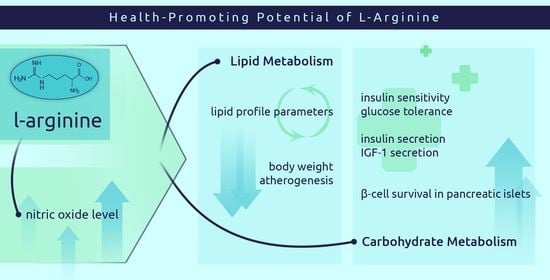
The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review
Abstract
1. Introduction
2. Transport and Absorption of L-Arginine
3. Current Uses and Potential Properties of L-Arginine
4. Effects of Nitric Oxide on Carbohydrate and Lipid Metabolism
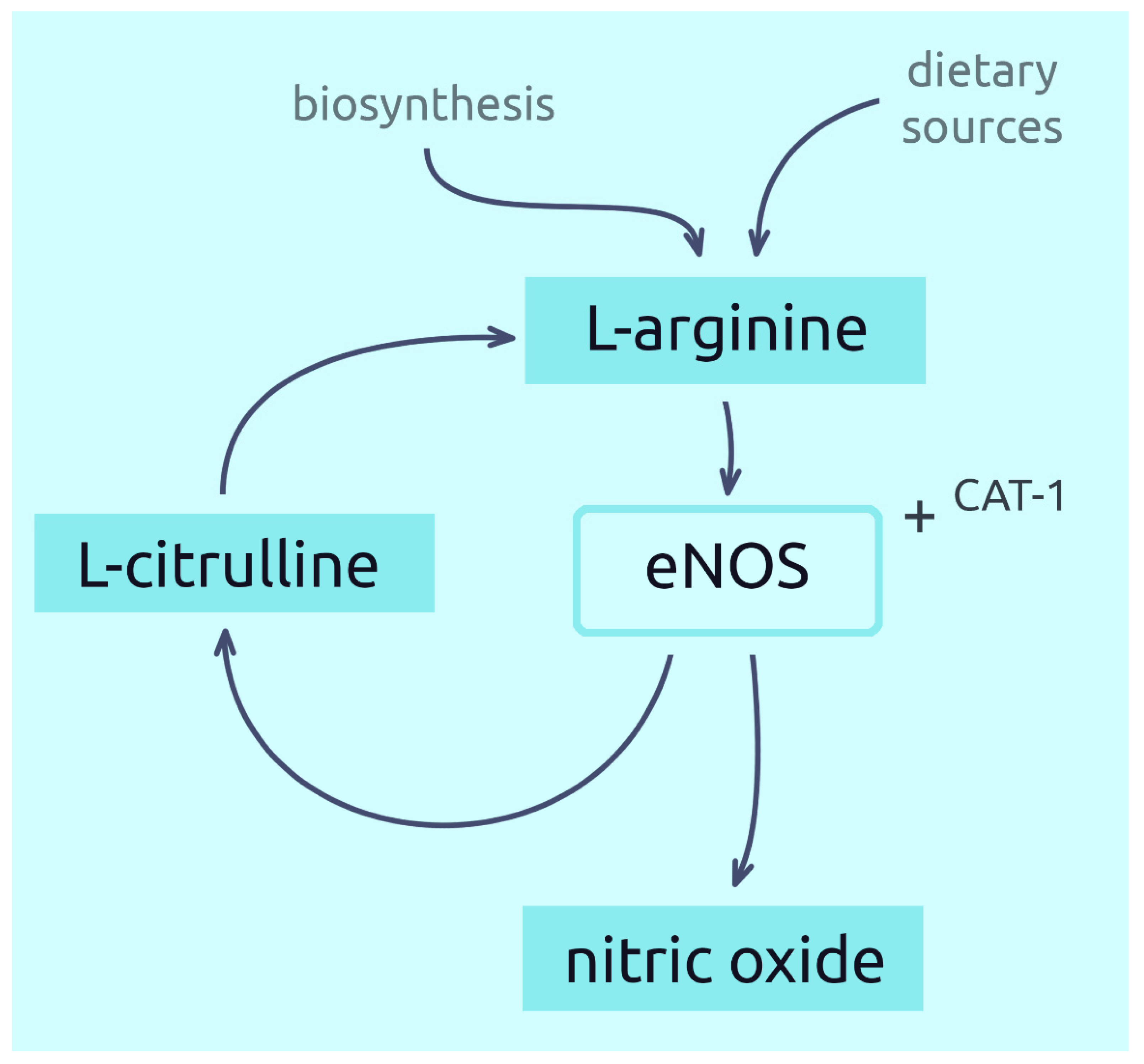
5. The Role of L-Arginine in the Nitric Acid Synthesis
6. Potential of L-Arginine in the Treatment of Carbohydrate Metabolism Disorders
6.1. Cell Testing
6.2. Animal Testing
6.3. Human Research
7. Potential of L-Arginine in the Treatment of Lipid Metabolism Disorders
7.1. Cell Testing
7.2. Animal Testing
7.3. Human Research
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blantz, R.C.; Satriano, J.; Gabbai, F.; Kelly, C. Biological Effects of Arginine Metabolites: Effects of Arginine Metabolites. Acta Physiol. Scand. 2000, 168, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.E.; Meininger, C.J.; Haynes, T.E.; Wu, G. The Metabolic Basis of Arginine Nutrition and Pharmacotherapy. Biomed. Pharmacother. 2002, 56, 427–438. [Google Scholar] [CrossRef]
- Morris, S.M. Regulation of Enzymes of the Urea Cycle and Arginine Metabolism. Annu. Rev. Nutr. 2002, 22, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Mathé, G.; Couvreur, P.; Tew, K.D.I. Arginine. Biomed. Pharmacother. 2002, 56, 439–445. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J.; Knabe, D.A.; Baze, F.W.; Rhoads, J.M. Arginine Nutrition in Development, Health and Disease. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 59–66. [Google Scholar] [CrossRef]
- Lokhande, P.D.; Kuchekar, B.S.; Chabukswar, A.R.; Jagdale, S.C. Nitric Oxide: Role in Biological System. Asian J. Biochem. 2005, 1, 1–17. [Google Scholar] [CrossRef][Green Version]
- Garlichs, C.D.; Beyer, J.; Zhang, H.; Schmeisser, A.; Plötze, K.; Mügge, A.; Schellong, S.; Daniel, W.G. Decreased Plasma Concentrations of L-Hydroxy-Arginine as a Marker of Reduced NO Formation in Patients with Combined Cardiovascular Risk Factors. J. Lab. Clin. Med. 2000, 135, 419–425. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M. Arginine Metabolism: Nitric Oxide and Beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Devés, R.; Boyd, C.A.R. Transporters for Cationic Amino Acids in Animal Cells: Discovery, Structure, and Function. Physiol. Rev. 1998, 78, 487–545. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M. The Clinical Pharmacology of L-Arginine. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 79–99. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and Pharmacodynamic Properties of Oral L-Citrulline and L-Arginine: Impact on Nitric Oxide Metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nakaki, T.; Hishikawa, K. The arginine paradox. Folia Pharmacol. Jpn. 2002, 119, 7–14. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.; Harris, M.B.; Goolsby, J.M.; Venema, R.C. Interaction of the Endothelial Nitric Oxide Synthase with the CAT-1 Arginine Transporter Enhances NO Release by a Mechanism Not Involving Arginine Transport. Biochem. J. 2005, 386, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Mohan, S.; Fung, H.