Vitamin D Deficiency in Cushing’s Disease: Before and After Its Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Assays
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muscogiuri, G.; Altieri, B.; Annweiler, C.; Balercia, G.; Pal, H.B.; Boucher, B.J.; Cannell, J.J.; Foresta, C.; Grübler, M.R.; Kotsa, K.; et al. Vitamin D and chronic diseases: The current state of the art. Arch. Toxicol. 2017, 91, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Misra, M. Extra-skeletal effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, A.; Arefi, M. Molecular evidence of role of vitamin D deficiency in various extraskeletal diseases. J. Cell. Biochem. 2019, 120, 8829–8840. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action-addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef]
- Pivonello, R.; Isidori, A.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.K.; Colao, A. Complications of Cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef]
- Guarnotta, V.; Ferrigno, R.; Martino, M.; Barbot, M.; Isidori, A.M.; Scaroni, C.; Ferrante, A.; Arnaldi, G.; Pivonello, R.; Giordano, C. Glucocorticoid excess and COVID-19 disease. Rev. Endocr. Metab. Disord. 2020, 22, 703–714. [Google Scholar] [CrossRef]
- Giordano, C.; Guarnotta, V.; Pivonello, R.; Amato, M.C.; Simeoli, C.; Ciresi, A.; Cozzolino, A.; Colao, A. Is diabetes in Cushing’s syndrome only a consequence of hypercortisolism? Eur. J. Endocrinol. 2014, 170, 311–319. [Google Scholar] [CrossRef]
- Drey, M.; Berr, C.M.; Reincke, M.; Fazel, J.; Seissler, J.; Schopohl, J.; Bidlingmaier, M.; Zopp, S.; Reisch, N.; Beuschlein, F.; et al. Cushing′s syndrome: A model for sarcopenic obesity. Endocrine 2017, 57, 481–485. [Google Scholar] [CrossRef]
- Guarnotta, V.; Prinzi, A.; Pitrone, M.; Pizzolanti, G.; Giordano, C. Circulating irisin levels as a marker of osteosarcopenic-obesity in Cushing’s disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1565–1574. [Google Scholar] [CrossRef]
- Hakami, O.A.; Ahmed, S.; Karavitaki, N. Epidemiology and mortality of Cushing′s syndrome. Best Pr. Res. Clin. Endocrinol. Metab. 2021, 35, 101521. [Google Scholar] [CrossRef]
