Characterization of Vitamin D Status in Older Persons with Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Frailty Index
2.3. Vitamin D
2.4. Statistical Analysis
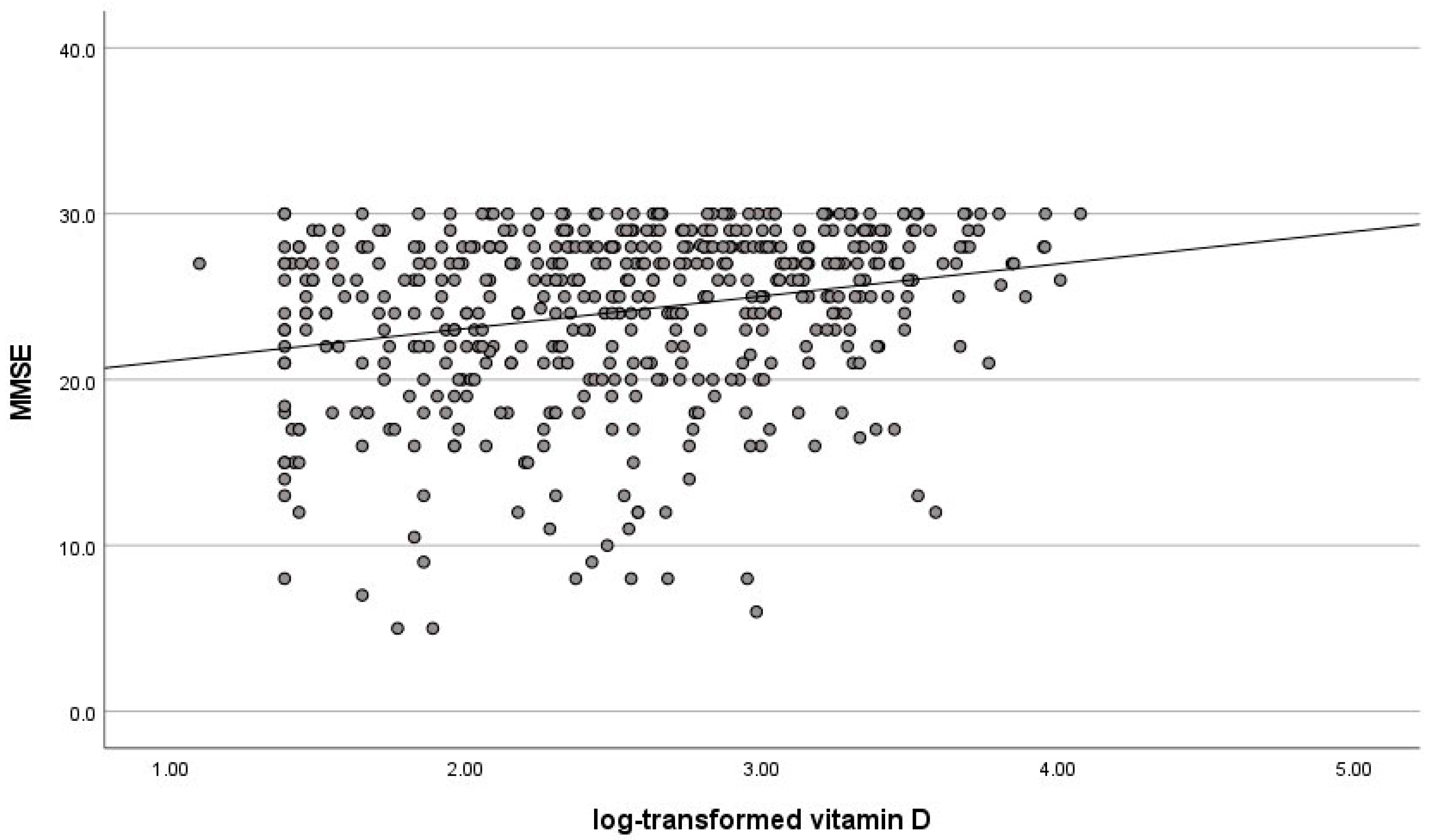
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vitale, G.; Salvioli, S.; Franceschi, C. Oxidative stress and the ageing endocrine system. Nat. Rev. Endocrinol. 2013, 9, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Lanske, B.; Razzaque, M.S. Vitamin D and aging: Old concepts and new insights. J. Nutr. Biochem. 2007, 18, 771–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arosio, B.; Guerini, F.R.; Costa, A.S.; Dicitore, A.; Ferri, E.; Mari, D.; Torresani, E.; Clerici, M.; Cesari, M.; Vitale, G. Vitamin D receptor polymorphisms in sex-frailty paradox. Nutrients 2020, 12, 2714. [Google Scholar] [CrossRef]
- Buchebner, D.; Bartosch, P.; Malmgren, L.; McGuigan, F.E.; Gerdhem, P.; Akesson, K.E. Association between vitamin D, frailty, and progression of frailty in community-dwelling older women. J. Clin. Endocrinol. Metab. 2019, 104, 6139–6147. [Google Scholar] [CrossRef] [Green Version]
- Kamwa, V.; Welch, C.; Hassan-Smith, Z.K. The endocrinology of sarcopenia and frailty. Minerva Endocrinol. 2020. [Google Scholar] [CrossRef]
- Spira, D.; Buchmann, N.; Konig, M.; Rosada, A.; Steinhagen-Thiessen, E.; Demuth, I.; Norman, K. Sex-specific differences in the association of vitamin D with low lean mass and frailty: Results from the Berlin aging study II. Nutrition 2019, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Frias-Toral, E.; Pugliese, G.; Garcia-Velasquez, E.; Carignano, M.D.L.A.; Savastano, S.; Colao, A.; Muscogiuri, G. Vitamin D in obesity and obesity-related diseases: An overview. Minerva Endocrinol. 2021, 46, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Bellia, C.; Iacolino, G.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Non-skeletal activities of vitamin D: From physiology to brain pathology. Medicina 2019, 55, 341. [Google Scholar] [CrossRef] [Green Version]
- Calsolaro, V.; Bottari, M.; Coppini, G.; Lemmi, B.; Monzani, F. Endocrine dysfunction and cognitive impairment. Minerva Endocrinol. 2021, 46, 335–349. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Beck, J.; da Silva Teixeira, S.; Harrison, K.; Xu, Y.; Sisley, S. Defining vitamin D receptor expression in the brain using a novel VDR(Cre) mouse. J. Comp. Neurol. 2021, 529, 2362–2375. [Google Scholar] [CrossRef]
- Sutherland, M.K.; Somerville, M.J.; Yoong, L.K.; Bergeron, C.; Haussler, M.R.; McLachlan, D.R. Reduction of vitamin D hormone receptor mRNA levels in alzheimer as compared to Huntington hippocampus: Correlation with calbindin-28k mRNA levels. Brain Res. Mol. Brain Res. 1992, 13, 239–250. [Google Scholar] [CrossRef]
