Anti-Obesity Effect of Nostoc commune Ethanol Extract In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Analysis of Phytochemicals
2.3.1. Total Polyphenol Quantitative Method
2.3.2. Total Flavonoid Quantitative Method
2.3.3. Total Terpenoid Quantitative Method
2.3.4. 3T3-L1 Preadipocyte Cell Culture and Differentiation
2.4. Cell Proliferation Analysis in 3T3-L1 Preadipocytes
2.5. Cell Viability Analysis in 3T3-L1 Preadipocytes
2.6. Lipid Accumulation Test in Differentiated 3T3-L1 Preadipocytes
2.6.1. Oil Red O Staining and Quantitative Analysis in Differentiated 3T3-L1 Preadipocytes
2.6.2. Triglyceride Deposition Test in Differentiated 3T3-L1 Preadipocytes
2.7. Animal Care and Treatment
2.8. Animal Serum Biochemical Parameters
2.9. Histopathological Analysis
2.10. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.11. Statistical Analysis
3. Results
3.1. Phytochemical Composition in NEE
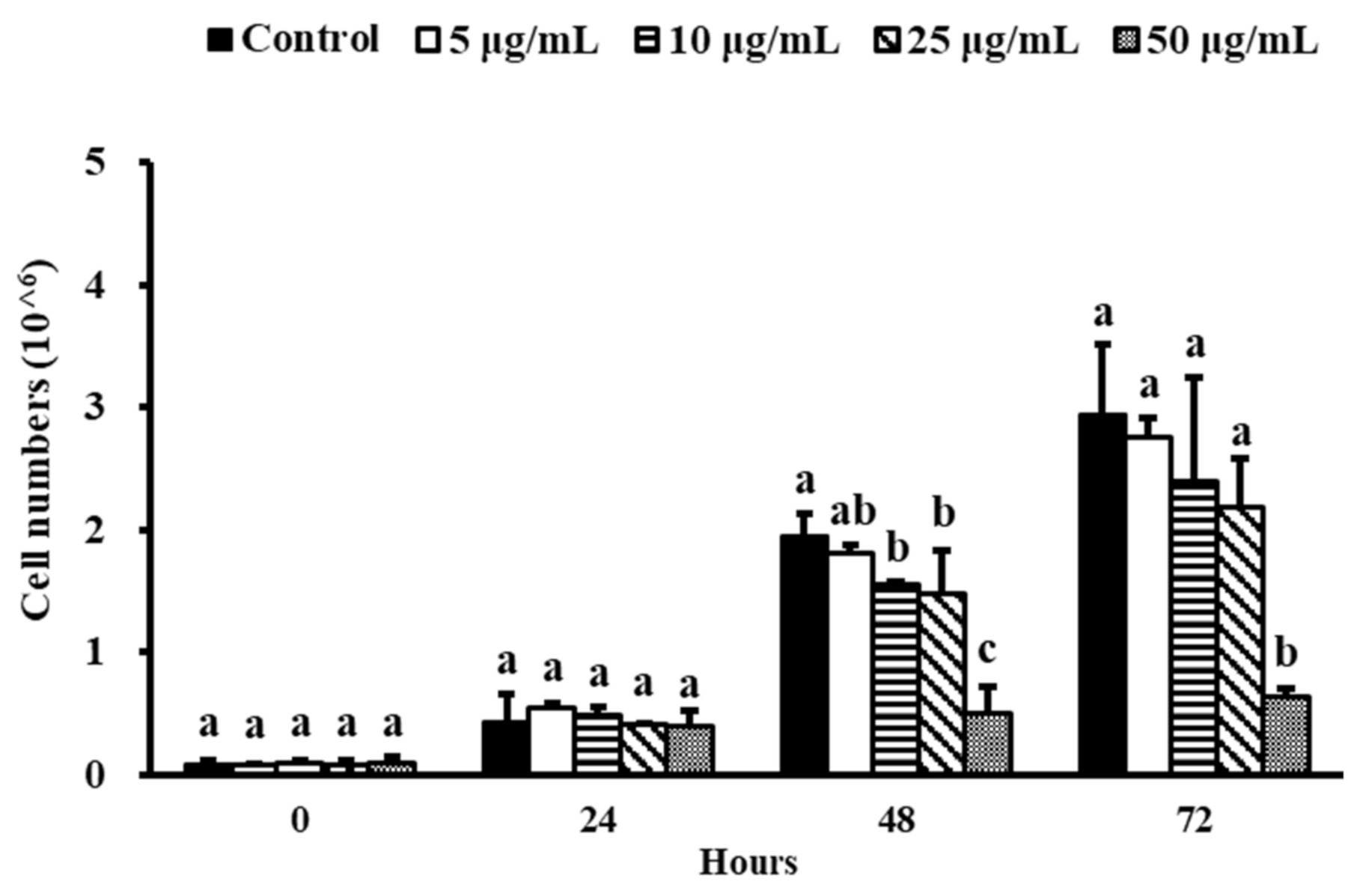
3.2. Effect of NEE on Cell Proliferation in 3T3-L1 Preadipocytes
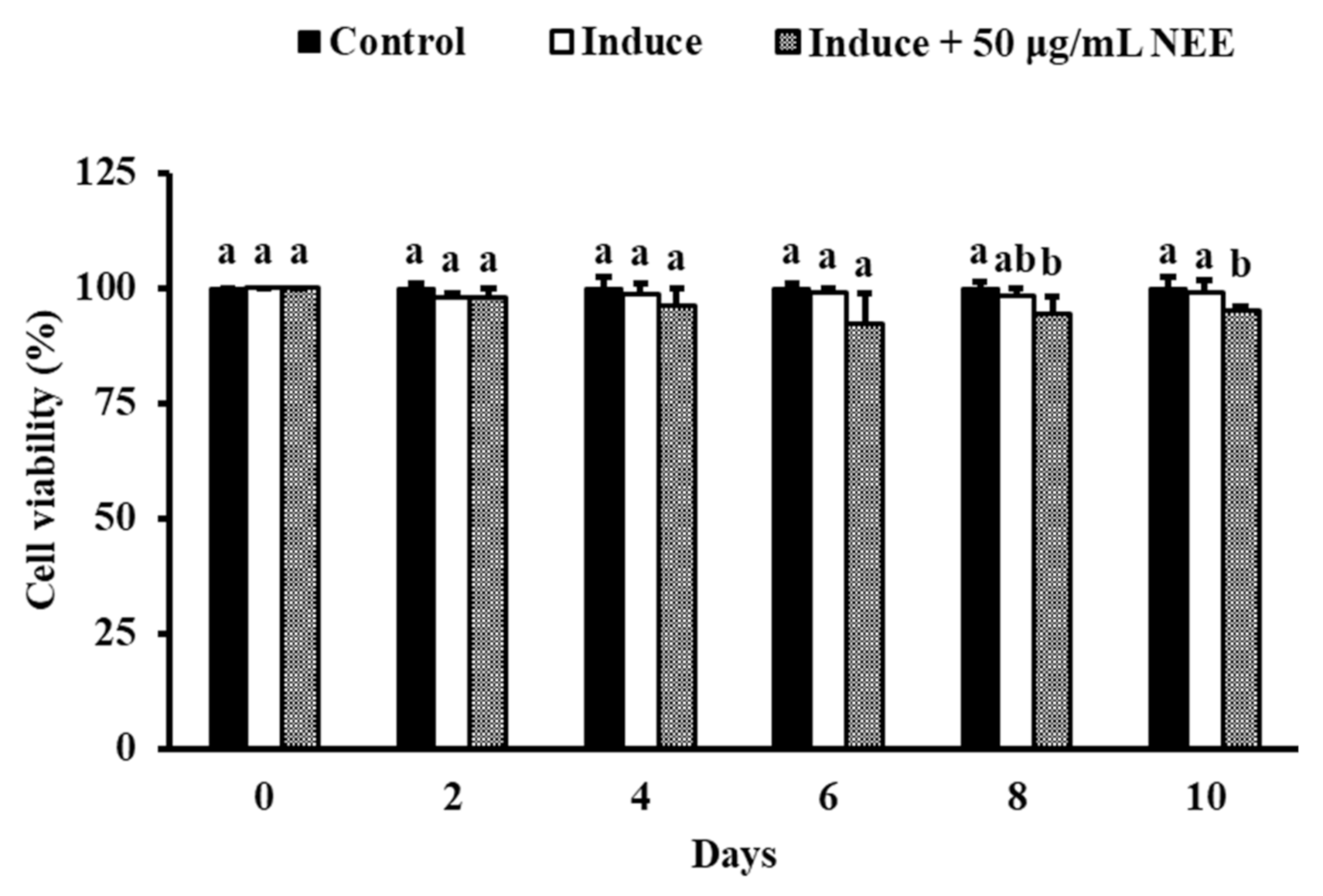
3.3. Effect of NEE on Cell Viability in 3T3-L1 Preadipocytes
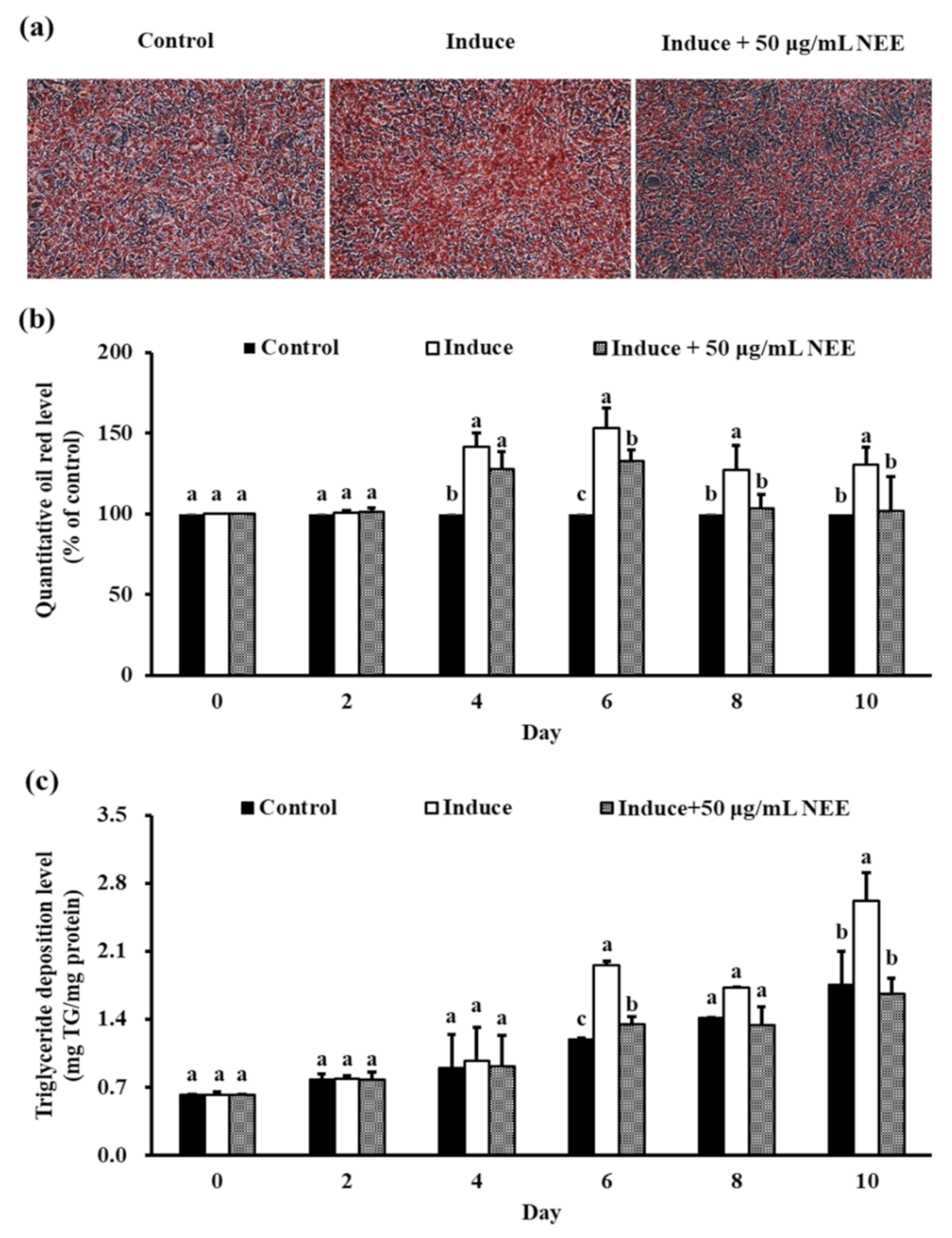
3.4. Effects of NEE on Lipid Accumulation in 3T3-L1 Preadipocytes
3.5. Effects of NEE on Growth Parameters and Serum Parameters in High Calorie Diet–Induced Obese Rats
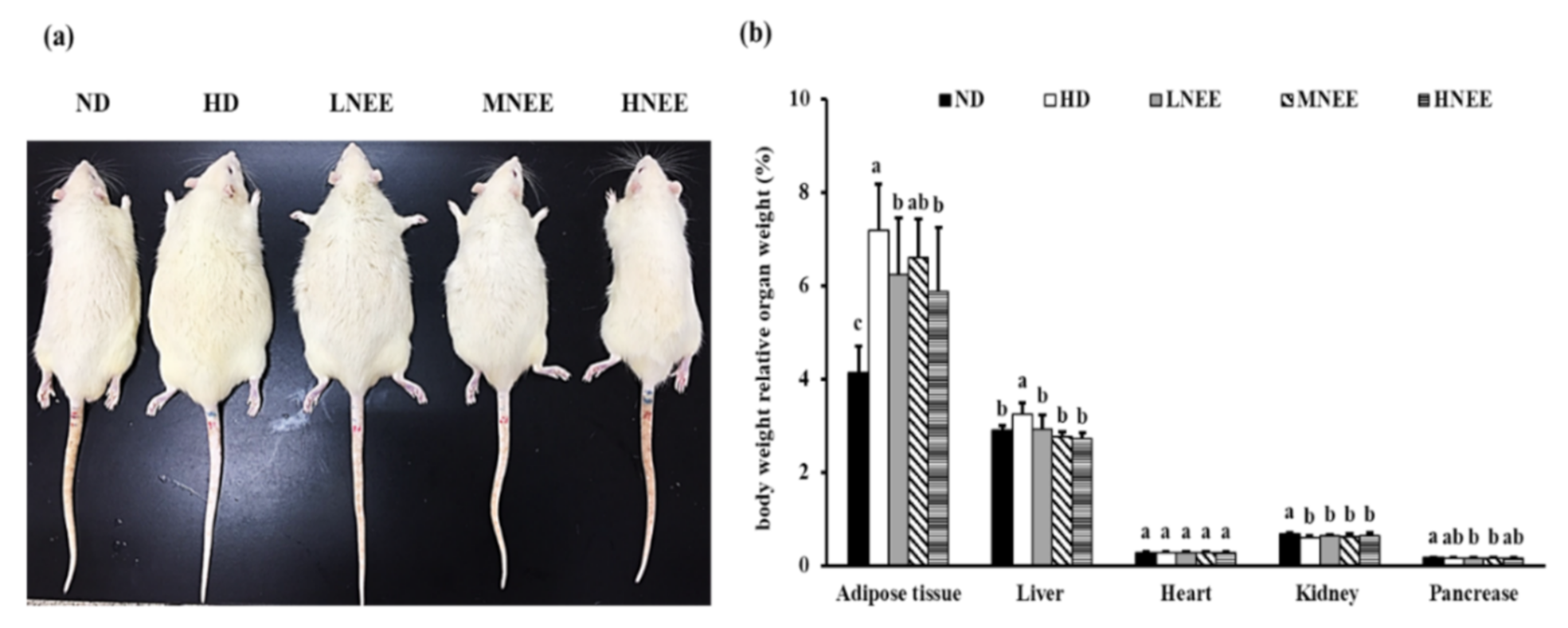
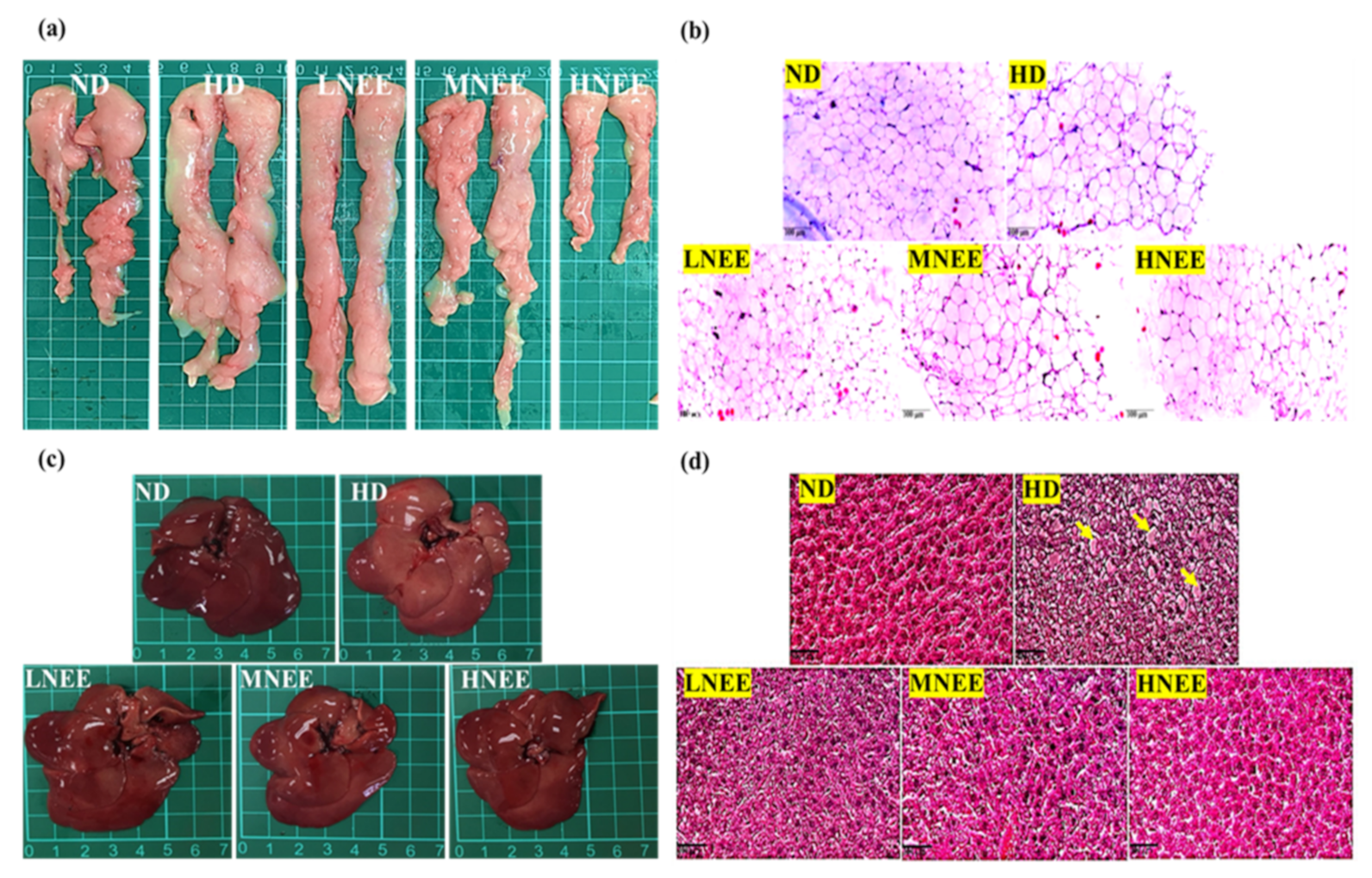
3.6. Effects of NEE on Animal Characteristics, Body Weight, and Relative Organ Weight in High Calorie Diet–Induced Obese Rat
3.7. Effects of NEE on Epididymal Adipose Tissue and Liver Histology in High Calorie Diet–Induced Obese Rat
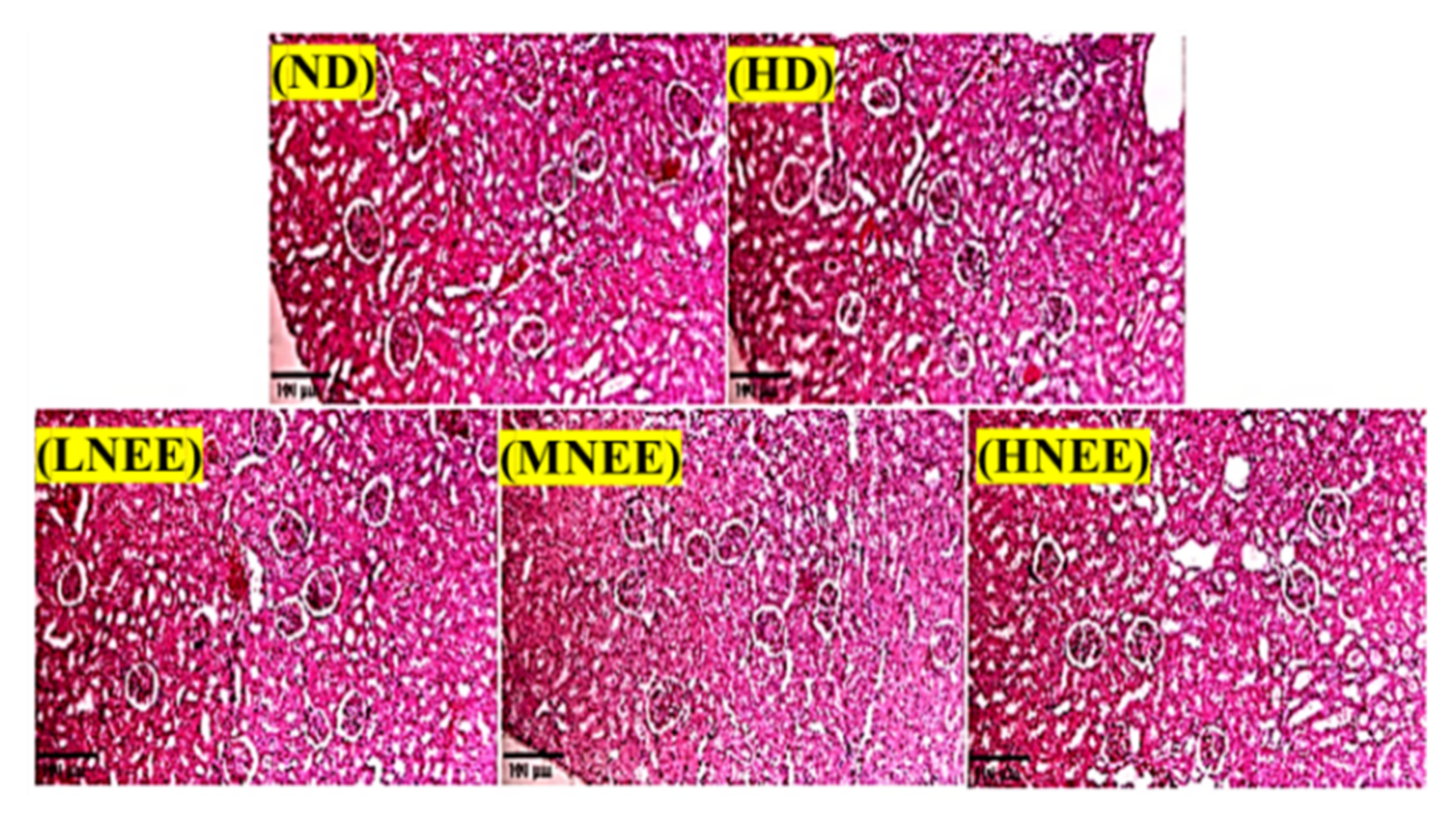
3.8. Effects of NEE on Kidney Histological in High Calorie Diet–Induced Obese Rat
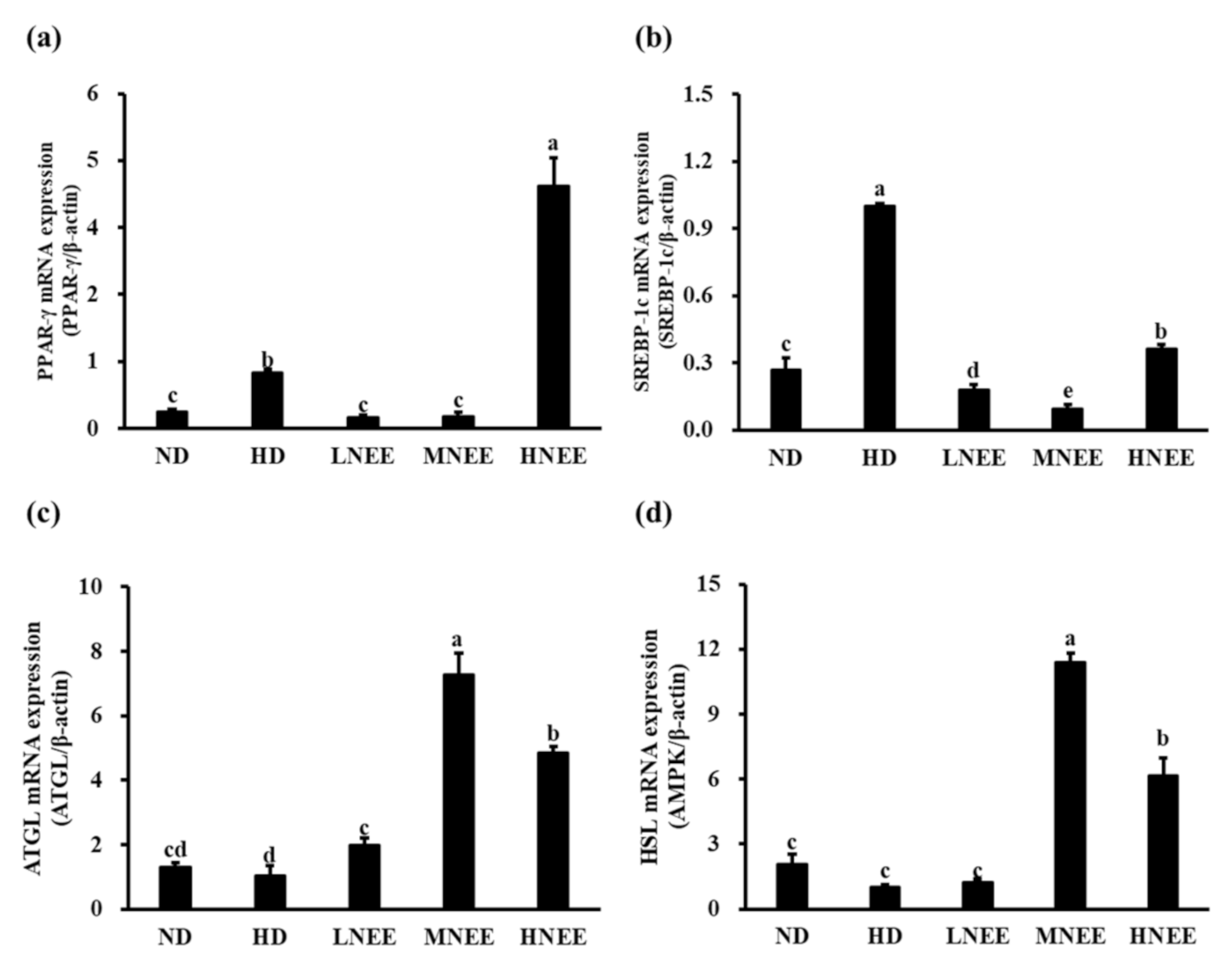
3.9. Effects of NEE on mRNA Expression of Adipogenesis and Lipid Lysis-Related Gene in Epididymal Adipose Tissue of High Calorie Diet–Induced Obese Rat