- Javanmard, P.; Duan, D.; Geer, E.B. Mortality in patients with endogenous Cushing′s Syndrome. Endocrinol. Metab. Clin. North Am. 2018, 47, 313–333. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.I.; Cidlowski, J.A. Physiologic and pharmacologic effects of corticosteroids. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock., R.E., Weichselbaum, R.R., Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Tirabassi, G.; Salvio, G.; Altieri, B.; Ronchi, C.L.; Della Casa, S.; Pontecorvi, A.; Balercia, G. Adrenal disorders: Is there any role for Vitamin D? Rev. Endocr. Metab. Disord. 2016, 18, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Skversky, A.L.; Kumar, J.; Abramowitz, M.K.; Kaskel, F.J.; Melamed, M.L. Association of glucocorticoid use and low 25-Hydroxyvitamin D levels: Results from the National Health and Nutrition Examination Survey (NHANES): 2001–2006. J. Clin. Endocrinol. Metab. 2011, 96, 3838–3845. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Altieri, B.; Penna-Martinez, M.; Badenhoop, K. Focus on Vitamin D and the Adrenal Gland. Horm. Metab. Res. 2015, 47, 239–246. [Google Scholar] [CrossRef]
- Davidson, Z.E.; Walker, K.Z.; Truby, H. Clinical review: Do Glucocorticosteroids alter Vitamin D status? A systematic review with meta-analyses of observational studies. J. Clin. Endocrinol. Metab. 2012, 97, 738–744. [Google Scholar] [CrossRef]
- Hidalgo, A.A.; Trump, D.L.; Johnson, C.S. Glucocorticoid regulation of the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2010, 121, 372–375. [Google Scholar] [CrossRef]
- Hidalgo, A.A.; Deeb, K.K.; Pike, J.W.; Johnson, C.S.; Trump, D.L. Dexamethasone enhances 1α,25-Dihydroxyvitamin D3 effects by increasing Vitamin D receptor transcription. J. Biol. Chem. 2011, 286, 36228–36237. [Google Scholar] [CrossRef]
- Favus, M.J.; Kimberg, D.V.; Millar, G.N.; Gershon, E. Effects of cortisone administration on the metabolism and localization of 25-Hydroxycholecalciferol in the rat. J. Clin. Investig. 1973, 52, 1328–1335. [Google Scholar] [CrossRef][Green Version]