- Harms, L.R.; Burne, T.H.; Eyles, D.W.; McGrath, J.J. Vitamin D and the brain. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Gussago, C.; Arosio, B.; Guerini, F.R.; Ferri, E.; Costa, A.S.; Casati, M.; Bollini, E.M.; Ronchetti, F.; Colombo, E.; Bernardelli, G.; et al. Impact of vitamin D receptor polymorphisms in centenarians. Endocrine 2016, 53, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Fernandes de Abreu, D.A.; Eyles, D.; Feron, F. Vitamin D, a neuro-immunomodulator: Implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S265–S277. [Google Scholar] [CrossRef]
- Eyles, D.; Almeras, L.; Benech, P.; Patatian, A.; Mackay-Sim, A.; McGrath, J.; Feron, F. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J. Steroid Biochem. Mol. Biol. 2007, 103, 538–545. [Google Scholar] [CrossRef]
- Grecksch, G.; Ruthrich, H.; Hollt, V.; Becker, A. Transient prenatal vitamin D deficiency is associated with changes of synaptic plasticity in the dentate gyrus in adult rats. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S258–S264. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Agnello, L.; Lo Sasso, B.; Scazzone, C.; Butera, D.; Gambino, C.M.; Iacolino, G.; Bellia, C.; Ciaccio, M. Vitamin D in malaria: More hypotheses than clues. Heliyon 2019, 5, e01183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcion, E.; Sindji, L.; Leblondel, G.; Brachet, P.; Darcy, F. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J. Neurochem. 1999, 73, 859–866. [Google Scholar] [CrossRef]
- Garcion, E.; Sindji, L.; Montero-Menei, C.; Andre, C.; Brachet, P.; Darcy, F. Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia 1998, 22, 282–294. [Google Scholar] [CrossRef]
- Bivona, G.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V.; Scazzone, C.; Agnello, L.; Ciaccio, M. The role of vitamin D as a biomarker in alzheimer’s disease. Brain Sci. 2021, 11, 334. [Google Scholar] [CrossRef]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Jimenez-Castillo, G.A.; Bracho-Vela, L.A.; Yeverino-Castro, S.G.; Avila-Funes, J.A. Association of vitamin D with mild cognitive impairment and alzheimer’s dementia in older Mexican adults. Rev. Investig. Clin. 2019, 71, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Buell, J.S.; Dawson-Hughes, B.; Scott, T.M.; Weiner, D.E.; Dallal, G.E.; Qui, W.Q.; Bergethon, P.; Rosenberg, I.H.; Folstein, M.F.; Patz, S.; et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 2010, 74, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Feart, C.; Helmer, C.; Merle, B.; Herrmann, F.R.; Annweiler, C.; Dartigues, J.F.; Delcourt, C.; Samieri, C. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and alzheimer’s disease in older adults. Alzheimers Dement. 2017, 13, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Licher, S.; de Bruijn, R.; Wolters, F.J.; Zillikens, M.C.; Ikram, M.A.; Ikram, M.K. Vitamin D and the risk of dementia: The rotterdam study. J. Alzheimers Dis. 2017, 60, 989–997. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Karakis, I.; Pase, M.P.; Beiser, A.; Booth, S.L.; Jacques, P.F.; Rogers, G.; DeCarli, C.; Vasan, R.S.; Wang, T.J.; Himali, J.J.; et al. Association of serum vitamin D with the risk of incident dementia and subclinical indices of brain aging: The framingham heart study. J. Alzheimers Dis. 2016, 51, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Chon, J.; Kim, Y.; Seo, Y.K.; Park, E.J.; Won, C.W.; Soh, Y. Association between vitamin D deficiency and cognitive function in the elderly Korean population: A Korean frailty and aging cohort study. Medicine 2020, 99, e19293. [Google Scholar] [CrossRef]