-L. Intracellular L-Arginine Concentration Does Not Determine NO Production in Endothelial Cells: Implications on the “L-Arginine Paradox”. Biochem. Biophys. Res. Commun. 2011, 414, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, K. Inhibition of Arginine Synthesis by Urea: A Mechanism for Arginine Deficiency in Renal Failure Which Leads to Increased Hydroxyl Radical Generation. In Guanidino Compounds in Biology and Medicine; Clark, J.F., Ed.; Springer: Boston, MA, USA, 2003; pp. 11–15. [Google Scholar]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory Role for the Arginine–Nitric Oxide Pathway in Metabolism of Energy Substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryu, H.; Ferrante, R.J.; Morris, S.M.; Ratan, R.R. Translational Control of Inducible Nitric Oxide Synthase Expression by Arginine Can Explain the Arginine Paradox. Proc. Natl. Acad. Sci. USA 2003, 100, 4843–4848. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Shi, W.; Meininger, C.J.; Haynes, T.E.; Hatakeyama, K.; Wu, G. Regulation of Tetrahydrobiopterin Synthesis and Bioavailability in Endothelial Cells. Cell Biochem. Biophys. 2004, 41, 415–434. [Google Scholar] [CrossRef]
- Böger, R.H. The Pharmacodynamics of L-Arginine. J. Nutr. 2007, 137, 1650S–1655S. [Google Scholar] [CrossRef]
- Lorin, J.; Zeller, M.; Guilland, J.-C.; Cottin, Y.; Vergely, C.; Rochette, L. Arginine and Nitric Oxide Synthase: Regulatory Mechanisms and Cardiovascular Aspects. Mol. Nutr. Food Res. 2014, 58, 101–116. [Google Scholar] [CrossRef]
- Banjarnahor, S.; Rodionov, R.N.; König, J.; Maas, R. Transport of L-Arginine Related Cardiovascular Risk Markers. J. Clin. Med. 2020, 9, 3975. [Google Scholar] [CrossRef]
- Grosse, G.M.; Schwedhelm, E.; Worthmann, H.; Choe, C. Arginine Derivatives in Cerebrovascular Diseases: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 1798. [Google Scholar] [CrossRef]
- Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Current Advances of Nitric Oxide in Cancer and Anticancer Therapeutics. Vaccines 2021, 9, 94. [Google Scholar] [CrossRef]
- Huynh, N.N.; Chin-Dusting, J. Amino Acids, Arginase and Nitric Oxide in Vascular Health. Clin. Exp. Pharmacol. Physiol. 2006, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Flam, B.R.; Eichler, D.C.; Solomonson, L.P. Endothelial Nitric Oxide Production Is Tightly Coupled to the Citrulline–NO Cycle. Nitric Oxide 2007, 17, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kedziora-Kornatowska, K.; Tkaczewski, W.; Blaszczyk, J.; Buczyn’ski, A.; Chojnacki, J.; Kedziora, J. Oxygen Metabolism in Blood of Patients with Gastric and Duodenal Ulcer Disease. Hepatogastroenterology 1995, 42, 246–249. [Google Scholar] [PubMed]
- Maher, T.J. L-Arginine. Continuing Education Module. New Hope Inst. Retail. 2000, 1–8. [Google Scholar]
- Merimee, T.J.; Rabinowitz, D.; Riggs, L.; Burgess, J.A.; Rimoin, D.L.; McKusick, V.A. Plasma Growth Hormone after Arginine Infusion: Clinical Experiences. N. Engl. J. Med. 1967, 276, 434–439. [Google Scholar] [CrossRef]
