Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways
Abstract
1. Introduction
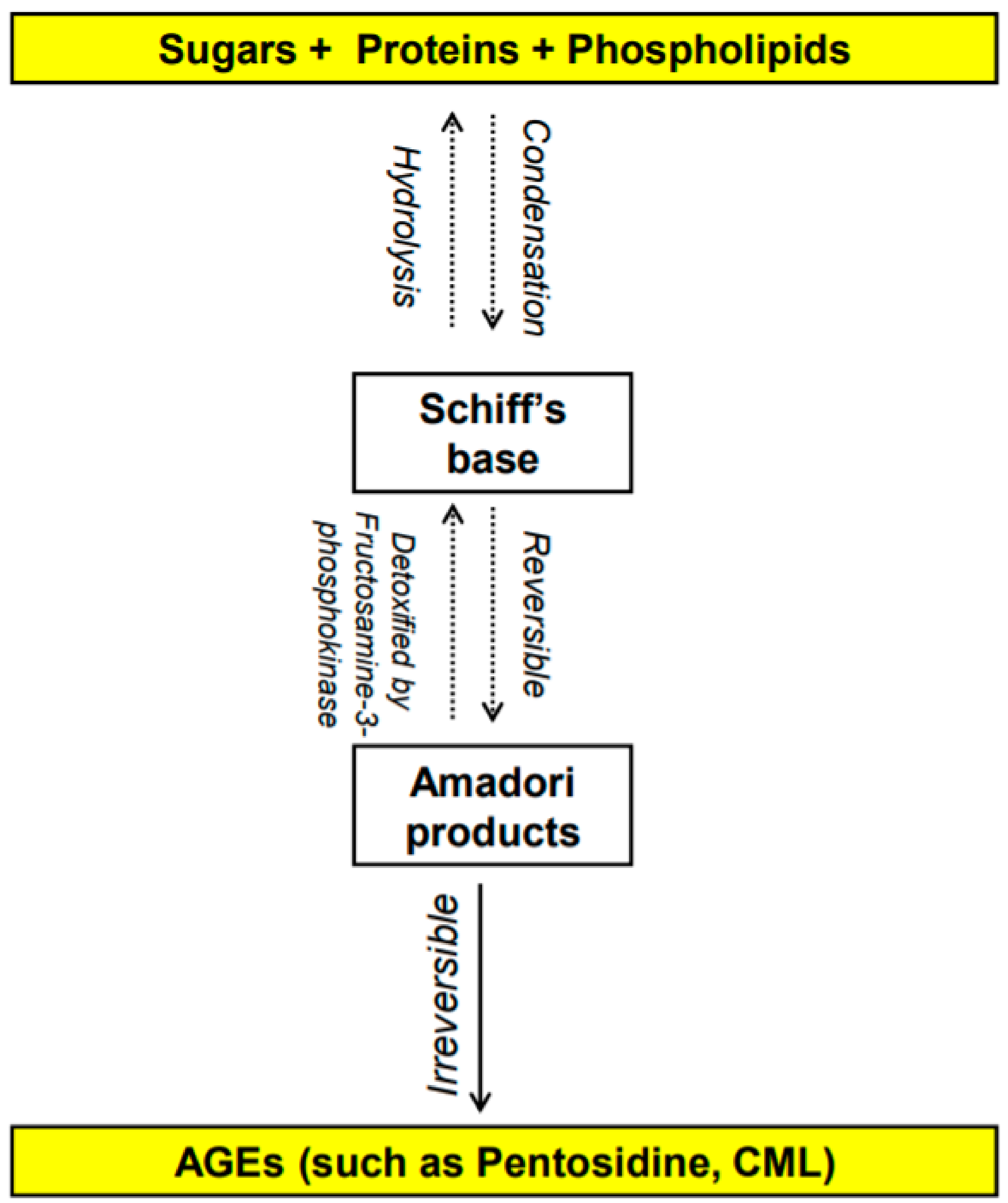
1.1. What Are AGEs? How Do They Form?
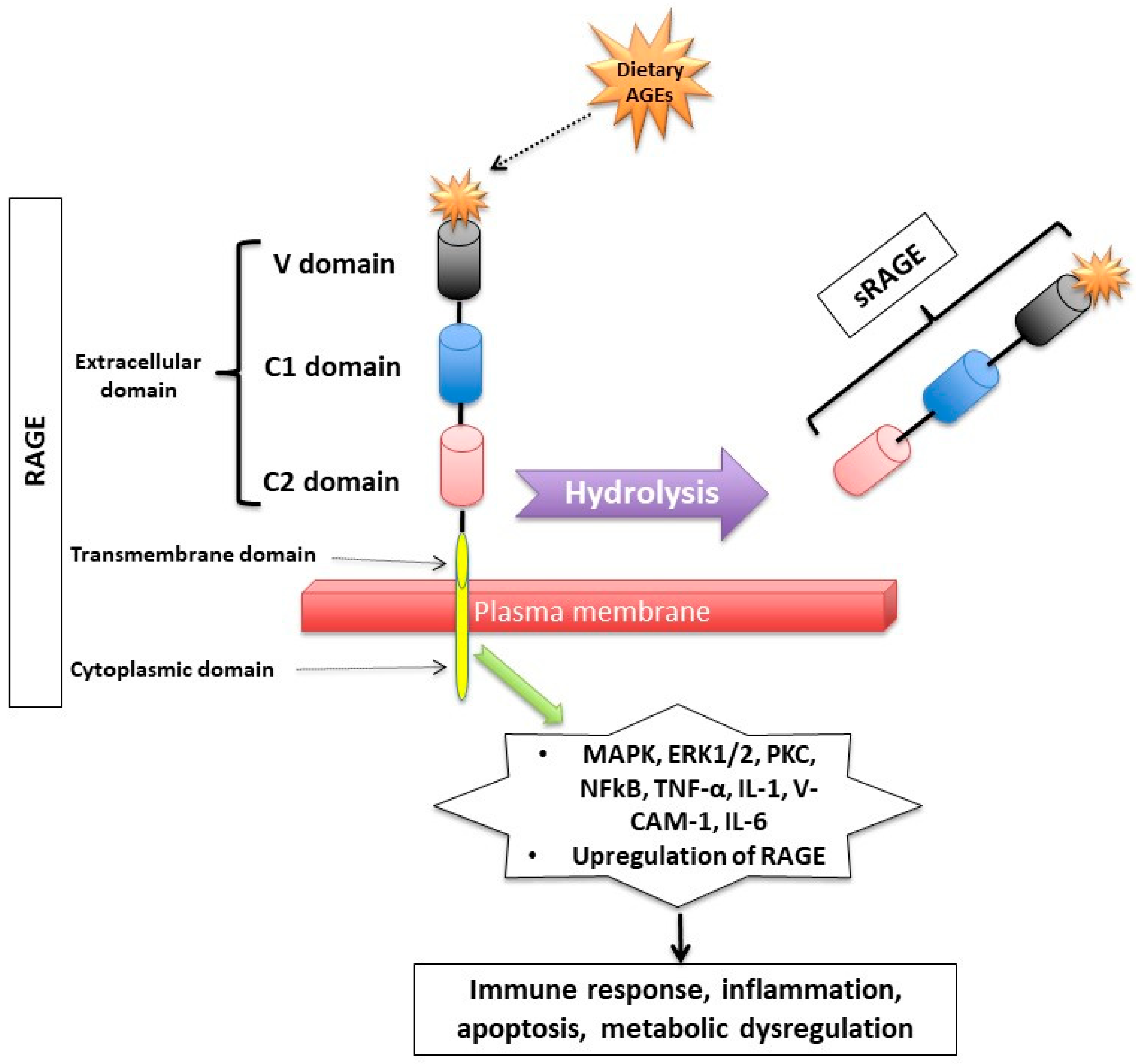
1.2. How Do Dietary AGEs Act?
1.3. How Are Dietary AGEs Cleared from the Body?
2. Polycystic Ovary Syndrome (PCOS) and Dietary AGEs
3. Ovarian Dysfunction
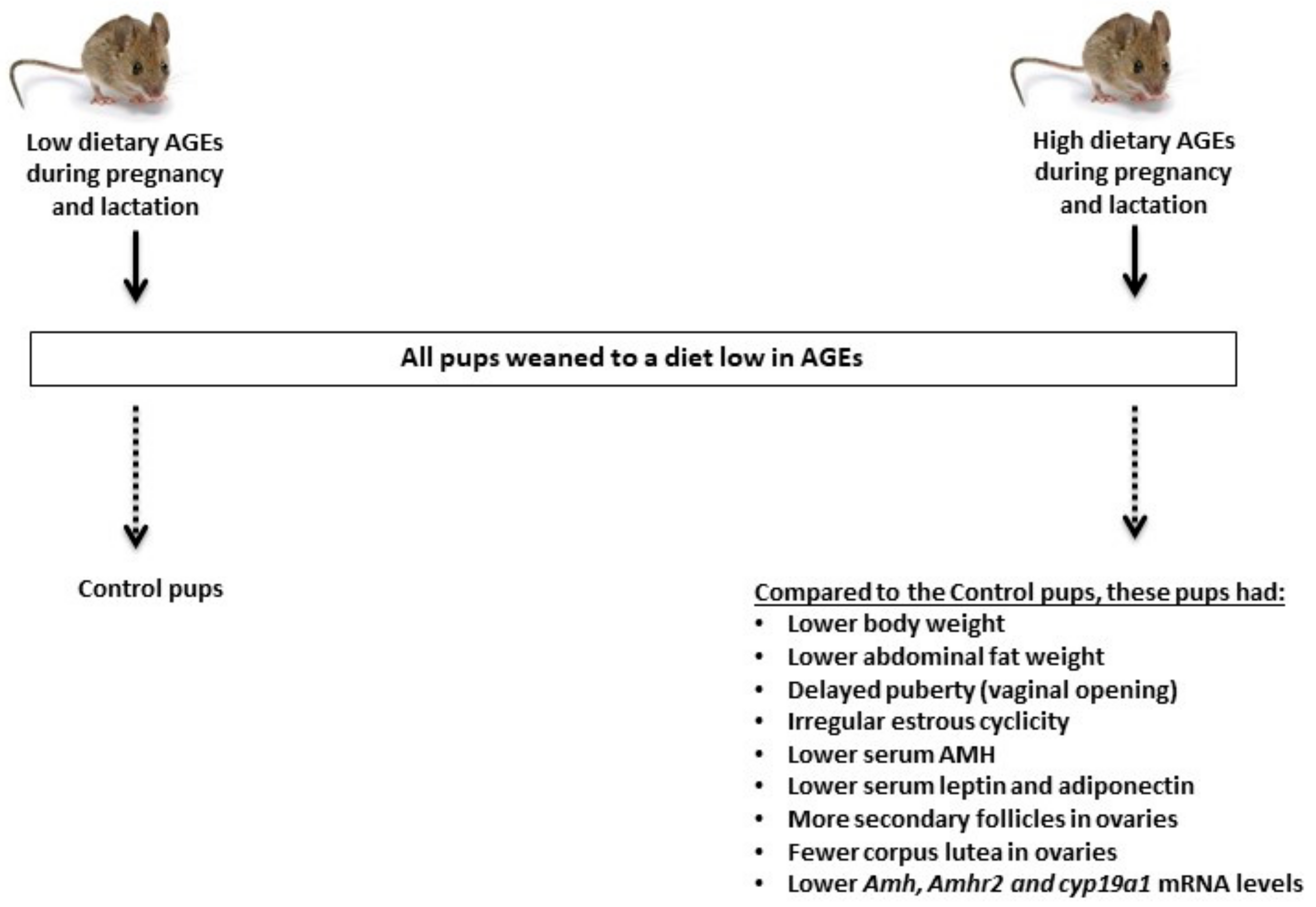
4. Perinatal Exposure to Elevated Dietary AGEs and Reproduction in Female Offspring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moschonas, D.P.; Piperi, C.; Korkolopoulou, P.; Levidou, G.; Kavantzas, N.; Trigka, E.-A.; Vlachos, I.; Arapostathi, C.; Perrea, D.; Mitropoulos, D.; et al. Impact of diet-induced obesity in male mouse reproductive system: The role of advanced glycation end product–receptor for advanced glycation end product axis. Exp. Biol. Med. 2014, 239, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Maillard, L.C. Action des acides aminés sur les sucres; formation des méla-noidines par voie methodique. Comptes Rendus Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Unoki, H.; Yamagishi, S. Advanced glycation end products and insulin resistance. Curr. Pharm. Des. 2008, 14, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar] [CrossRef]
- Brownlee, M. Glycosylation products as toxic mediators of diabetic complications. Annu. Rev. Med. 1991, 42, 159–166. [Google Scholar] [CrossRef]
- Bucala, R.; Cerami, A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Stud. Surf. Sci. Catal. 1992, 23, 1–34. [Google Scholar] [CrossRef]
- Monnier, V.M.; Stevens, V.J.; Cerami, A. Maillard reactions involving proteins and carbohydrates in vivo: Relevance to diabetes mellitus and aging. Prog. Food Nutr. Sci. 1981, 5, 315–327. [Google Scholar]
- Yamagishi, S.-I.; Nakamura, K.; Imaizumi, T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr. Diabetes Rev. 2005, 1, 93–106. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Patsouris, E.; Korkolopoulou, P.; Panidis, D.; Pawelczyk, L.; Papavassiliou, A.G.; Duleba, A.J. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem. Cell Biol. 2007, 127, 581–589. [Google Scholar] [CrossRef]
- Tatone, C.; Amicarelli, F. The aging ovary—the poor granulosa cells. Fertil. Steril. 2013, 99, 12–17. [Google Scholar] [CrossRef]