- Wilson, V.K.; Houston, D.K.; Kilpatrick, L.; Lovato, J.; Yaffe, K.; Cauley, J.A.; Harris, T.B.; Simonsick, E.M.; Ayonayon, H.N.; Kritchevsky, S.B.; et al. Relationship between 25-hydroxyvitamin D and cognitive function in older adults: The health, aging and body composition study. J. Am. Geriatr. Soc. 2014, 62, 636–641. [Google Scholar] [CrossRef] [Green Version]
- Kuzma, E.; Soni, M.; Littlejohns, T.J.; Ranson, J.M.; van Schoor, N.M.; Deeg, D.J.; Comijs, H.; Chaves, P.H.; Kestenbaum, B.R.; Kuller, L.H.; et al. Vitamin D and memory decline: Two population-based prospective studies. J. Alzheimers Dis. 2016, 50, 1099–1108. [Google Scholar] [CrossRef] [Green Version]
- Breitling, L.P.; Perna, L.; Muller, H.; Raum, E.; Kliegel, M.; Brenner, H. Vitamin D and cognitive functioning in the elderly population in Germany. Exp. Gerontol. 2012, 47, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Perna, L.; Mons, U.; Kliegel, M.; Brenner, H. Serum 25-hydroxyvitamin D and cognitive decline: A longitudinal study among non-demented older adults. Dement. Geriatr. Cogn. Disord. 2014, 38, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, S.; Hofer, E.; Meinitzer, A.; Fritz-Petrin, E.; Simstich, S.; Goessler, W.; Schmidt, R.; Herrmann, M. Association of vitamin D metabolites with cognitive function and brain atrophy in elderly individuals—The Austrian stroke prevention study. Aging 2021, 13, 9455–9467. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Sasso, B.L.; Iacolino, G.; Gambino, C.M.; Scazzone, C.; Agnello, L.; Ciaccio, M. Standardized measurement of circulating vitamin D [25(OH)D] and its putative role as a serum biomarker in alzheimer’s disease and parkinson’s disease. Clin. Chim. Acta 2019, 497, 82–87. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-mental state examination: A normative study in Italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Graf, C. The lawton instrumental activities of daily living scale. Am. J. Nurs. 2008, 108, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Marmarou, A.; Black, P.; Bergsneider, M.; Klinge, P.; Relkin, N. Guidelines for management of idiopathic normal pressure hydrocephalus: Progress to date. Acta Neurochir. Suppl. 2005, 95, 237–240. [Google Scholar] [CrossRef]
- Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010, 120, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwood, K.; Song, X.; Mitnitski, A. Changes in relative fitness and frailty across the adult lifespan: Evidence from the Canadian national population health survey. CMAJ 2011, 183, E487–E494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogeser, M.; Kyriatsoulis, A.; Huber, E.; Kobold, U. Candidate reference method for the quantification of circulating 25-hydroxyvitamin D3 by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2004, 50, 1415–1417. [Google Scholar] [CrossRef] [Green Version]