- Kikuta, K.; Sawamura, T.; Miwa, S.; Hashimoto, N.; Masaki, T. High-Affinity Arginine Transport of Bovine Aortic Endothelial Cells Is Impaired by Lysophosphatidylcholine. Circ. Res. 1998, 83, 1088–1096. [Google Scholar] [CrossRef]
- Kurose, I.; Wolf, R.; Grisham, M.B.; Aw, T.Y.; Specian, R.D.; Granger, D.N. Microvascular Responses to Inhibition of Nitric Oxide Production: Role of Active Oxidants. Circ. Res. 1995, 76, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meininger, C.J. Arginine Nutrition and Cardiovascular Function. J. Nutr. 2000, 130, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Preli, R.B.; Klein, K.P.; Herrington, D.M. Vascular Effects of Dietary L-Arginine Supplementation. Atherosclerosis 2002, 162, 1–15. [Google Scholar] [CrossRef]
- Schulman, S.P.; Becker, L.C.; Kass, D.A.; Champion, H.C.; Terrin, M.L.; Forman, S.; Ernst, K.V.; Kelemen, M.D.; Townsend, S.N.; Capriotti, A.; et al. L-Arginine Therapy in Acute Myocardial Infarction: The Vascular Interaction with Age in Myocardial Infarction (VINTAGE MI) Randomized Clinical Trial. JAMA 2006, 295, 58. [Google Scholar] [CrossRef]
- Meru, A.V.; Mittra, S.; Thyagarajan, B.; Chugh, A. Intermittent Claudication: An Overview. Atherosclerosis 2006, 187, 221–237. [Google Scholar] [CrossRef]
- Casino, P.R.; Kilcoyne, C.M.; Quyyumi, A.A.; Koeg, J.M.; Panza, J.A. Investigation of Decreased Availability of Nitric Oxide Precursor as the Mechanism Responsible for Impaired Endothelium-Dependent Vasodilation in Hypercholesterolemic Patients. J. Am. Coll. Cardiol. 1994, 23, 844–850. [Google Scholar] [CrossRef][Green Version]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric Dimethylarginine (ADMA): A Novel Risk Factor for Endothelial Dysfunction: Its Role in Hypercholesterolemia. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Freeman, B.A. Interactions between Nitric Oxide and Lipid Oxidation Pathways: Implications for Vascular Disease. Circ. Res. 2001, 88, 12–21. [Google Scholar] [CrossRef]
- Stroes, E.S.G.; Koomans, H.A.; Rabelink, T.J.; de Bruin, T.W.A. Vascular Function in the Forearm of Hypercholesterolaemic Patients off and on Lipid-Lowering Medication. Lancet 1995, 346, 467–471. [Google Scholar] [CrossRef]
- Williams, S.B.; Cusco, J.A.; Roddy, M.-A.; Johnstone, M.T.; Creager, M.A. Impaired Nitric Oxide-Mediated Vasodilation in Patients with Non-Insulin-Dependent Diabetes Mellitus. J. Am. Coll. Cardiol. 1996, 27, 567–574. [Google Scholar] [CrossRef]
- Gokce, N. L-Arginine and Hypertension. J. Nutr. 2004, 134, 2807S–2811S. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Ischiropoulos, H.; Cuzzocrea, S. Roles of Nitric Oxide and Superoxide in Inflammation. In Inflammation Protocols; Humana Press: Passaic, NJ, USA, 2003; Volume 225, pp. 291–304. ISBN 978-1-59259-374-3. [Google Scholar]