- Pertynska-Marczewska, M.; Merhi, Z. Relationship of advanced glycation end products with cardiovascular disease in menopausal women. Reprod. Sci. 2015, 22, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z. Advanced glycation end-products: Pathway of potentially significant pathophysiological and therapeutic relevance for metabolic syndrome in menopausal women. J. Clin. Endocrinol. Metab. 2014, 99, 1146–1148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khalifah, R.G.; Baynes, J.W.; Hudson, B. Amadorins: Novel post-amadori inhibitors of advanced glycation reactions. Biochem. Biophys. Res. Commun. 1999, 257, 251–258. [Google Scholar] [CrossRef]
- Inagi, R. Inhibitors of advanced glycation and endoplasmic reticulum stress. Methods Enzymol. 2011, 491, 361–380. [Google Scholar] [CrossRef]
- Piperi, C.; Adamopoulos, C.; Dalagiorgou, G.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab. 2012, 97, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Piperi, C.; Korkolopoulou, P.; Kandaraki, E.; Levidou, G.; Papalois, A.; Patsouris, E.; Papavassiliou, A.G. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. Klin. Wochenschr. 2007, 85, 1413–1420. [Google Scholar] [CrossRef]
- Takahashi, M.; Oikawa, M.; Nagano, A. Effect of age and menopause on serum concentrations of pentosidine, an advanced glycation end product. J. Gerontol. Ser. A 2000, 55, M137–M140. [Google Scholar] [CrossRef]
- Merhi, Z. Advanced glycation end products and their relevance in female reproduction. Hum. Reprod. 2014, 29, 135–145. [Google Scholar] [CrossRef]
- Desai, K.; Wu, L. Methylglyoxal and advanced glycation endproducts: New therapeutic horizons? Recent patents on cardiovascular drug discovery. Recent Pat. Cardiovasc. Drug Discov. (Discontin.) 2007, 2, 89–99. [Google Scholar] [CrossRef]
- Kerkeni, M.; Saïdi, A.; Bouzidi, H.; Ben Yahya, S.; Hammami, M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc. Res. 2012, 84, 378–383. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Bone quality. Nihon Rinsho 2015, 73, 1665–1672. [Google Scholar] [PubMed]
- Saeki, C.; Saito, M.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Saruta, M.; Tsubota, A. Plasma pentosidine levels are associated with prevalent fractures in patients with chronic liver disease. PLoS ONE 2021, 16, e0249728. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.A.; Elewa, A.; Arafa, L.F. Pentosidine and N-Carboxymethyl-Lysine: Biomarkers for Type 2 diabetic retinopathy. Eur. J. Ophthalmol. 2011, 21, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-i reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J. Biol. Chem. 2011, 286, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, L.; Zhang, R.; Liu, G.; Xiao, S.; Qiao, X.; Wu, Y.; Gong, Z. Toxicological evaluation of advanced glycation end product Nε-(carboxymethyl)lysine: Acute and subacute oral toxicity studies. Regul. Toxicol. Pharmacol. 2016, 77, 65–74. [Google Scholar] [CrossRef]