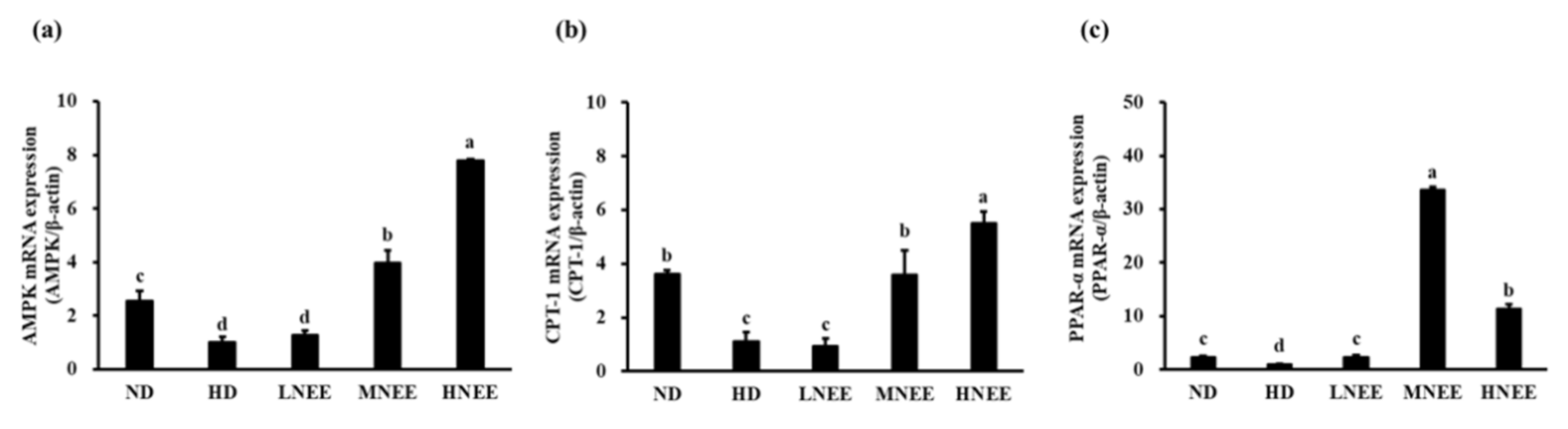
3.10. Effects of NEE on mRNA Expression of the β-Oxidation Related Gene in the Liver of High Calorie Diet–Induced Obese Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bermano, G.; Stoyanova, T.; Hennequart, F.; Wainwright, C.L. Seaweed-derived bioactives as potential energy regulators in obesity and type 2 diabetes. Adv. Pharmacol. 2020, 87, 205–256. [Google Scholar] [PubMed]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr. Physiol. 2011, 9, 1–58. [Google Scholar]
- Ràfols, M.E. Adipose tissue: Cell Heterogeneity and functional diversity. Endocrinol. Nutr. (Engl. Ed.) 2014, 61, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Bitter, A.; Nüssler, A.K.; Thasler, W.E.; Klein, K.; Zanger, U.M.; Schwab, M.; Burk, O. Human sterol regulatory element-binding protein 1a contributes significantly to hepatic lipogenic gene expression. Cell. Physiol. Biochem. 2015, 35, 803–815. [Google Scholar] [CrossRef]
- Lee, J.W.; Choe, S.S.; Jang, H.; Kim, J.; Jeong, H.W.; Jo, H.; Jeong, K.H.; Tadi, S.; Park, M.G.; Kwak, T.H. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J. Lipid Res. 2012, 53, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Yeaman, S.J.; Smith, G.M.; Jepson, C.A.; Wood, S.L.; Emmison, N. The multifunctional role of hormone-sensitive lipase in lipid metabolism. Adv. Enzym. Regul. 1994, 34, 355–370. [Google Scholar] [CrossRef]
- Trites, M.J.; Clugston, R.D. The role of adipose triglyceride lipase in lipid and glucose homeostasis: Lessons from transgenic mice. Lipids Health Dis. 2019, 18, 204. [Google Scholar] [CrossRef] [Green Version]
- Brejchova, K.; Radner, F.P.W.; Balas, L.; Paluchova, V.; Cajka, T.; Chodounska, H.; Kudova, E.; Schratter, M.; Schreiber, R.; Durand, T. Distinct roles of adipose triglyceride lipase and hormone-sensitive lipase in the catabolism of triacylglycerol estolides. Proc. Natl. Acad. Sci. USA 2021, 118, e2020999118. [Google Scholar] [CrossRef]