- Kugai, N.; Koide, Y.; Yamashita, K.; Shimauchi, T.; Nagata, N.; Takatani, O. Impaired mineral metabolism in Cushing’s syndrome: Parathyroid function, vitamin D metabolites and osteopenia. Endocrinol. Jpn. 1986, 33, 345–352. [Google Scholar] [CrossRef]
- Aloia, J.F.; Roginsky, M.; Ellis, K.; Shukla, K.; Cohn, S. Skeletal metabolism and body composition in Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 1974, 39, 981–985. [Google Scholar] [CrossRef]
- Findling, J.W.; Adams, N.D.; Lemann, J., Jr.; Gray, R.W.; Thomas, C.J.; Tyrrell, J.B. Vitamin D metabolites and parathyroid hormone in Cushing’s Syndrome: Relationship to calcium and phosphorus homeostasis. J. Clin. Endocrinol. Metab. 1982, 54, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Kumar, R.; Hunder, G.G.; Scott, M.; Heath, H., 3rd; Riggs, B.L. Production, degradation, and circulating levels of 1,25-dihydroxyvitamin D in health and in chronic glucocorticoid excess. J. Clin. Investig. 1980, 66, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.G.; Arnaud, S.B.; Gallagher, J.C.; DeLuca, H.F.; Riggs, B.L. Intestinal calcium absorption in exogenous Hypercortisonism. J. Clin. Investig. 1977, 60, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Chaiamnuay, S.; Chailurkit, L.-O.; Narongroeknawin, P.; Asavatanabodee, P.; Laohajaroensombat, S.; Chaiamnuay, P. Current daily glucocorticoid use and serum creatinine levels are associated with lower 25(OH) Vitamin D levels in Thai patients with systemic lupus erythematosus. JCR J. Clin. Rheumatol. 2013, 19, 121–125. [Google Scholar] [CrossRef]
- Slovik, D.M.; Neer, R.M.; Ohman, J.L.; Lowell, F.C.; Clark, M.B.; Segre, G.V.; Potts, J.T., Jr. Parathyroid hormone and 25-hydroxyvitamin D levels in glucocorticoid-treated patients. Clin. Endocrinol. 1980, 12, 243–248. [Google Scholar] [CrossRef]
- Lindgren, J.U.; Merchant, C.R.; DeLuca, H.F. Effect of 1,25-dihydroxyvitamin D3 on osteopenia induced by prednisolone in adult rats. Calcif. Tissue Res. 1982, 34, 253–257. [Google Scholar] [CrossRef]