- Jud, P.; Sourij, H. Therapeutic options to reduce advanced glycation end products in patients with diabetes mellitus: A review. Diabetes Res. Clin. Pract. 2019, 148, 54–63. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.-J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef]
- Tantalaki, E.; Piperi, C.; Livadas, S.; Kollias, A.; Adamopoulos, C.; Koulouri, A.; Christakou, C.; Diamanti-Kandarakis, E. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones 2014, 13, 65–73. [Google Scholar] [CrossRef]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Schmidt, A.M.; Hudson, B.I. RAGE: A novel biological and genetic marker for vascular disease. Clin. Sci. 2009, 116, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Kasteler, S.D.; Cosio, M.G.; Sturrock, A.; Huecksteadt, T.; Hoidal, J.R. RAGE: Developmental expression and positive feedback regulation by Egr-1 during cigarette smoke exposure in pulmonary epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2008, 294, L1094–L1101. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Nakatsuka, M.; Chekir, C.; Noguchi, S.; Kamada, Y.; Sasaki, A.; Hiramatsu, Y. Advanced glycation end products induce secretion of chemokines and apoptosis in human first trimester trophoblasts. Hum. Reprod. 2004, 19, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Basta, G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Fujii, E.Y.; Nakayama, M. The measurements of RAGE, VEGF, and AGEs in the plasma and follicular fluid of reproductive women: The influence of aging. Fertil. Steril. 2010, 94, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular interplay between glycation, inflammation, and hallmarks of cancer—A review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef] [PubMed]
- Kandaraki, E.A.; Chatzigeorgiou, A.; Papageorgiou, E.; Piperi, C.; Adamopoulos, C.; Papavassiliou, A.G.; Koutsilieris, M.; Diamanti-Kandarakis, E. Advanced glycation end products interfere in luteinizing hormone and follicle stimulating hormone signaling in human granulosa KGN cells. Exp. Biol. Med. 2017, 243, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Manna, S.K. Advanced Glycation End Products (AGE) potently induce autophagy through activation of RAF protein kinase and nuclear factor κB (NF-κB). J. Biol. Chem. 2016, 291, 1481–1491. [Google Scholar] [CrossRef]
- Guimarães, E.L.; Empsen, C.; Geerts, A.; van Grunsven, L.A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010, 52, 389–397. [Google Scholar] [CrossRef]