- Araki, M.; Nakagawa, Y.; Oishi, A.; Han, S.I.; Wang, Y.; Kumagai, K.; Ohno, H.; Mizunoe, Y.; Iwasaki, H.; Sekiya, M. The peroxisome proliferator-activated receptor α (PPARα) agonist pemafibrate protects against diet-induced obesity in mice. Int. J. Mol. Sci. 2018, 19, 2148. [Google Scholar] [CrossRef] [Green Version]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [Green Version]
- Heinecke, F.; Mazzucco, M.B.; Fornes, D.; Roberti, S.; Jawerbaum, A.; White, V. The offspring from rats fed a fatty diet display impairment in the activation of liver peroxisome proliferator activated receptor alpha and features of fatty liver disease. Mol. Cell. Endocrinol. 2020, 511, 110818. [Google Scholar] [CrossRef] [PubMed]
- Miotto, P.M.; Steinberg, G.R.; Holloway, G.P. Controlling skeletal muscle CPT-I malonyl-CoA sensitivity: The importance of AMPK-independent regulation of intermediate filaments during exercise. Biochem. J. 2017, 474, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, C.; Wang, J.; Lin, C.; Chang, Y.; Li, J.; Liang, J. Effects of base-catalysis on determination of total polyphenols with Folin-Ciocalteu reagent MC-transaction on biotechnology. Retrieved Website 2012, 4, 10–19. [Google Scholar]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Li, Z.; Guo, M. Healthy efficacy of Nostoc commune Vaucher. Oncotarget 2018, 9, 14669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, L.L.; Bi, Y.; Zhang, S.G.; Liu, L. Study on antimicrobial capability of different solvent extracts from Nostoc commune Vauch. Sci. Technol. Food Ind. 2010, 31, 68–70. [Google Scholar]
- Qian, S.H.; Li, R.Y.; Wei, M.; Yang, C.Y. Antimicrobial activities of Nostoc commune extracts on several common microorganisms. Hunan Agric. Sci. 2011, 17. [Google Scholar]