- Corbee, R.; Tryfonidou, M.; Meij, B.; Kooistra, H.; Hazewinkel, H. Vitamin D status before and after hypophysectomy in dogs with pituitary-dependent hypercortisolism. Domest. Anim. Endocrinol. 2012, 42, 43–49. [Google Scholar] [CrossRef]
- Park, J.E.; Pichiah, P.T.; Cha, Y.-S. Vitamin D and metabolic diseases: Growing roles of Vitamin D. J. Obes. Metab. Syndr. 2018, 27, 223–232. [Google Scholar] [CrossRef]
- Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J.A. Vitamin D: Effect on Haematopoiesis and immune system and clinical applications. Int. J. Mol. Sci. 2018, 19, 2663. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V. The diagnosis of Cushing’s Syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Guarnotta, V.; Amato, M.C.; Pivonello, R.; Arnaldi, G.; Ciresi, A.; Trementino, L.; Citarrella, R.; Iacuaniello, D.; Michetti, G.; Simeoli, C.; et al. The degree of urinary hypercortisolism is not correlated with the severity of cushing’s syndrome. Endocrine 2016, 55, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of Vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Fiebrich, H.-B.; Berg, G.V.D.; Kema, I.P.; Links, T.P.; Kleibeuker, J.H.; Van Beek, A.P.; Walenkamp, A.M.E.; Sluiter, W.J.; De Vries, E.G.E. Deficiencies in fat-soluble vitamins in long-term users of somatostatin analogue. Aliment. Pharmacol. Ther. 2010, 32, 1398–1404. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Prigeon, R.L.; Faulenbach, M.V.; Tong, J.; Carr, D.B.; Boyko, E.J.; Leonetti, D.L.; McNeely, M.J.; Fujimoto, W.Y.; Kahn, S.E. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009, 32, 335–341. [Google Scholar] [CrossRef]
- Glendenning, P.; Zhu, K.; Inderjeeth, C.; Howat, P.; Lewis, J.R.; Prince, R.L. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: A randomized controlled trial. J. Bone Miner. Res. 2011, 27, 170–176. [Google Scholar] [CrossRef]
- Kearns, M.D.; Alvarez, J.A.; Tangpricha, V. Large, single-dose, oral Vitamin D supplementation in adult populations: A systematic review. Endocr. Pract. 2014, 20, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, J.; Norlin, M.; Wikvall, K. 1α,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells. Biochim. Biophys. Acta 2010, 1801, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.