- Dunaif, A.; Segal, K.R.; Futterweit, W.; Dobrjansky, A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989, 38, 1165–1174. [Google Scholar] [CrossRef]
- Ramasamy, R.; Yan, S.F.; D’Agati, V.; Schmidt, A.M. Receptor for Advanced Glycation Endproducts (RAGE): A formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging. Curr. Mol. Med. 2007, 7, 699–710. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Piperi, C.; Kalofoutis, A.; Creatsas, G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin. Endocrinol. 2005, 62, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bukulin, M.; Kojro, E.; Roth, A.; Metz, V.V.; Fahrenholz, F.; Nawroth, P.P.; Bierhaus, A.; Postina, R. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J. Biol. Chem. 2008, 283, 35507–35516. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E. Insulin resistance in PCOS. Endocrine 2006, 30, 13–17. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), receptor for AGEs, diabetes, and bone: Review of the literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015, 72, 1–8. [Google Scholar] [CrossRef]
- Selvin, E.; Halushka, M.K.; Rawlings, A.M.; Hoogeveen, R.C.; Ballantyne, C.M.; Coresh, J.; Astor, B.C. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 2013, 62, 2116–2121. [Google Scholar] [CrossRef]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef]
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315. [Google Scholar] [CrossRef]
- Semba, R.D.; Ang, A.; Talegawkar, S.A.; Crasto, C.; Dalal, M.; Jardack, P.; Traber, M.; Ferrucci, L.; Arab, L. Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: The Energetics Study. Eur. J. Clin. Nutr. 2011, 66, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Förster, A.; Kühne, Y.; Henle, T. Studies on absorption and elimination of dietary maillard reaction products. Ann. N. Y. Acad. Sci. 2005, 1043, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Palace, M. Diabetes and advanced glycation endproducts. J. Intern. Med. 2002, 251, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Araki, N.; Higashi, T.; Mori, T.; Shibayama, R.; Kawabe, Y.; Kodama, T.; Takahashi, K.; Shichiri, M.; Horiuchi, S. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur. J. Biochem. 1995, 230, 408–415. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Kouli, C.R.; Bergiele, A.T.; Filandra, F.A.; Tsianateli, T.C.; Spina, G.G.; Zapanti, E.D.; Bartzis, M.I. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999, 84, 4006–4011. [Google Scholar] [CrossRef]