- Hameed, M.A.; Hassan, S.; Mohammed, R.; Gamal, R. Isolation and characterization of antimicrobial active compounds from the cyanobacterium Nostoc commune Vauch. J. Pure Appl. Microbiol. 2013, 7, 109–116. [Google Scholar]
- DiaoYi, Y.Z. Study on Antibacterial Effects of Fat-Soluble Compositions in Nostoc commune. Biotechnol. Bull. 2014, 83–86. [Google Scholar]
- Jaki, B.; Orjala, J.; Sticher, O. A novel extracellular diterpenoid with antibacterial activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 1999, 62, 502–503. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Orjala, J.; Heilmann, J.; Linden, A.; Vogler, B.; Sticher, O. Novel Extracellular diterpenoids with biological activity from the cyanobacterium Nostoc commune. J. Nat. Prod. 2000, 63, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Heilmann, J.; Sticher, O. New antibacterial metabolites from the cyanobacterium Nostoc commune (Eawag 122b). J. Nat. Prod. 2000, 63, 1283–1285. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.F.; Wu, T.J.; Tai, J.L.; Chi, M.C.; Lin, L.L. Immunomodulatory potential of the polysaccharide-rich extract from edible cyanobacterium Nostoc commune. Med. Sci. 2015, 3, 112–123. [Google Scholar] [CrossRef]
- Matsui, K.; Nazifi, E.; Hirai, Y.; Wada, N.; Matsugo, S.; Sakamoto, T. The cyanobacterial Uv-absorbing pigment scytonemin displays radical-scavenging activity. J. Gen. Appl. Microbiol. 2012, 58, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Qian, S.; Li, R.; Wei, M.; Yang, C. Extraction and antibacterial activity of polysaccharides from Nostoc commune. Food Sci. 2012, 33, 96–99. [Google Scholar]
- Li, R.; Qian, S.; Dong, Q.; Liu, H. Extraction of total flavonoids from Nostoc Commune and preliminary determination of its antimicrobial effects. Acta Acad. Med. Wannan 2012, 31, 186–190. [Google Scholar]