- Phinney, K.W. Development of a standard reference material for vitamin D in serum. Am. J. Clin. Nutr. 2008, 88, 511S–512S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, S.; Taimuri, U.; Basnan, S.A.; Ai-Orabi, W.K.; Awadallah, A.; Almowald, F.; Hazazi, A. Low vitamin D and its association with cognitive impairment and dementia. J. Aging Res. 2020, 2020, 6097820. [Google Scholar] [CrossRef]
- Graf, C.E.; Rossi, C.; Giannelli, S.V.; Nobari, B.H.; Gold, G.; Herrmann, F.R.; Zekry, D. Vitamin D is not associated with cognitive status in a cohort of very old hospitalized patients. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S53–S61. [Google Scholar] [CrossRef]
- Knekt, P.; Saaksjarvi, K.; Jarvinen, R.; Marniemi, J.; Mannisto, S.; Kanerva, N.; Heliovaara, M. Serum 25-hydroxyvitamin d concentration and risk of dementia. Epidemiology 2014, 25, 799–804. [Google Scholar] [CrossRef]
- Lefevre-Arbogast, S.; Dhana, K.; Aggarwal, N.T.; Zhang, S.; Agarwal, P.; Liu, X.; Laranjo, N.; Carey, V.; Sacks, F.; Barnes, L.L.; et al. Vitamin D intake and brain cortical thickness in community-dwelling overweight older adults: A cross-sectional study. J. Nutr. 2021, 151, 2760–2767. [Google Scholar] [CrossRef]
- Schneider, A.L.; Lutsey, P.L.; Alonso, A.; Gottesman, R.F.; Sharrett, A.R.; Carson, K.A.; Gross, M.; Post, W.S.; Knopman, D.S.; Mosley, T.H.; et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: The ARIC brain MRI study. Eur. J. Neurol. 2014, 21, 1211-e70. [Google Scholar] [CrossRef] [Green Version]
- Bartali, B.; Devore, E.; Grodstein, F.; Kang, J.H. Plasma vitamin D levels and cognitive function in aging women: The nurses’ health study. J. Nutr. Health Aging 2014, 18, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, L.; Houston, D.K.; Wilson, V.K.; Lovato, J.; Ayonayon, H.N.; Cauley, J.A.; Harris, T.; Simonsick, E.M.; Yaffe, K.; Kritchevsky, S.B.; et al. Low 25-hydroxyvitamin D concentrations and risk of incident cognitive impairment in black and white older adults: The health ABC study. J. Nutr. Gerontol. Geriatr. 2018, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.J.; Lang, I.A.; Langa, K.M.; Muniz-Terrera, G.; Phillips, C.L.; Cherubini, A.; Ferrucci, L.; Melzer, D. Vitamin D and risk of cognitive decline in elderly persons. Arch. Intern. Med. 2010, 170, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Slinin, Y.; Paudel, M.; Taylor, B.C.; Ishani, A.; Rossom, R.; Yaffe, K.; Blackwell, T.; Lui, L.Y.; Hochberg, M.; Ensrud, K.E. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1092–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slinin, Y.; Paudel, M.L.; Taylor, B.C.; Fink, H.A.; Ishani, A.; Canales, M.T.; Yaffe, K.; Barrett-Connor, E.; Orwoll, E.S.; Shikany, J.M.; et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology 2010, 74, 33–41. [Google Scholar] [CrossRef]