- Maas, R.; Schwedhelm, E.; Kahl, L.; Li, H.; Benndorf, R.; Lüneburg, N.; Förstermann, U.; Böger, R.H. Simultaneous Assessment of Endothelial Function, Nitric Oxide Synthase Activity, Nitric Oxide–Mediated Signaling, and Oxidative Stress in Individuals with and without Hypercholesterolemia. Clin. Chem. 2008, 54, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, H.; Leusink, J.; Bos, I.S.T.; Zaagsma, J.; Meurs, H. Arginase Strongly Impairs Neuronal Nitric Oxide-Mediated Airway Smooth Muscle Relaxation in Allergic Asthma. Respir. Res. 2006, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.R.; Böger, R.H.; Bode-Böger, S.M.; Tangphao, O.; Tsao, P.S.; Blaschke, T.F.; Cooke, J.P. Asymmetric Dimethylarginine Increases Mononuclear Cell Adhesiveness in Hypercholesterolemic Humans. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Kojda, G. Interactions between NO and Reactive Oxygen Species: Pathophysiological Importance in Atherosclerosis, Hypertension, Diabetes and Heart Failure. Cardiovasc. Res. 1999, 43, 562–571. [Google Scholar] [CrossRef]
- Jeong, H.; Vacanti, N.M. Systemic Vitamin Intake Impacting Tissue Proteomes. Nutr. Metab. 2020, 17, 73. [Google Scholar] [CrossRef]
- Chin-Dusting, J.P.F.; Alexander, C.T.; Arnold, P.J.; Hodgson, W.C.; Lux, A.S.; Jennings, G.L.R. Effects of In Vivo and In Vitro L-Arginine Supplementation on Healthy Human Vessels: J. Cardiovasc. Pharmacol. 1996, 28, 158–166. [Google Scholar] [CrossRef]
- van de Poll, M.C.; Siroen, M.P.; van Leeuwen, P.A.; Soeters, P.B.; Melis, G.C.; Boelens, P.G.; Deutz, N.E.; Dejong, C.H. Interorgan Amino Acid Exchange in Humans: Consequences for Arginine and Citrulline Metabolism. Am. J. Clin. Nutr. 2007, 85, 167–172. [Google Scholar] [CrossRef]
- Weiner, C.P.; Lizasoain, I.; Baylis, S.A.; Knowles, R.G.; Charles, I.G.; Moncada, S. Induction of Calcium-Dependent Nitric Oxide Synthases by Sex Hormones. Proc. Natl. Acad. Sci. USA 1994, 91, 5212–5216. [Google Scholar] [CrossRef]
- Tracey, W.R.; Xue, C.; Klinghofer, V.; Barlow, J.; Pollock, J.S.; Forstermann, U.; Johns, R.A. Immunochemical Detection of Inducible NO Synthase in Human Lung. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1994, 266, L722–L727. [Google Scholar] [CrossRef]
- Marletta, M.A. Nitric Oxide Synthase Structure and Mechanism. J. Biol. Chem. 1993, 268, 12231–12234. [Google Scholar] [CrossRef]
- Adeghate, E.; Ponery, A.S.; El-Sharkawy, T.; Parvez, H. L-Arginine Stimulates Insulin Secretion from the Pancreas of Normal and Diabetic Rats. Amino Acids 2001, 21, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Wu, Y.; Lenchik, N.I.; Gerling, I.; Quarles, L.D. GPRC6A Mediates the Effects of L-Arginine on Insulin Secretion in Mouse Pancreatic Islets. Endocrinology 2012, 153, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Smajilovic, S.; Clemmensen, C.; Johansen, L.D.; Wellendorph, P.; Holst, J.J.; Thams, P.G.; Ogo, E.; Bräuner-Osborne, H. The L-α-Amino Acid Receptor GPRC6A Is Expressed in the Islets of Langerhans but Is Not Involved in L-Arginine-Induced Insulin Release. Amino Acids 2013, 44, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.S.; McClenaghan, N.H.; Flatt, P.R.; Homem de Bittencourt, P.I.; Murphy, C.; Newsholme, P. L-Arginine Is Essential for Pancreatic β-Cell Functional Integrity, Metabolism and Defense from Inflammatory Challenge. J. Endocrinol. 2011, 211, 87–97. [Google Scholar] [CrossRef]