- Kajiyama, S.I.; Kanzaki, H.; Kawazu, K.; Kobayashi, A. Nostofungicidine, an antifungal lipopeptide from the field-grown terrestrial blue-green alga Nostoc commune. Tetrahedron Lett. 1998, 39, 3737–3740. [Google Scholar] [CrossRef]
- Olafsdottir, A.; Thorlacius, G.E.; Omarsdottir, S.; Olafsdottir, E.S.; Vikingsson, A.; Freysdottir, J.; Hardardottir, I. A heteroglycan from the cyanobacterium Nostoc commune modulates LPS-induced inflammatory cytokine secretion by THP-1 monocytes through phosphorylation of ERK1/2 and Akt. Phytomedicine 2014, 21, 1451–1457. [Google Scholar] [CrossRef]
- Itoh, T.; Koketsu, M.; Yokota, N.; Touho, S.; Ando, M.; Tsukamasa, Y. Reduced scytonemin isolated from Nostoc commune suppresses LPS/IFNγ-induced no production in murine macrophage Raw264 cells by inducing hemeoxygenase-1 expression via the Nrf2/Are pathway. Food Chem. Toxicol. 2014, 69, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Da Silva, L.A.L.; Pezzini, B.R.; Soares, L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L. (Lamiaceae) leaves. Pharmacogn. Mag. 2015, 11, 96. [Google Scholar]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.E.; Blobaum, K.R.; Jesch, E.D.; Ku, C.S.; Park, Y.K.; Lu, F.; Carr, T.P.; Lee, J.Y. Hypocholesterolemic effect of Nostoc commune var. sphaeroides Kützing, an edible blue-green alga. Eur. J. Nutr. 2009, 48, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, J.; Liu, Y.; Ai, S.; Qin, F.; Li, Z.; Zhang, H.; Huang, Z. Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohydr. Polym. 2011, 83, 1821–1827. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A review on obesity management through natural compounds and a green nanomedicine-based approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, W.T.; Lin, A.H.; Tsai, C.W.; Huang, C.S.; Chen, Y.T.; Chen, H.W.; Lii, C.K. Andrographolide Inhibits Adipogenesis of 3t3-L1 Cells by Suppressing C/Ebpβ Expression and Activation. Toxicol. Appl. Pharmacol. 