- Carmina, E.; Lobo, R.A. Polycystic ovary syndrome (PCOS): Arguably the most common endocrinopathy is associated with significant morbidity in women. J. Clin. Endocrinol. Metab. 1999, 84, 1897–1899. [Google Scholar] [CrossRef]
- Cussons, A.J.; Stuckey, B.G.; Watts, G.F. Cardiovascular disease in the polycystic ovary syndrome: New insights and perspectives. Atherosclerosis 2006, 185, 227–239. [Google Scholar] [CrossRef]
- Garg, D.; Merhi, Z. Advanced glycation end products: Link between diet and ovulatory dysfunction in PCOS? Nutrients 2015, 7, 10129–10144. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Katsikis, I.; Piperi, C.; Kandaraki, E.; Piouka, A.; Papavassiliou, A.G.; Panidis, D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2008, 69, 634–641. [Google Scholar] [CrossRef]
- Rutkowska, A.Z.; Diamanti-Kandarakis, E. Do Advanced Glycation End Products (AGEs) contribute to the comorbidities of Polycystic Ovary Syndrome (PCOS)? Curr. Pharm. Des. 2016, 22, 5558–5571. [Google Scholar] [CrossRef]
- Pertynska-Marczewska, M.; Diamanti-Kandarakis, E.; Zhang, J.; Merhi, Z. Advanced glycation end products: A link between metabolic and endothelial dysfunction in polycystic ovary syndrome? Metabolism 2015, 64, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z. Crosstalk between advanced glycation end products and vitamin D: A compelling paradigm for the treatment of ovarian dysfunction in PCOS. Mol. Cell. Endocrinol. 2019, 479, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Kandaraki, E.A.; Diamanti-Kandarakis, E. Implications and future perspectives of AGEs in PCOS pathophysiology. Trends Endocrinol. Metab. 2019, 30, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, J.; Yang, Q.; Zhang, F.; Hao, M.; Guo, Y. Decreased levels of sRAGE in follicular fluid from patients with PCOS. Reproduction 2017, 153, 285–292. [Google Scholar] [CrossRef]
- Garg, D.; Grazi, R.; Lambert-Messerlian, G.M.; Merhi, Z. Correlation between follicular fluid levels of sRAGE and vitamin D in women with PCOS. J. Assist. Reprod. Genet. 2017, 34, 1507–1513. [Google Scholar] [CrossRef]
- Irani, M.; Minkoff, H.; Seifer, D.; Merhi, Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J. Clin. Endocrinol. Metab. 2014, 99, E886–E890. [Google Scholar] [CrossRef]
- Merhi, Z.; Irani, M.; Doswell, A.D.; Ambroggio, J. Follicular fluid soluble receptor for advanced glycation end-products (sRAGE): A potential indicator of ovarian reserve. J. Clin. Endocrinol. Metab. 2014, 99, E226–E233. [Google Scholar] [CrossRef]