- Tsugawa, Y.; Handa, H.; Imai, T. Arginine Induces IGF-1 Secretion from the Endoplasmic Reticulum. Biochem. Biophys. Res. Commun. 2019, 514, 1128–1132. [Google Scholar] [CrossRef]
- Cho, J.; Hiramoto, M.; Masaike, Y.; Sakamoto, S.; Imai, Y.; Imai, Y.; Handa, H.; Imai, T. UGGT1 Retains Proinsulin in the Endoplasmic Reticulum in an Arginine Dependent Manner. Biochem. Biophys. Res. Commun. 2020, 527, 668–675. [Google Scholar] [CrossRef]
- Kohli, R.; Meininger, C.J.; Haynes, T.E.; Yan, W.; Self, J.T.; Wu, G. Dietary L-Arginine Supplementation Enhances Endothelial Nitric Oxide Synthesis in Streptozotocin-Induced Diabetic Rats. J. Nutr. 2004, 134, 600–608. [Google Scholar] [CrossRef]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.E.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary L-Arginine Supplementation Reduces Fat Mass in Zucker Diabetic Fatty Rats. J. Nutr. 2005, 135, 714–721. [Google Scholar] [CrossRef]
- Clemmensen, C.; Smajilovic, S.; Smith, E.P.; Woods, S.C.; Bräuner-Osborne, H.; Seeley, R.J.; D’Alessio, D.A.; Ryan, K.K. Oral L-Arginine Stimulates GLP-1 Secretion to Improve Glucose Tolerance in Male Mice. Endocrinology 2013, 154, 3978–3983. [Google Scholar] [CrossRef]
- El-Missiry, M.A.; Othman, A.I.; Amer, M.A. L-Arginine Ameliorates Oxidative Stress in Alloxan-Induced Experimental Diabetes Mellitus. J. Appl. Toxicol. 2004, 24, 93–97. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Ortiz, M.; Lores-Arnaiz, S.; Albertoni Borghese, M.F.; Balonga, S.; Lavagna, A.; Filipuzzi, A.L.; Cicerchia, D.; Majowicz, M.; Bustamante, J. Mitochondrial Dysfunction in Brain Cortex Mitochondria of STZ-Diabetic Rats: Effect of L-Arginine. Neurochem. Res. 2013, 38, 2570–2580. [Google Scholar] [CrossRef]
- Pai, M.-H.; Huang, K.-H.; Wu, C.-H.; Yeh, S.-L. Effects of Dietary Arginine on Inflammatory Mediator and Receptor of Advanced Glycation Endproducts (RAGE) Expression in Rats with Streptozotocin-Induced Type 2 Diabetes. Br. J. Nutr. 2010, 104, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Wascher, T.C.; Graier, W.F.; Dittrich, P.; Hussain, M.A.; Bahadori, B.; Wallner, S.; Toplak, H. Effects of Low-Dose L-Arginine on Insulin-Mediated Vasodilatation and Insulin Sensitivity. Eur. J. Clin. Investig. 1997, 27, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Piatti, P.; Monti, L.D.; Valsecchi, G.; Magni, F.; Setola, E.; Marchesi, F.; Galli-Kienle, M.; Pozza, G.; Alberti, K.G.M.M. Long-Term Oral L-Arginine Administration Improves Peripheral and Hepatic Insulin Sensitivity in Type 2 Diabetic Patients. Diabetes Care 2001, 24, 875–880. [Google Scholar] [CrossRef]
- Lucotti, P.; Setola, E.; Monti, L.D.; Galluccio, E.; Costa, S.; Sandoli, E.P.; Fermo, I.; Rabaiotti, G.; Gatti, R.; Piatti, P. Beneficial Effects of a Long-Term Oral L-Arginine Treatment Added to a Hypocaloric Diet and Exercise Training Program in Obese, Insulin-Resistant Type 2 Diabetic Patients. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E906–E912. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, P.; Monti, L.; Setola, E.; La Canna, G.; Castiglioni, A.; Rossodivita, A.; Pala, M.G.; Formica, F.; Paolini, G.; Catapano, A.L.; et al. Oral L-Arginine Supplementation Improves Endothelial Function and Ameliorates Insulin Sensitivity and Inflammation in Cardiopathic Nondiabetic Patients after an Aortocoronary Bypass. Metabolism 2009, 58, 1270–1276. [Google Scholar] [CrossRef]