2016, 307, 115–122. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, S.G.; Kim, M.; Hahn, D.; Jung, S.K.; Cho, T.O.; Nam, J.O. Sargassum miyabei Yendo brown algae exert anti-oxidative and anti-adipogenic effects on 3T3-L1 Adipocytes by downregulating PPARγ. Medicina 2020, 56, 634. [Google Scholar] [CrossRef]
- Li, Z.S.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The Green Algal Carotenoid Siphonaxanthin Inhibits Adipogenesis in 3t3-L1 Preadipocytes and the Accumulation of Lipids in White Adipose Tissue of Kk-Ay Mice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.M.; Jang, M.S. Anti-Obesity Effects of Laminaria japonica Fermentation on 3T3-L1 Adipocytes Are Mediated by the Inhibition of C/Ebp-A/Β and Ppar-Γ. Cell. Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.E.; Blobaum, K.R.; Park, Y.K.; Ehlers, S.J.; Lu, F.; Lee, J.Y. Lipid extract of Nostoc commune var. sphaeroides Kützing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells. J. Nutr. 2008, 138, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazifi, E.; Wada, N.; Asano, T.; Nishiuchi, T.; Iwamuro, Y.; Chinaka, S.; Matsugo, S.; Sakamoto, T. Characterization of the chemical diversity of glycosylated mycosporine-like amino acids in the terrestrial cyanobacterium Nostoc commune. J. Photochem. Photobiol. B Biol. 2015, 142, 154–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Jeepipalli, S.P.; Du, B.; Sabitaliyevich, U.Y.; Xu, B. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 2020, 318, 126474. [Google Scholar] [CrossRef]
- Yuan, X.; Nakao, T.; Satone, H.; Ohara, K.; Kominami, Y.; Ito, M.; Aizawa, T.; Ueno, T.; Ushio, H. The effects of brown algae-derived monosaccharide l-fucose on lipid metabolism in C57BL/6J obese mice. Nutrients 2020, 12, 3798. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.; Lee, J.S. Fucoidan Prevents High-Fat Diet-Induced Obesity in Animals by Suppression of Fat Accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef]