-S.; Morisset, A.-S.; Carreau, A.-M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: A randomised, placebo-controlled trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tong, C.H.; Rowland, C.M.; Radcliff, J.; Bare, L.A.; McPhaul, M.J.; Devlin, J.J. Association of changes in lipid levels with changes in vitamin D levels in a real-world setting. Sci. Rep. 2021, 11, 21536. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Povaliaeva, A.; Bogdanov, V.; Pigarova, E.; Zhukov, A.; Dzeranova, L.; Belaya, Z.; Rozhinskaya, L.; Mel’Nichenko, G.; Mokrysheva, N. Assessment of Vitamin D metabolism in patients with Cushing’s disease in response to 150,000 IU cholecalciferol treatment. Nutrients 2021, 13, 4329. [Google Scholar] [CrossRef]
| Controls | Cushing’s Disease | p | |
|---|---|---|---|
| (No. = 48) | (No. = 50) | ||
| Subjects (%) | Subjects (%) | ||
| Gender | |||
| Male | 9 (18.7%) | 7 (14%) | 0.475 |
| Female | 39 (81.3%) | 43 (86%) | |
| Arterial hypertension | 18 (37.5%) | 32 (64%) | 0.009 |
| Osteoporosis/osteopenia | 7 (14.6%) | 21 (42%) | 0.002 |
| Visceral obesity | 38 (79.1%) | 44 (88%) | 0.224 |
| Metabolic syndrome | 19 (39.6%) | 29 (58%) | 0.069 |
| Hypercholesterolemia | 14 (29.1%) | 30 (60%) | 0.002 |
| Hypertriglyceridemia | 11 (22.9%) | 13 (26%) | 0.486 |
| Low HDL | 14 (29.1%) | 19 (38%) | 0.361 |
| Cardiovascular disease | 0 | 5 (10%) | 0.118 |
| Peripheral vascular disease | 0 | 1 (2%) | 0.489 |
| Diabetes mellitus | 6 (12.5%) | 24 (48%) | 0.026 |
| IFG | 0 | 6 (12%) | 0.622 |
| IGT | 6 (12.5%) | 7 (14%) | 0.678 |
| IFG + IGT | 1 (2%) | 3 (6%) | 0.457 |
| Moon face | 24 (50%) | 33 (66%) | 0.108 |
| Myopathy | 12 (25%) | 36 (72%) | <0.001 |
| Facial rubor | 9 (18.7%) | 23 (46%) | 0.005 |
| Buffalo hump | 17 (35.4%) | 33 (66%) | 0.002 |
| Purple striae | 11 (22.9%) | 15 (30%) | 0.245 |
| Hypovitaminosis D | |||
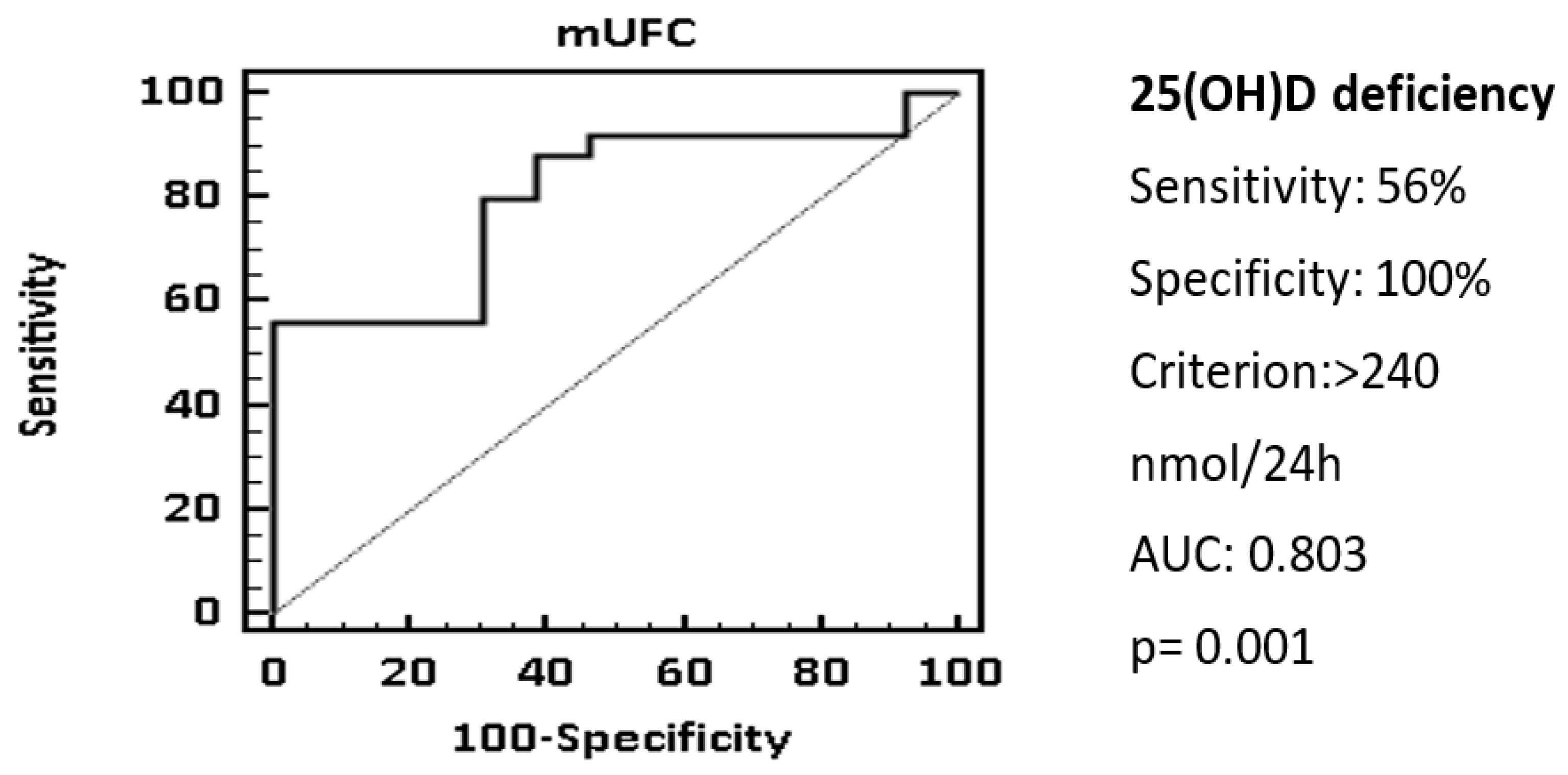
| Deficiency | 4 (8.4%) | 26 (52%) | 0.001 |
| Insufficiency | 10 (20.8%) | 14 (28%) | 0.545 |
| Sufficiency | 34 (70.8%) | 10 (20%) | 0.004 |