- Chatzigeorgiou, A.; Kandaraki, E.; Piperi, C.; Livadas, S.; Papavassiliou, A.G.; Koutsilieris, M.; Papalois, A.; Diamanti-Kandarakis, E. Dietary glycotoxins affect scavenger receptor expression and the hormonal profile of female rats. J. Endocrinol. 2013, 218, 331–337. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Piperi, C.; Levidou, G.; Korkolopoulou, P.; Pawelczyk, L.; Diamanti-Kandarakis, E.; Papavassiliou, A.G. Lysyl oxidase interacts with AGE signalling to modulate collagen synthesis in polycystic ovarian tissue. J. Cell. Mol. Med. 2010, 14, 2460–2469. [Google Scholar] [CrossRef]
- Kandaraki, E.; Chatzigeorgiou, A.; Piperi, C.; Palioura, E.; Palimeri, S.; Korkolopoulou, P.; Koutsilieris, M.; Papavassiliou, A.G. Reduced ovarian glyoxalase-i activity by dietary glycotoxins and androgen excess: A causative link to polycystic ovarian syndrome. Mol. Med. 2012, 18, 1183–1189. [Google Scholar] [CrossRef]
- Garg, D.; Merhi, Z. Relationship between advanced glycation end products and steroidogenesis in PCOS. Reprod. Biol. Endocrinol. 2016, 14, 1–13. [Google Scholar] [CrossRef]
- Thornton, K.; Merhi, Z.; Jindal, S.; Goldsammler, M.; Charron, M.J.; Buyuk, E. Dietary Advanced Glycation End Products (AGEs) could alter ovarian function in mice. Mol. Cell. Endocrinol. 2020, 510, 110826. [Google Scholar] [CrossRef]
- Su, Y.Q.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002, 143, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Kayampilly, P.P.; Menon, K.M.J. Follicle-stimulating hormone inhibits adenosine 5′-Monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an akt-dependent pathway. Endocrinology 2009, 150, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Fadiel, A.; Buyuk, E.; Naftolin, F.; Cipolla, M. Vitamin D attenuates the adverse effect of advanced glycation end products on human granulosa cells: Implications for women with PCOS. Fertil. Steril. 2015, 104, e106. [Google Scholar] [CrossRef]
- Josso, N.; Clemente, N. Transduction pathway of anti-Mullerian hormone, a sex-specific member of the TGF-beta family. Trends Endocrinol. Metab. 2003, 14, 91–97. [Google Scholar] [CrossRef]
- Josso, N.; di Clemente, N.; Gouédard, L. Anti-müllerian hormone and its receptors. Mol. Cell. Endocrinol. 2001, 179, 25–32. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.J.; Grootegoed, J.A.; Themmen, A.P. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- Merhi, Z.; Wang, S.; Cipolla, M. Special research presentation: Vitamin d reverses the adverse effects of advanced glycation end products on granulosa cells. Fertil. Steril. 2016, 106, e76. [Google Scholar] [CrossRef]