- Bogdanski, P.; Suliburska, J.; Grabanska, K.; Musialik, K.; Cieslewicz, A.; Skoluda, A.; Jablecka, A. Effect of 3-Month L-Arginine Supplementation on Insulin Resistance and Tumor Necrosis Factor Activity in Patients with Visceral Obesity. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 816–823. [Google Scholar]
- Jabłecka, A.; Bogda, P.; Balcer, N.; Cie, A.; Skołuda, A.; Musialik, K. The Effect of Oral L-Arginine Supplementation on Fasting Glucose, HbA1c, Nitric Oxide and Total Antioxidant Status in Diabetic Patients with Atherosclerotic Peripheral Arterial Disease of Lower Extremities. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 342–350. [Google Scholar]
- Bogdanski, P.; Szulinska, M.; Suliburska, J.; Pupek-Musialik, D.; Jablecka, A.; Witmanowski, H. Supplementation with L-Arginine Favorably Influences Plasminogen Activator Inhibitor Type 1 Concentration in Obese Patients. A Randomized, Double Blind Trial. J. Endocrinol. Investig. 2013, 36, 221–226. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Pupek-Musialik, D.; Jablecka, A. Changes in Mineral Status Are Associated with Improvements in Insulin Sensitivity in Obese Patients Following L-Arginine Supplementation. Eur. J. Nutr. 2014, 53, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.D.; Casiraghi, M.C.; Setola, E.; Galluccio, E.; Pagani, M.A.; Quaglia, L.; Bosi, E.; Piatti, P. L-Arginine Enriched Biscuits Improve Endothelial Function and Glucose Metabolism: A Pilot Study in Healthy Subjects and a Cross-Over Study in Subjects with Impaired Glucose Tolerance and Metabolic Syndrome. Metabolism 2013, 62, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.D.; Setola, E.; Lucotti, P.C.G.; Marrocco-Trischitta, M.M.; Comola, M.; Galluccio, E.; Poggi, A.; Mammì, S.; Catapano, A.L.; Comi, G.; et al. Effect of a Long-Term Oral L-Arginine Supplementation on Glucose Metabolism: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Obes. Metab. 2012, 14, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.D.; Galluccio, E.; Villa, V.; Fontana, B.; Spadoni, S.; Piatti, P.M. Decreased Diabetes Risk over 9 Year after 18-Month Oral L-Arginine Treatment in Middle-Aged Subjects with Impaired Glucose Tolerance and Metabolic Syndrome (Extension Evaluation of L-Arginine Study). Eur. J. Nutr. 2018, 57, 2805–2817. [Google Scholar] [CrossRef]
- Assumpção, C.R.L.; Brunini, T.M.C.; Pereira, N.R.; Godoy-Matos, A.F.; Siqueira, M.A.S.; Mann, G.E.; Mendes-Ribeiro, A.C. Insulin Resistance in Obesity and Metabolic Syndrome: Is There a Connection with Platelet L-Arginine Transport? Blood Cells. Mol. Dis. 2010, 45, 338–342. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Chong, A.L.; Zhang, W.-Z.; Kaye, D.M. Insulin-Mediated Activation of the L-Arginine Nitric Oxide Pathway in Man, and Its Impairment in Diabetes. PLoS ONE 2013, 8, e61840. [Google Scholar] [CrossRef]