| Controls Baseline (No. = 48) | Cushing’s Disease Baseline (No. = 50) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (yrs) | 48.2 ± 13.4 | 50.9 ± 17.4 | 0.815 |
| Anthropometric parameters | |||
| BMI (kg/m2) | 31.9 ± 5.01 | 33.1 ± 6.41 | 0.321 |
| Waist circumference (cm) | 105.4 ± 12.7 | 110.7 ± 8.97 | 0.031 |
| Metabolic parameters | |||
| Creatinine (mg/dL) | 0.78 ± 0.25 | 0.81 ± 0.31 | 0.601 |
| Calcium (mg/dL) | 9.43 ± 0.46 | 9.46 ± 0.61 | 0.841 |
| Phosphorus (mg/dL) | 3.83 ± 0.67 | 3.46 ± 0.54 | 0.125 |
| Parathyroid hormone (pg/mL) | 33.8 ± 8.03 | 54.1 ± 22.7 | 0.003 |
| 25(OH)D (ng/mL) | 28.7 ± 8.49 | 16.7 ± 8.18 | <0.001 |
| Glycaemia (mmol/L) | 4.97 ± 2.77 | 6.66 ± 2.19 | 0.010 |
| HbA1c (%) | 5.79 ± 0.73 | 6.73 ± 1.09 | 0.004 |
| Total cholesterol (mmol/L) | 4.51 ± 0.82 | 5.34 ± 1.07 | <0.001 |
| HDL cholesterol (mmol/L) | 1.15 ± 0.29 | 1.19 ± 0.45 | 0.184 |
| Triglycerides (mmol/L) | 1.66 ± 0.43 | 1.73 ± 0.67 | 0.585 |
| LDL cholesterol (mmol/L) | 2.62 ± 0.91 | 3.31 ± 0.99 | 0.002 |
| HOMA-IR | 3.07 ± 1.01 | 4.67 ± 2.83 | 0.051 |
| ISI-Matsuda | 4.14 ± 1.59 | 3.02 ± 2.18 | 0.007 |
| Oral disposition index | 3.75 ± 0.54 | 2.25 ± 2.04 | 0.003 |
| Hormonal parameters | |||
| ACTH (pmol/L) | 7.72 ± 2.19 | 15.1 ± 6.56 | <0.001 |
| Mean urinary free cortisol (nmol/24 h) | 310.2 ± 104.1 | 604.7 ± 65.6 | 0.001 |
| Cortisol after low dose of dexamethasone suppression test (nmol/L) | 44.4 ± 11.5 | 361.4 ± 98.4 | 0.001 |
| Cushing’s Disease (No. = 50) | p | ||
|---|---|---|---|
| Baseline | Six Weeks After Cholecalciferol | ||
| Mean ± SD | Mean ± SD | ||
| Anthropometric parameters | |||
| BMI (kg/m2) | 33.1 ± 6.41 | 32.9 ± 7.43 | 0.880 |
| Waist circumference (cm) | 110.7 ± 8.97 | 109.8 ± 7.08 | 0.586 |
| Metabolic parameters | |||
| Creatinine (mg/dL) | 0.81 ± 0.32 | 0.78 ± 0.26 | 0.615 |
| Calcium (mg/dL) | 9.46 ± 0.61 | 9.75 ± 0.56 | 0.017 |
| Phosphorus (mg/dL) | 3.46 ± 0.54 | 3.54 ± 0.43 | 0.424 |
| Parathyroid hormone (pg/mL) | 54.1 ± 22.7 | 40.5 ± 11.5 | 0.004 |
| 25(OH)D (ng/mL) | 16.7 ± 8.18 | 30.7 ± 9.65 | <0.001 |
| Glycaemia (mmol/L) | 6.66 ± 2.19 | 6.02 ± 1.65 | 0.109 |