- Toffanello, E.D.; Coin, A.; Perissinotto, E.; Zambon, S.; Sarti, S.; Veronese, N.; De Rui, M.; Bolzetta, F.; Corti, M.C.; Crepaldi, G.; et al. Vitamin D deficiency predicts cognitive decline in older men and women: The pro.V.A. study. Neurology 2014, 83, 2292–2298. [Google Scholar] [CrossRef]
- van Schoor, N.M.; Comijs, H.C.; Llewellyn, D.J.; Lips, P. Cross-sectional and longitudinal associations between serum 25-hydroxyvitamin D and cognitive functioning. Int. Psychogeriatr. 2016, 28, 759–768. [Google Scholar] [CrossRef]
- Malabanan, A.; Veronikis, I.E.; Holick, M.F. Redefining vitamin D insufficiency. Lancet 1998, 351, 805–806. [Google Scholar] [CrossRef]
- Burke, W.J.; Houston, M.J.; Boust, S.J.; Roccaforte, W.H. Use of the geriatric depression scale in dementia of the alzheimer type. J. Am. Geriatr. Soc. 1989, 37, 856–860. [Google Scholar] [CrossRef]
- Gilley, D.W.; Wilson, R.S. Criterion-related validity of the geriatric depression scale in alzheimer’s disease. J. Clin. Exp. Neuropsychol. 1997, 19, 489–499. [Google Scholar] [CrossRef]
- Perrotte, M.; Le Page, A.; Fournet, M.; Le Sayec, M.; Rassart, E.; Fulop, T.; Ramassamy, C. Blood-based redox-signature and their association to the cognitive scores in MCI and alzheimer’s disease patients. Free Radic. Biol. Med. 2019, 130, 499–511. [Google Scholar] [CrossRef]
- Ferri, E.; Casati, M.; Cesari, M.; Vitale, G.; Arosio, B. Vitamin D in physiological and pathological aging: Lesson from centenarians. Rev. Endocr. Metab. Disord. 2019, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Lim, L.L.; Yee, A.; Loh, H.S.; Vethakkan, S.R. Effect of vitamin D replacement in primary hyperparathyroidism with concurrent vitamin D deficiency: A systematic review and meta-analysis. Minerva Endocrinol. 2019, 44, 221–231. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Hammami, S.; Zarrouk, A.; Piron, C.; Almas, I.; Sakly, N.; Latteur, V. Prevalence and factors associated with frailty in hospitalized older patients. BMC Geriatr. 2020, 20, 144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Liu, L.K.; Chen, W.T.; Lee, W.J.; Peng, L.N.; Wang, P.N.; Chen, L.K. Cognitive function in individuals with physical frailty but without dementia or cognitive complaints: Results from the I-lan longitudinal aging study. J. Am. Med. Dir. Assoc. 2015, 16, 899.e9–899.e16. [Google Scholar] [CrossRef]
- Wyskida, M.; Wieczorowska-Tobis, K.; Chudek, J. Prevalence and factors promoting the occurrence of vitamin D deficiency in the elderly. Postepy Hig. I Med. Dosw. Online 2017, 71, 198–204. [Google Scholar] [CrossRef]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of vitamin D in atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef] [Green Version]
- Perez, L.; Heim, L.; Sherzai, A.; Jaceldo-Siegl, K. Nutrition and vascular dementia. J. Nutr. Health Aging 2012, 16, 319–324. [Google Scholar] [CrossRef]
| Deficit | |
|---|---|
| Biochemical Parameters | |
| Cholesterol | ≤200 mg/dL |
| CRP | <0.5 mg/dL |
| Vitamin B12 | 191–663 ng/L |
| Folate | 4.6–18.7 µg/L |
| TSH | 0.28–4.30 mIU/L |
| Signs | |
| Pain | |
| Bowel incontinence | |
| Sleep disorders | |