- Merhi, Z.; Büyük, E.; Cipolla, M.J. Advanced glycation end products alter steroidogenic gene expression by granulosa cells: An effect partially reversible by vitamin D. Mol. Hum. Reprod. 2018, 24, 318–326. [Google Scholar] [CrossRef]
- Barker, D.J. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 1997, 13, 807–813. [Google Scholar] [CrossRef]
- Dicken, C.L.; Israel, D.D.; Davis, J.B.; Sun, Y.; Shu, J.; Hardin, J.; Neal-Perry, G. Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol. Reprod. 2012, 87, 51. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 507–519. [Google Scholar] [CrossRef]
- Edelstein, D.; Brownlee, M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes 1992, 41, 26–29. [Google Scholar] [CrossRef]
- Horiuchi, S.; Araki, N.; Morino, Y. Immunochemical approach to characterize advanced glycation end products of the Maillard reaction. Evidence for the presence of a common structure. J. Biol. Chem. 1991, 266, 7329–7332. [Google Scholar] [CrossRef]
- Csongová, M.; Gurecká, R.; Koborová, I.; Celec, P.; Domonkos, E.; Uličná, O.; Somoza, V.; Šebeková, K. The effects of a maternal advanced glycation end product-rich diet on somatic features, reflex ontogeny and metabolic parameters of offspring mice. Food Funct. 2018, 9, 3432–3446. [Google Scholar] [CrossRef]
- Borg, D.J.; Yap, F.Y.T.; Keshvari, S.; Simmons, D.; Gallo, L.A.; Fotheringham, A.; Zhuang, A.; Slattery, R.M.; Hasnain, S.; Coughlan, M.; et al. Perinatal exposure to high dietary advanced glycation end products in transgenic NOD8.3 mice leads to pancreatic beta cell dysfunction. Islets 2017, 10, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Du, X.Q.; Charron, M.J. Perinatal exposure to high dietary advanced glycation end products affects the reproductive system in female offspring in mice. Mol. Hum. Reprod. 2020, 26, 615–623. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouanness, M.; Merhi, Z. Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways. Nutrients 2022, 14, 966. https://doi.org/10.3390/nu14050966
Mouanness M, Merhi Z. Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways. Nutrients. 2022; 14(5):966. https://doi.org/10.3390/nu14050966
Chicago/Turabian StyleMouanness, Marco, and Zaher Merhi. 2022. "Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways" Nutrients 14, no. 5: 966. https://doi.org/10.3390/nu14050966
APA StyleMouanness, M., & Merhi, Z. (2022). Impact of Dietary Advanced Glycation End Products on Female Reproduction: Review of Potential Mechanistic Pathways. Nutrients, 14(5), 966. https://doi.org/10.3390/nu14050966