| Total cholesterol (mmol/L) | 5.34 ± 1.07 | 4.87 ± 0.81 | 0.017 |
| HDL cholesterol (mmol/L) | 1.19 ± 0.45 | 1.21 ± 0.38 | 0.465 |
| Triglycerides (mmol/L) | 1.73 ± 0.67 | 1.68 ± 0.41 | 0.660 |
| LDL cholesterol (mmol/L) | 3.31 ± 0.99 | 2.98 ± 0.75 | 0.068 |
| HOMA-IR | 4.67 ± 2.83 | 3.97 ± 2.02 | 0.166 |
| ISI-Matsuda | 3.02 ± 2.18 | 3.76 ± 1.12 | 0.035 |
| Oral disposition index | 2.25 ± 2.04 | 2.97 ± 1.89 | 0.045 |
| Hormonal parameters | |||
| ACTH (pmol/L) | 15.1 ± 6.56 | 14.3 ± 6.36 | 0.519 |
| Mean urinary free cortisol (nmol/24 h) | 604.7 ± 65.6 | 582.5 ± 54.9 | 0.075 |
| Cortisol after low dose of dexamethasone suppression test (nmol/L) | 361.4 ± 98.4 | 363.9 ± 89.6 | 0.895 |
| 25(OH)D | ||||
|---|---|---|---|---|
| Cushing’s Disease | Controls | |||
| r | p | r | p | |
| Glycaemia (mmol/L) | −0.385 | 0.019 | −0.737 | 0.097 |
| HbA1c (%) | −0.391 | 0.017 | 0.213 | 0.355 |
| BMI (kg/m2) | −0.221 | 0.189 | 0.007 | 0.976 |
| WC (cm) | −0.373 | 0.023 | −0.130 | 0.042 |
| ACTH (pmol/L) | −0.133 | 0.440 | −0.198 | 0.567 |
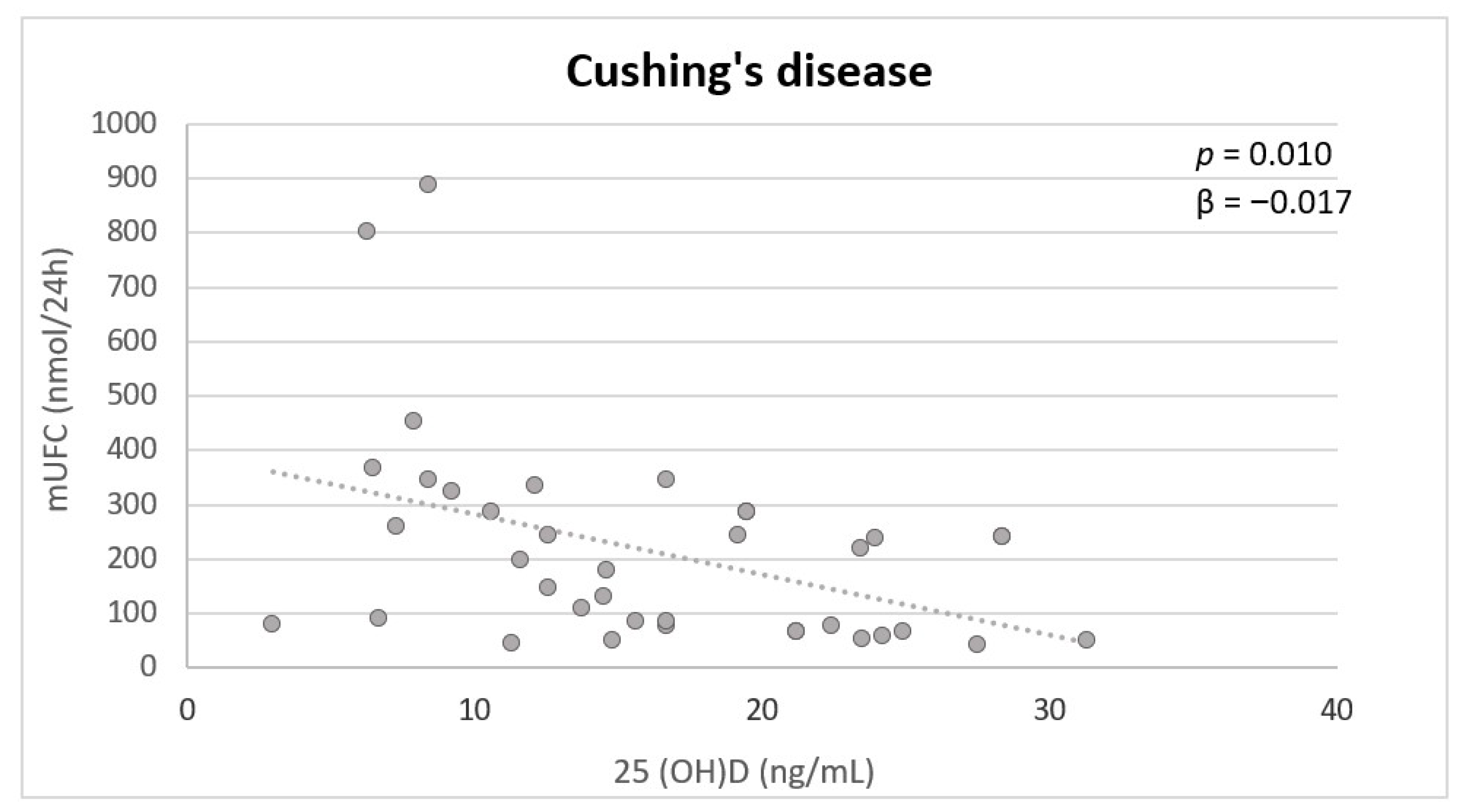
| Urinary free cortisol (nmol/24 h) | −0.466 | 0.033 | 0.040 | 0.862 |
| Cortisol after low dose of dexamethasone suppression test (nmol/L) | −0.299 | 0.049 | 0.260 | 0.255 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarnotta, V.; Di Gaudio, F.; Giordano, C. Vitamin D Deficiency in Cushing’s Disease: Before and After Its Supplementation. Nutrients 2022, 14, 973. https://doi.org/10.3390/nu14050973
Guarnotta V, Di Gaudio F, Giordano C. Vitamin D Deficiency in Cushing’s Disease: Before and After Its Supplementation. Nutrients. 2022; 14(5):973. https://doi.org/10.3390/nu14050973
Chicago/Turabian StyleGuarnotta, Valentina, Francesca Di Gaudio, and Carla Giordano. 2022. "Vitamin D Deficiency in Cushing’s Disease: Before and After Its Supplementation" Nutrients 14, no. 5: 973. https://doi.org/10.3390/nu14050973
APA StyleGuarnotta, V., Di Gaudio, F., & Giordano, C. (2022). Vitamin D Deficiency in Cushing’s Disease: Before and After Its Supplementation. Nutrients, 14(5), 973. https://doi.org/10.3390/nu14050973






