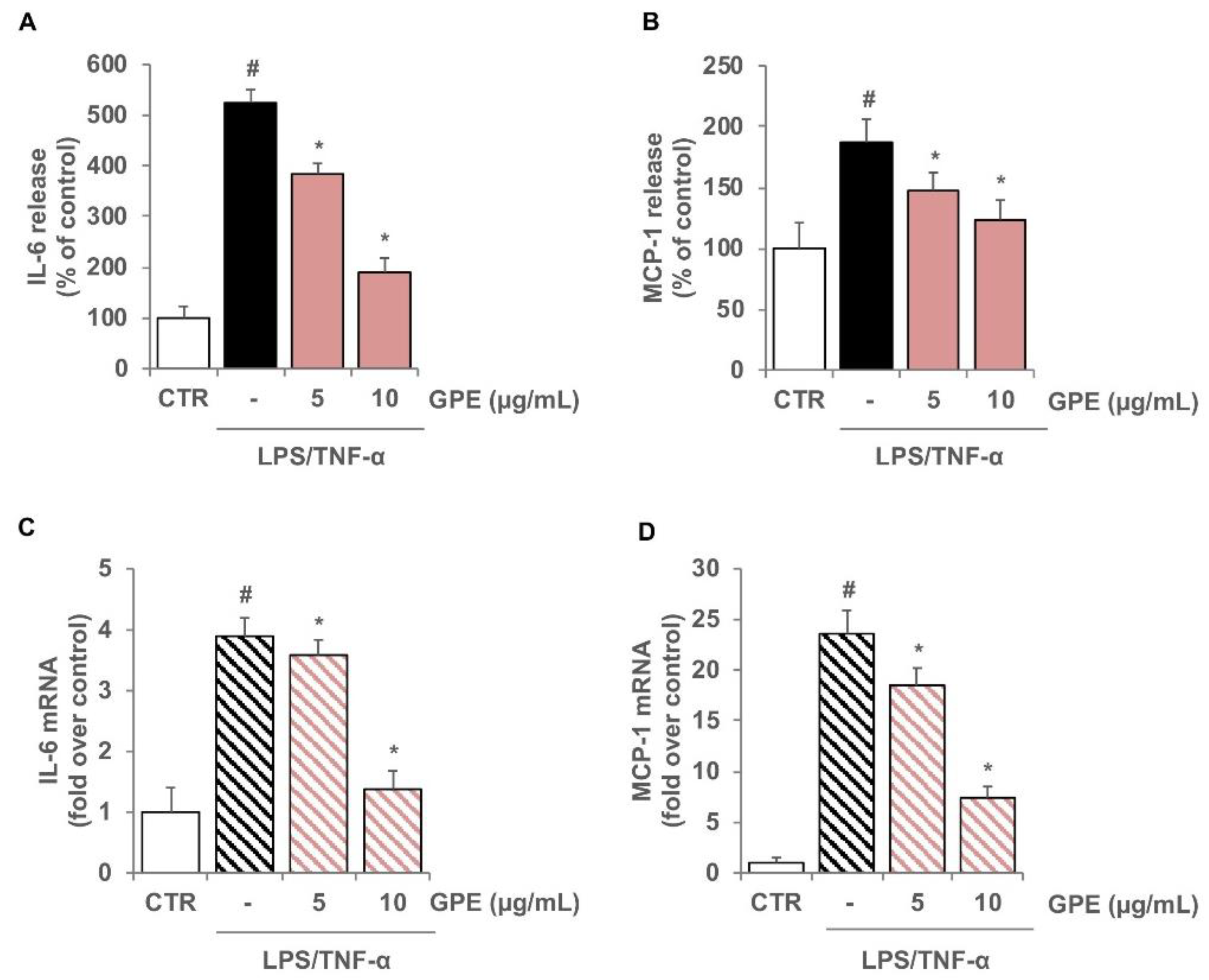
3.2. GPE Prevents the Stimulated Expression of Proinflammatory Mediators in Caco-2 Cells
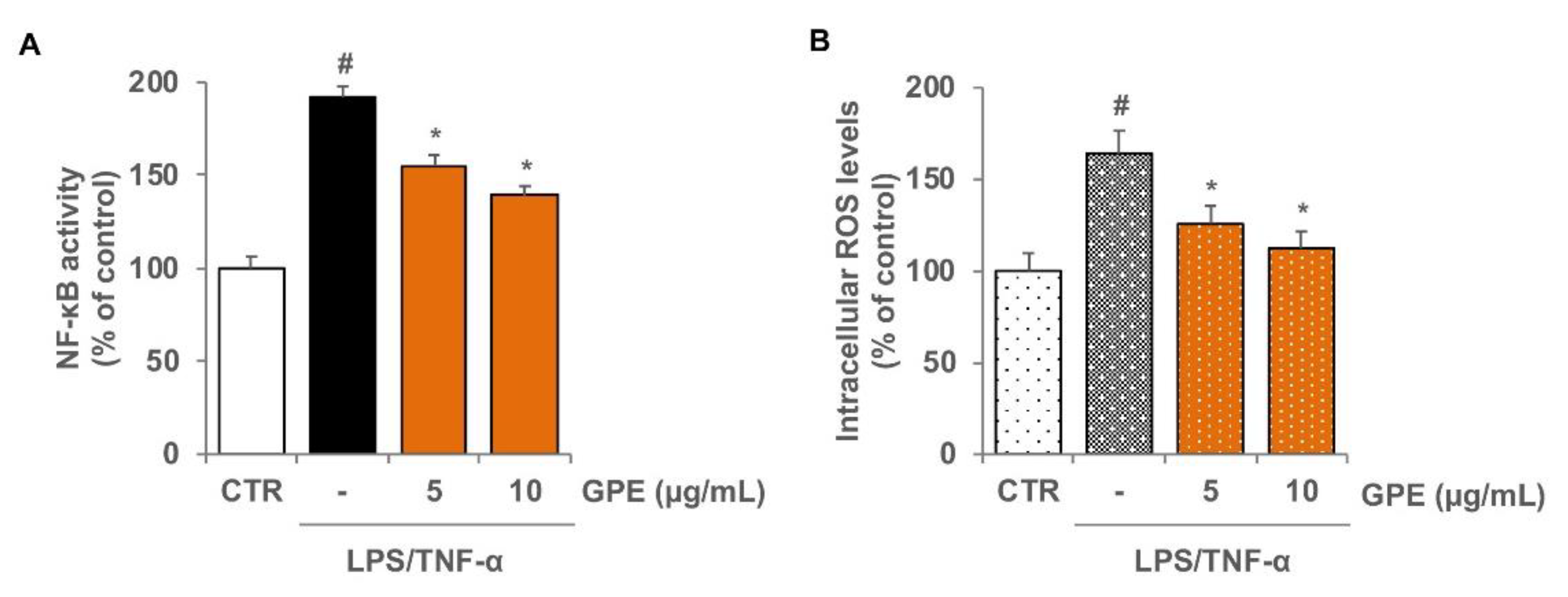
3.3. GPE Reduced the Stimulated Activation of NF-κB and the Intracellular ROS Levels in Caco-2 Cells
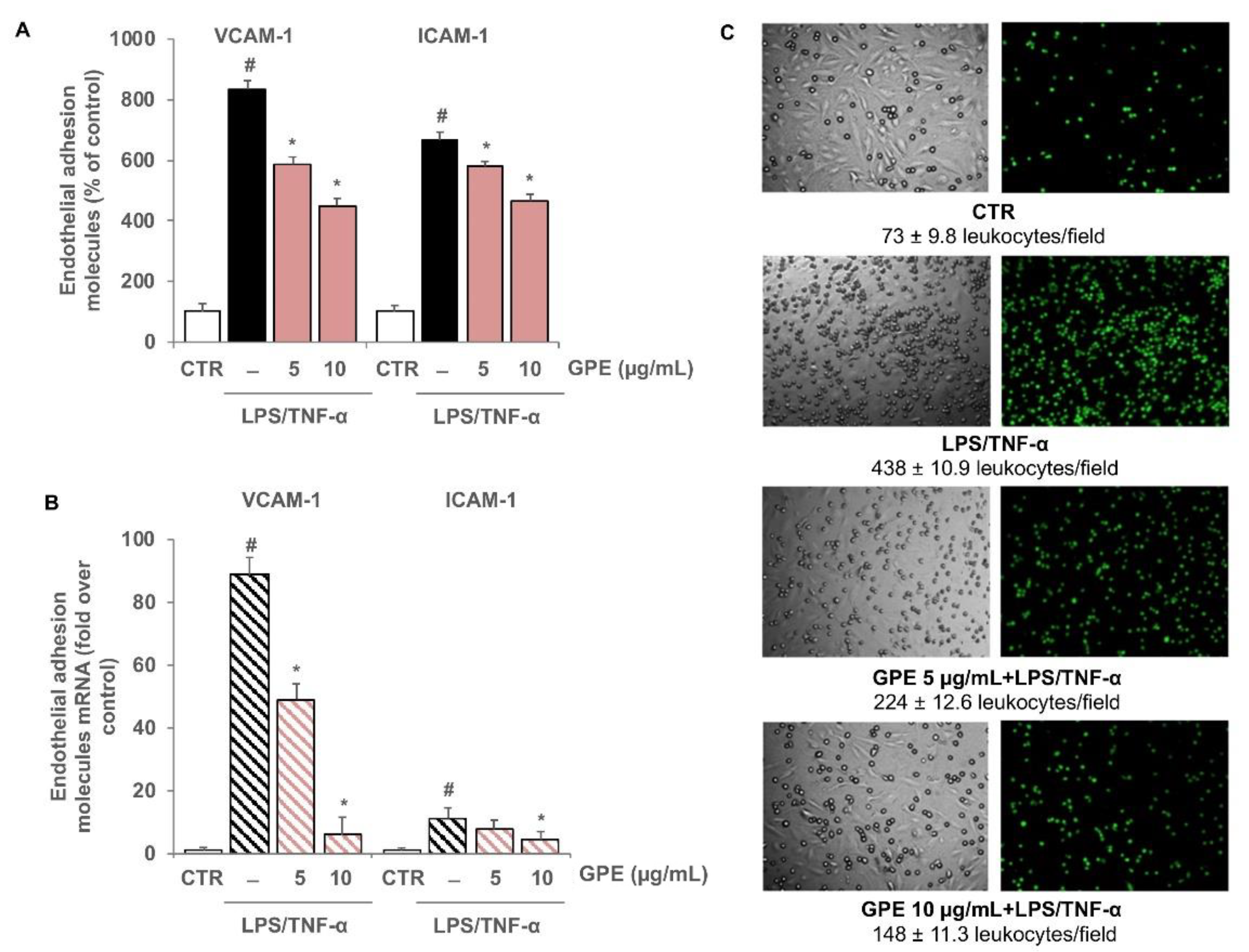
3.4. GPE Inhibited Endothelial Activation and Monocyte Adhesion in Intestinal Epithelial/Endothelial Co-Culture Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jia, F.; Zhang, B.; Zhang, P. Risk of cardiovascular disease in inflammatory bowel disease. Exp. Ther. Med. 2017, 13, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Biondi, R.B.; Salmazo, P.S.; Bazan, S.G.Z.; Hueb, J.C.; de Paiva, S.A.R.; Sassaki, L.Y. Cardiovascular Risk in Individuals with Inflammatory Bowel Disease. Clin. Exp. Gastroenterol. 2020, 13, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bunu, D.M.; Timofte, C.E.; Ciocoiu, M.; Floria, M.; Tarniceriu, C.C.; Barboi, O.B.; Tanase, D.M. Cardiovascular Manifestations of Inflammatory Bowel Disease: Pathogenesis, Diagnosis, and Preventive Strategies. Gastroenterol. Res. Pract. 2019, 2019, 3012509. [Google Scholar] [CrossRef] [PubMed]
- Cibor, D.; Domagala-Rodacka, R.; Rodacki, T.; Jurczyszyn, A.; Mach, T.; Owczarek, D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J. Gastroenterol. 2016, 22, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III27–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Sans, M.; de la Motte, C.; Graziani, C.; West, G.; Phillips, M.H.; Pola, R.; Rutella, S.; Willis, J.; Gasbarrini, A.; et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology 2006, 130, 2060–2073. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Purushothaman, M.; Purushothaman, K.R. Plaque neovascularization: Defense mechanisms, betrayal, or a war in progress. Ann. N. Y. Acad. Sci. 2012, 1254, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Schicho, R.; Marsche, G.; Storr, M. Cardiovascular complications in inflammatory bowel disease. Curr. Drug Targets 2015, 16, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Rastelli, S.; Inserra, G.; Lentini, P.; Valvo, E.; Calcagno, E.; Boutouyrie, P.; Laurent, S.; Castellino, P. Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs. Atherosclerosis 2014, 234, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Chidlow, J.H., Jr.; Shukla, D.; Grisham, M.B.; Kevil, C.G. Pathogenic angiogenesis in IBD and experimental colitis: New ideas and therapeutic avenues. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G5–G18. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, J.; Vilovic, M.; Zivkovic, P.M.; Tadin Hadjina, I.; Rusic, D.; Bukic, J.; Borovac, J.A.; Bozic, J. Mediterranean Diet Adherence and Dietary Attitudes in Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 3429. [Google Scholar] [CrossRef] [PubMed]
- Olejar, K.J.; Ricci, A.; Swift, S.; Zujovic, Z.; Gordon, K.C.; Fedrizzi, B.; Versari, A.; Kilmartin, P.A. Characterization of an Antioxidant and Antimicrobial Extract from Cool Climate, White Grape Marc. Antioxidants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, C.; Pinto, L.; Baruzzi, F.; Giovinazzo, G. Comparison of Antibacterial and Antioxidant Properties of Red (cv. Negramaro) and White (cv. Fiano) Skin Pomace Extracts. Molecules 2021, 26, 5918. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—a review. Int. J. Food Sci. Tech. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Gerardi, C.; D’Amico, L.; Migoni, D.; Santino, A.; Salomone, A.; Carluccio, M.A.; Giovinazzo, G. Strategies for Reuse of Skins Separated From Grape Pomace as Ingredient of Functional Beverages. Front. Bioeng. Biotechnol. 2020, 8, 645. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; D’Acuna, S.; Perez, D.; Dicenta, S.; Echeverria, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Costabile, G.; Vitale, M.; Luongo, D.; Naviglio, D.; Vetrani, C.; Ciciola, P.; Tura, A.; Castello, F.; Mena, P.; Del Rio, D.; et al. Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: A pilot study. Clin. Nutr. 2019, 38, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Pellegrino, M.; Storelli, C.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Multiple anti-inflammatory and anti-atherosclerotic properties of red wine polyphenolic extracts: Differential role of hydroxycinnamic acids, flavonols and stilbenes on endothelial inflammatory gene expression. Eur. J. Nutr. 2016, 55, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Pellegrino, M.; Ingrosso, I.; Giovinazzo, G.; Carluccio, M.A. Red Grape Skin Polyphenols Blunt Matrix Metalloproteinase-2 and -9 Activity and Expression in Cell Models of Vascular Inflammation: Protective Role in Degenerative and Inflammatory Diseases. Molecules 2016, 21, 1147. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Romero, S.; Martinez-Maqueda, D.; Hereu, M.; Amezqueta, S.; Torres, J.L.; Perez-Jimenez, J. Modifications of Gut Microbiota after Grape Pomace Supplementation in Subjects at Cardiometabolic Risk: A Randomized Cross-Over Controlled Clinical Trial. Foods 2020, 9, 1279. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. In Vitro 2010, 24, 1441–1449. [Google Scholar] [CrossRef]
- Grootaert, C.; Kamiloglu, S.; Capanoglu, E.; Van Camp, J. Cell Systems to Investigate the Impact of Polyphenols on Cardiovascular Health. Nutrients 2015, 7, 9229–9255. [Google Scholar] [CrossRef]
- Magalhaes, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L. Rapid microplate high-throughput methodology for assessment of Folin-Ciocalteu reducing capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Melis, M.P.; Corona, G.; Deiana, M. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites: Insight into the mechanism of action. Food Chem. Toxicol. 2019, 125, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxid. Med. Cell Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Maaser, C.; Schoeppner, S.; Kucharzik, T.; Kraft, M.; Schoenherr, E.; Domschke, W.; Luegering, N. Colonic epithelial cells induce endothelial cell expression of ICAM-1 and VCAM-1 by a NF-kappaB-dependent mechanism. Clin. Exp. Immunol. 2001, 124, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Asseburg, H.; Dold, S.; Rompp, A.; Frohling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Toaldo, I.M.; Van Camp, J.; Gonzales, G.B.; Kamiloglu, S.; Bordignon-Luiz, M.T.; Smagghe, G.; Raes, K.; Capanoglu, E.; Grootaert, C. Resveratrol improves TNF-alpha-induced endothelial dysfunction in a coculture model of a Caco-2 with an endothelial cell line. J. Nutr. Biochem. 2016, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A. Transcription factor NF-kappa B as a potential biomarker for oxidative stress. Br. J. Nutr. 2001, 86, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lomillo, J.; Gonzalez-SanJose, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Tech. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.L. Characterisation of Mediterranean grape pomace seed and skin extracts: Polyphenolic content and antioxidant activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.R.; Wallace, J.L. Gastrointestinal inflammation: A central component of mucosal defense and repair. Exp. Biol. Med. 2006, 231, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Saredy, J.; Yang, W.L.Y.; Sun, Y.; Lu, Y.F.; Saaoud, F.; Drummer, C.; Johnson, C.; Xu, K.M.; Jiang, X.H.; et al. Vascular Endothelial Cells and Innate Immunity. Arterioscl. Throm. Vas. 2020, 40, E138–E152. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory bowel disease: Mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Spalinger, M.R.; Gottier, C.; Biedermann, L.; Zeitz, J.; Lang, S.; Weber, A.; Rogler, G.; Scharl, M. Bilberry-Derived Anthocyanins Modulate Cytokine Expression in the Intestine of Patients with Ulcerative Colitis. PLoS ONE 2016, 11, e0154817. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Thangaraj, P.; Lima, B.D.; Trindade, G.G.G.; Narain, N.; Silva, A.M.D.E.; Santin, J.R.; Broering, M.F.; Serafini, M.R.; Quintans, L.J.; et al. Protective effects of flavonoid composition rich P. subpeltata Ortega. on indomethacin induced experimental ulcerative colitis in rat models of inflammatory bowel diseases. J. Ethnopharmacol. 2020, 248, 112350. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Chieppa, M.; Santino, A. Plant Polyphenols-Biofortified Foods as a Novel Tool for the Prevention of Human Gut Diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- McCormack, G.; Moriarty, D.; O’Donoghue, D.P.; McCormick, P.A.; Sheahan, K.; Baird, A.W. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm. Res. 2001, 50, 491–495. [Google Scholar] [CrossRef]
- Mudter, J.; Neurath, M.F. Il-6 signaling in inflammatory bowel disease: Pathophysiological role and clinical relevance. Inflamm. Bowel Dis. 2007, 13, 1016–1023. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol. Asp. Med. 2005, 26, 379–390. [Google Scholar] [CrossRef]
- Garg, P.; Vijay-Kumar, M.; Wang, L.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G175–184. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Pender, S.L.; Jackson, C.L.; Prothero, J.D.; Gordon, J.N.; Picariello, L.; Rovedatti, L.; Docena, G.; Monteleone, G.; Rampton, D.S.; et al. Functional modulation of Crohn’s disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 2007, 133, 137–149. [Google Scholar] [CrossRef]