- Tsao, P.S.; McEvoy, L.M.; Drexler, H.; Butcher, E.C.; Cooke, J.P. Enhanced Endothelial Adhesiveness in Hypercholesterolemia Is Attenuated by L-Arginine. Circulation 1994, 89, 2176–2182. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Peng, F.; Wang, X.; Gong, H. L-Arginine Ameliorates High-Fat Diet-Induced Atherosclerosis by Downregulating MiR-221. BioMed Res. Int. 2020, 2020, 4291327. [Google Scholar] [CrossRef]
- Cooke, J.P.; Singer, A.H.; Tsao, P.; Zera, P.; Rowan, R.A.; Billingham, M.E. Antiatherogenic Effects of L-Arginine in the Hypercholesterolemic Rabbit. J. Clin. Investig. 1992, 90, 1168–1172. [Google Scholar] [CrossRef]
- Tsao, P.S.; Theilmeier, G.; Singer, A.H.; Leung, L.L.; Cooke, J.P. L-Arginine Attenuates Platelet Reactivity in Hypercholesterolemic Rabbits. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1529–1533. [Google Scholar] [CrossRef]
- Nematbakhsh, M.; Haghjooyjavanmard, S.; Mahmoodi, F.; Monajemi, A. The Prevention of Endothelial Dysfunction through Endothelial Cell Apoptosis Inhibition in a Hypercholesterolemic Rabbit Model: The Effect of L-Arginine Supplementation. Lipids Health Dis. 2008, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Méndez, J.D.; Balderas, F. Regulation of Hyperglycemia and Dyslipidemia by Exogenous L-Arginine in Diabetic Rats. Biochimie 2001, 83, 453–458. [Google Scholar] [CrossRef]
- El-Kirsh, A.A.A.; Abd El-Wahab, H.M.F.; Abd-Ellah Sayed, H.F. The Effect of L-Arginine or L-Citrulline Supplementation on Biochemical Parameters and the Vascular Aortic Wall in High-Fat and High-Cholesterol-Fed Rats: Role of L-Arginine or L-Citrulline on Hfc-Fed Rats. Cell Biochem. Funct. 2011, 29, 414–428. [Google Scholar] [CrossRef]
- Aly, O.; El-Matty, D.A.; Badawy, E.A.; Sherif, H.W.E.; Megahed, H.A. Regulation of Hyperglycemia and Dyslipidemia by Exogenous L-Arginine in Streptozotocin-Induced Diabetic Rats. Glob. J. Pharmacol. 2014, 8, 525–531. [Google Scholar] [CrossRef]
- Hurson, M.; Regan, M.C.; Kirk, S.J.; Wasserkrug, H.L.; Barbul, A. Metabolic Effects of Arginine in a Healthy Elderly Population. J. Parenter. Enter. Nutr. 1995, 19, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.; Adams, M.R.; Powe, A.J.; Donald, A.E.; McCredie, R.; Robinson, J.; McCarthy, S.N.; Keech, A.; Celermajer, D.S.; Deanfield, J.E. Oral L-Arginine Improves Endothelium-Dependent Dilation in Hypercholesterolemic Young Adults. J. Clin. Investig. 1996, 97, 1989–1994. [Google Scholar] [CrossRef]