- Ilavenil, S.; Kim, D.H.; Vijayakumar, M.; Srigopalram, S.; Roh, S.G.; Arasu, M.V.; Lee, J.S.; Choi, K.C. Potential role of marine algae extract on 3T3-L1 cell proliferation and differentiation: An in vitro approach. Biol. Res. 2016, 49, 38. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Petalonia Binghamiae extract and its constituent fucoxanthin ameliorate high-fat diet-induced obesity by activating amp-activated protein kinase. J. Agric. Food Chem. 2012, 60, 3389–3395. [Google Scholar] [CrossRef]
| Total Polyphenols (GAE µg/NEE mg) | Total Flavonoids (RUT µg/NEE mg) | Total Terpenoids (OAH µg/NEE mg) |
|---|---|---|
| 25.89 ± 1.18 | 19.32 ± 0.45 | 926.53 ± 0.03 |
| ND | HD | LNEE | MNEE | HNEE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth parameters | |||||||||||||||
| Food intake (g/rat/day) | 30.39 | ± | 1.58 a | 22.41 | ± | 2.00 c | 23.59 | ± | 1.59 c | 22.68 | ± | 1.23 c | 26.36 | ± | 5.26 b |
| FCR (%) | 8.53 | ± | 0.73 a | 5.34 | ± | 0.75 c | 5.80 | ± | 0.54 c | 5.95 | ± | 0.93 c | 7.09 | ± | 1.55 b |
| Weight gain (%) | 136.20 | ± | 9.00 b | 166.20 | ± | 20.80 a | 148.10 | ± | 14.70 b | 139.20 | ± | 21.9 b | 144.00 | ± | 17.40 b |
| Serum parameters | |||||||||||||||
| AST (U/L) | 99.25 | ± | 3.81 a,b | 106.67 | ± | 15.13 a | 96.00 | ± | 7.52 a | 94.75 | ± | 7.65 b | 92.09 | ± | 9.01 b |
| ALT (U/L) | 38.50 | ± | 2.27 a | 34.30 | ± | 5.10 b | 29.50 | ± | 4.28 c | 29.17 | ± | 4.09 c | 31.86 | ± | 4.02 b,c |
| Creatinine (mg/dL) | 0.64 | ± | 0.02 a | 0.64 | ± | 0.03 a | 0.61 | ± | 0.05 a,b | 0.59 | ± | 0.03 b | 0.59 | ± | 0.03 b |
| Uric acid (mg/dL) | 3.37 | ± | 0.41 a | 3.38 | ± | 0.43 a | 2.70 | ± | 0.62 b | 3.38 | ± | 0.52 a | 3.31 | ± | 0.81 a,b |
| FFA (mmol/L) | 1.05 | ± | 0.17 b | 1.34 | ± | 0.24 a | 1.01 | ± | 0.16 b | 1.16 | ± | 0.20 b | 1.07 | ± | 0.11 b |