- Wang, S.; Mateos, R.; Goya, L.; Amigo-Benavent, M.; Sarria, B.; Bravo, L. A phenolic extract from grape by-products and its main hydroxybenzoic acids protect Caco-2 cells against pro-oxidant induced toxicity. Food Chem. Toxicol. 2016, 88, 65–74. [Google Scholar] [CrossRef]
- Carullo, G.; Governa, P.; Spizzirri, U.G.; Biagi, M.; Sciubba, F.; Giorgi, G.; Loizzo, M.R.; Di Cocco, M.E.; Aiello, F.; Restuccia, D. Sangiovese cv Pomace Seeds Extract-Fortified Kefir Exerts Anti-Inflammatory Activity in an In Vitro Model of Intestinal Epithelium Using Caco-2 Cells. Antioxidants 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: Effects in Caco-2 cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Martins, I.M.; Roberto, B.S.; Blumberg, J.B.; Chen, C.O.; Macedo, G.A. Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res. Int. 2016, 89, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients 2020, 12, 1623. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.T.Y.; Tang, Q.; Xue, C.; Li, R.W.; Wu, V.C.H. Malvidin 3-Glucoside Modulated Gut Microbial Dysbiosis and Global Metabolome Disrupted in a Murine Colitis Model Induced by Dextran Sulfate Sodium. Mol. Nutr. Food Res. 2019, 63, e1900455. [Google Scholar] [CrossRef] [PubMed]
- Noe, V.; Penuelas, S.; Lamuela-Raventos, R.M.; Permanyer, J.; Ciudad, C.J.; Izquierdo-Pulido, M. Epicatechin and a cocoa polyphenolic extract modulate gene expression in human Caco-2 cells. J. Nutr. 2004, 134, 2509–2516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Effect of Different Anthocyanidin Glucosides on Lutein Uptake by Caco-2 Cells, and Their Combined Activities on Anti-Oxidation and Anti-Inflammation In Vitro and Ex Vivo. Molecules 2018, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Sang, L.X.; Jiang, M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Romier, B.; Schneider, Y.J.; Larondelle, Y.; During, A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr. Rev. 2009, 67, 363–378. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharm. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Lingua, M.S.; Theumer, M.G.; Kruzynski, P.; Wunderlin, D.A.; Baroni, M.V. Bioaccessibility of polyphenols and antioxidant properties of the white grape by simulated digestion and Caco-2 cell assays: Comparative study with its winemaking product. Food Res. Int. 2019, 122, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Grootaert, C.; Capanoglu, E.; Ozkan, C.; Smagghe, G.; Raes, K.; Van Camp, J. Anti-inflammatory potential of black carrot (Daucus carota L.) polyphenols in a co-culture model of intestinal Caco-2 and endothelial EA.hy926 cells. Mol. Nutr. Food Res. 2017, 61, 1600455. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Cimino, F.; Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Busa, R.; Saija, A.; Speciale, A. Cyanidin-3-O-Glucoside Modulates the In Vitro Inflammatory Crosstalk between Intestinal Epithelial and Endothelial Cells. Mediat. Inflamm. 2017, 2017, 3454023. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Saiko, P.; Szakmary, A.; Jaeger, W.; Szekeres, T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. 2008, 658, 68–94. [Google Scholar] [CrossRef]
- Menet, M.C.; Cottart, C.H.; Taghi, M.; Nivet-Antoine, V.; Dargere, D.; Vibert, F.; Laprevote, O.; Beaudeux, J.L. Ultra high performance liquid chromatography-quadrupole-time of flight analysis for the identification and the determination of resveratrol and its metabolites in mouse plasma. Anal. Chim. Acta 2013, 761, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Barrington, R.; Williamson, G.; Bennett, R.N.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, Conjugation and Efflux of the Flavonoids, Kaempferol and Galangin, Using the Intestinal CACO-2/TC7 Cell Model. J. Funct. Foods 2009, 1, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, H.; Jana, S. Evaluation of physicochemical properties and intestinal permeability of six dietary polyphenols in human intestinal colon adenocarcinoma Caco-2 cells. Eur. J. Drug Metab. Pharm. 2016, 41, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Oliveira, H.; Bras, N.F.; Fernandes, I.; Cruz, L.; De Freitas, V.; Mateus, N. In vitro gastrointestinal absorption of red wine anthocyanins—Impact of structural complexity and phase II metabolization. Food Chem. 2020, 317, 126398. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Accession Number | Forward Primer | Reverse Primer | Size (bp) |
|---|---|---|---|---|
| IL1B | NM_000576.2 | 5′-CTGTCCTGCGTGTTGAAAGA-3′ | 5′-AGTTATATCCTGGCCGCCTT-3′ | 228 |
| IL6 | NM_000600.3 | 5′-AGGAGACTTGCCTGGTGAAA-3′ | 5′-CAGGGGTGGTTATTGCATCT-3′ | 180 |
| TNF | NM_000594.2 | 5′-CCTGTGAGGAGGACGAACAT-3′ | 5′-AGGCCCCAGTTTGAATTCTT-3′ | 240 |