| BMI | 21–30 kg/m2 |
| Edema | |
| Tremor | |
| Disabilities | |
| Mobility impairment | |
| ADL-disability in self-feeding | |
| ADL-disability in dressing | |
| ADL-disability in bathing | |
| ADL-disability in transferring | |
| ADL-disability in toileting | |
| ADL-incontinence | |
| IADL-disability in using telephone | |
| IADL-disability in shopping | |
| IADL-disability in food preparation | |
| IADL-disability in housekeeping | |
| IADL-disability in doing laundry | |
| IADL-disability in travelling by car or public transportation | |
| IADL-disability in medication use | |
| IADL-disability in handling finances | |
| Diseases | |
| Hypertension | |
| Diabetes | |
| Congestive heart failure | |
| Coronary heart disease | |
| Cardiac arrhythmia | |
| Chronic obstructive pulmonary disease | |
| Decreased visual acuity | |
| Hearing loss | |
| Osteoarthritis | |
| Vascular endothelial abnormalities | |
| Chronic renal insufficiency | |
| Hepatopathy | |
| Depression | |
| Cerebrovascular disease | |
| Cancer | |
| Osteoporosis | |
| Anemia | |
| Diverticulosis | |
| Mean (SD) | Median (IQR) | |
|---|---|---|
| Age | 79 (5) | |
| Education (years) | 9.4 (4.6) | |
| MMSE score | 24 (5) | |
| ADL score | 5.0 (1.3) | |
| IADL score | 5.1 (2.4) | |
| GDS | 11 (6) | |
| FI | 0.27 (0.21–0.36) | |
| Vitamin D (ng/mL) | 13 (8–21) |
| Controls (n 115) | AD (n 59) | MCI (n 176) | iNPH (n 26) | MD (n 133) | p | |
|---|---|---|---|---|---|---|
| Age | 80 (6) | 78 (5) | 78 (5) | 81 (6) | 80 (5) | <0.001 |
| Education (years) | 10.1 (4.5) | 8.7 (4.8) | 10.3 (4.6) | 10.5 (4.4) | 7.9 (4.0) | <0.001 |
| MMSE | 27 (4) | 21 (5) | 27 (2) | 21 (5) | 20 (5) | <0.001 |
| ADL | 5.2 (1.0) | 5.1 (1.3) | 5.4 (0.8) | 4.0 (1.8) | 4.5 (1.5) | <0.001 |
| IADL | 5.9 (2.3) | 4.9 (2.6) | 5.9 (1.9) | 3.3 (1.9) | 3.8 (2.3) | <0.001 |
| GDS | 12.5 (6.6) | 7.8 (4.6) | 10.5 (5.7) | 12.4 (4.8) | 14.4 (7.0) | <0.001 |
| FI | 0.24 (0.17–0.32) | 0.25 (0.19–0.33) | 0.25 (0.19–0.31) | 0.34 (0.26–0.41) | 0.34 (0.24–0.42) | <0.001 |
| Vitamin D (ng/mL) | 12 (7–22) | 12 (8–20) | 17 (10–25) | 13 (5–19) | 10 (6–16) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arosio, B.; Rossi, P.D.; Ferri, E.; Cesari, M.; Vitale, G. Characterization of Vitamin D Status in Older Persons with Cognitive Impairment. Nutrients 2022, 14, 1142. https://doi.org/10.3390/nu14061142
Arosio B, Rossi PD, Ferri E, Cesari M, Vitale G. Characterization of Vitamin D Status in Older Persons with Cognitive Impairment. Nutrients. 2022; 14(6):1142. https://doi.org/10.3390/nu14061142
Chicago/Turabian StyleArosio, Beatrice, Paolo Dionigi Rossi, Evelyn Ferri, Matteo Cesari, and Giovanni Vitale. 2022. "Characterization of Vitamin D Status in Older Persons with Cognitive Impairment" Nutrients 14, no. 6: 1142. https://doi.org/10.3390/nu14061142
APA StyleArosio, B., Rossi, P. D., Ferri, E., Cesari, M., & Vitale, G. (2022). Characterization of Vitamin D Status in Older Persons with Cognitive Impairment. Nutrients, 14(6), 1142. https://doi.org/10.3390/nu14061142