| Lipase (mmol/L) | 26.68 | ± | 4.88 d | 42.50 | ± | 6.97 c | 47.18 | ± | 12.75 c | 66.35 | ± | 9.37 b | 78.64 | ± | 2.79 a |
| TG (mg/dL) | 85.00 | ± | 15.78 c | 113.14 | ± | 12.51 a | 103.33 | ± | 17.92 a,b | 98.22 | ± | 14.40 a,b,c | 89.83 | ± | 15.47 b,c |
| TC (mg/dL) | 84.33 | ± | 6.91 a | 82.17 | ± | 8.77 a | 75.50 | ± | 8.43 b | 71.31 | ± | 8.97 b | 70.70 | ± | 5.21 b |
| HDL (mg/dL) | 60.11 | ± | 4.26 a | 58.78 | ± | 8.15 a,b | 50.86 | ± | 6.36 b | 52.41 | ± | 8.38 b | 50.40 | ± | 5.34 b |
| LDL (mg/dL) | 10.79 | ± | 1.58 b | 13.33 | ± | 1.87 a | 11.50 | ± | 2.20 b | 11.00 | ± | 1.93 b | 10.92 | ± | 1.98 b |
| Glucose AC (mg/dL) | 128.33 | ± | 14.82 b | 165.20 | ± | 15.61 a | 94.33 | ± | 12.29 b,c | 113.20 | ± | 16.18 b,c | 119.00 | ± | 42.83 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-C.; Huang, Y.-W.; Wu, C.-C.; Wang, J.-J.; Chen, Y.-T.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D.; Hsieh, S.-L. Anti-Obesity Effect of Nostoc commune Ethanol Extract In Vitro and In Vivo. Nutrients 2022, 14, 968. https://doi.org/10.3390/nu14050968
Tsai S-C, Huang Y-W, Wu C-C, Wang J-J, Chen Y-T, Singhania RR, Chen C-W, Dong C-D, Hsieh S-L. Anti-Obesity Effect of Nostoc commune Ethanol Extract In Vitro and In Vivo. Nutrients. 2022; 14(5):968. https://doi.org/10.3390/nu14050968
Chicago/Turabian StyleTsai, Sheng-Chieh, Yu-Wen Huang, Chih-Chung Wu, Jyh-Jye Wang, Ya-Ting Chen, Reeta Rani Singhania, Chiu-Wen Chen, Cheng-Di Dong, and Shu-Ling Hsieh. 2022. "Anti-Obesity Effect of Nostoc commune Ethanol Extract In Vitro and In Vivo" Nutrients 14, no. 5: 968. https://doi.org/10.3390/nu14050968
APA StyleTsai, S.-C., Huang, Y.-W., Wu, C.-C., Wang, J.-J., Chen, Y.-T., Singhania, R. R., Chen, C.-W., Dong, C.-D., & Hsieh, S.-L. (2022). Anti-Obesity Effect of Nostoc commune Ethanol Extract In Vitro and In Vivo. Nutrients, 14(5), 968. https://doi.org/10.3390/nu14050968