| CXCL10 | NM_001565.2 | 5′-CAAGGATGGACCACACAGAG-3′ | 5′-GCAGGGTCAGAACATCCACT-3′ | 248 |
| CCL2/MCP1 | NM_002982.3 | 5′-CCCCAGTCACCTGCTGTTAT-3′ | 5′-TCCTGAACCCACTTCTGCTT-3′ | 166 |
| CSF1/MCSF | NM_000757.4 | 5′-TGGACGCACAGAACAGTCTC-3′ | 5′-CCTCCAGGGCTCACAATAAA-3′ | 235 |
| PTGS2/COX-2 | NM_000963.2 | 5′-TGCTGTGGAGCTGTATCCTG-3′ | 5′-GAAACCCACTTCTCCACCA-3′ | 176 |
| VCAM1 | NM_00107B.3 | 5′-CATGGAATTCGAACCCAAAC-3′ | 5′-CCTGGCTCAAGCATGTCATA-3′ | 140 |
| ICAM1 | NM_000201.2 | 5′-AGACATAGCCCCACCATGAG-3′ | 5′-CAAGGGTTGGGGTCAGTAGA-3′ | 190 |
| MMP9 | NM_004994.2 | 5′-AAAGCCTATTTCTGCCAGGAC-3′ | 5′-GTGGGGATTTACATGGCACT-3′ | 157 |
| MMP2 | NM_004530.4 | 5′-CACTTTCCTGGGCAACAAAT-3′ | 5′-TGATGTCATCCTGGGACAGA-3′ | 257 |
| TIMP1 | NM_003254.2 | 5′-TGACATCCGGTTCGTCTACA-3′ | 5′-CTGCAGTTTTCCAGCAATGA-3′ | 103 |
| TIMP2 | NM_003255.4 | 5′-CCAAGCAGGAGTTTCTCGAC-3′ | 5′-TTTCCAGGAAGGGATGTCAG-3′ | 121 |
| GAPDH | NM_002046.3 | 5′-ATCACTGCCACCCAGAAGAC-3′ | 5′-TTCTAGACGGCAGGTCAGGT-3′ | 210 |
| 18 ribosomal RNA | NR_003286.2 | 5′-AAACGGCTACCACATCCAAG-3′ | 5′-CCTCCAATGGATCCTCGTTA-3′ | 155 |
| TEAC | ORAC | TP |
|---|---|---|
| µmolTE/g | µmolTE/g | mgGAE/g |
| 794.98 ± 39.75 | 801.99 ± 40.09 | 61.40 ± 1.03 |
| Gene Name | LPS/TNF-α | GPE + LPS/TNF-α |
|---|---|---|
| IL1B | 9.3 ± 0.8 | 7.5 ± 0.7 * |
| TNF | 4.3 ± 0.7 | 0.9 ± 0.6 ** |
| CXCL10 | 14.6 ± 1.2 | 5.9 ± 0.8 ** |
| CSF1/MCSF | 4.0 ± 0.6 | 1.6 ± 0.7 ** |
| PTGS2/COX-2 | 3.8 ± 0.9 | 1.7 ± 0.6 * |
| VCAM1 | 15.1 ± 2.3 | 2.3 ± 0.8 ** |
| ICAM1 | 1.56 ± 0.3 | 1.0 ± 0.2 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients 2022, 14, 1175. https://doi.org/10.3390/nu14061175
Calabriso N, Massaro M, Scoditti E, Verri T, Barca A, Gerardi C, Giovinazzo G, Carluccio MA. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients. 2022; 14(6):1175. https://doi.org/10.3390/nu14061175
Chicago/Turabian StyleCalabriso, Nadia, Marika Massaro, Egeria Scoditti, Tiziano Verri, Amilcare Barca, Carmela Gerardi, Giovanna Giovinazzo, and Maria Annunziata Carluccio. 2022. "Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation" Nutrients 14, no. 6: 1175. https://doi.org/10.3390/nu14061175
APA StyleCalabriso, N., Massaro, M., Scoditti, E., Verri, T., Barca, A., Gerardi, C., Giovinazzo, G., & Carluccio, M. A. (2022). Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients, 14(6), 1175. https://doi.org/10.3390/nu14061175