- Blum, A.; Cannon, R.O.; Costello, R.; Schenke, W.H.; Csako, G. Endocrine and Lipid Effects of Oral L-Arginine Treatment in Healthy Postmenopausal Women. J. Lab. Clin. Med. 2000, 135, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Schulze, F.; Glos, S.; Petruschka, D.; Altenburg, C.; Maas, R.; Benndorf, R.; Schwedhelm, E.; Beil, U.; Böger, R.H. L-Arginine Enhances the Triglyceride-Lowering Effect of Simvastatin in Patients with Elevated Plasma Triglycerides. Nutr. Res. 2009, 29, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.A.; Higa, E.M.S.; de Mello, M.T.; Tufik, S.; Oyama, L.M.; Santos, R.V.; Amaya Farfan, J.; Risso, E.M.; De Souza, C.T.; Pimentel, G.D.; et al. Effects of Short-Term L-Arginine Supplementation on Lipid Profile and Inflammatory Proteins after Acute Resistance Exercise in Overweight Men. E-SPEN J. 2014, 9, e141–e145. [Google Scholar] [CrossRef][Green Version]
- Tripathi, P.; Misra, M.K.; Pandey, S. Role of L-Arginine on Dyslipidemic Conditions of Acute Myocardial Infarction Patients. Indian J. Clin. Biochem. 2012, 27, 296–299. [Google Scholar] [CrossRef]
- Pahlavani, N.; Jafari, M.; Sadeghi, O.; Rezaei, M.; Rasad, H.; Rahdar, H.A.; Entezari, M.H. L-Arginine Supplementation and Risk Factors of Cardiovascular Diseases in Healthy Men: A Double-Blind Randomized Clinical Trial. F1000Research 2017, 3, 306. [Google Scholar] [CrossRef] [PubMed]
- Dashtabi, A.; Mazloom, Z.; Fararouei, M.; Hejazi, N. Oral L-Arginine Administration Improves Anthropometric and Biochemical Indices Associated with Cardiovascular Diseases in Obese Patients: A Randomized, Single Blind Placebo Controlled Clinical Trial. Res. Cardiovasc. Med. 2015, 5, e29419. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Li, R.; Maimaitijiang, A.; Liu, R.; Yan, F.; Hu, H.; Gao, X.; Shi, H. MiR-221-3p Inhibits Oxidized Low-density Lipoprotein Induced Oxidative Stress and Apoptosis via Targeting a Disintegrin and Metalloprotease-22. J. Cell. Biochem. 2019, 120, 6304–6314. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Tahmasebinejad, Z.; Azizi, F. Dietary L-Arginine Intake and the Incidence of Coronary Heart Disease: Tehran Lipid and Glucose Study. Nutr. Metab. 2016, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Golzarand, M.; Davudabadi-Farahani, R.; Azizi, F. Dietary Animal-Derived L-Arginine Intakes and Risk of Chronic Kidney Disease: A 6-Year Follow-up of Tehran Lipid and Glucose Study. Iran. J. Kidney Dis. 2017, 11, 352–359. [Google Scholar]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z.; Azizi, F. Habitual Intake of Dietary L-Arginine in Relation to Risk of Type 2 Diabetes: A Prospective Study. BMC Endocr. Disord. 2021, 21, 113. [Google Scholar] [CrossRef]
| Cell Testing | ||||||
| Study (Year) | Cell Line/Model | Dose(s) of L-Arginine Tested | Control Culture | Outcome | ||
| Adeghate et al. (2001) [54] | pancreas fragments of diabetic rats | 100 mM | + | L-arginine stimulates insulin secretion | ||
| Pi et al. (2012) [55] | pancreatic islets of Gprc6a−/− mice | 10 mM | + | L-arginine stimulates insulin secretion in β-cells through GPRC6A activation of cAMP pathways | ||
| Smajilovic et al. (2013) [56] | pancreatic islets of Gprc6a−/− mice | 20 mM | + | L-arginine induces insulin secretion, but GPRC6A is not involved in the process | ||
| Krause et al. (2011) [57] | BRIN-BD11 | 0.1, 0.25, 1.15 mM | + | L-arginine induces insulin secretion, contributes to glutathione synthesis and has a protective effects in the presence of proinflammatory cytokines | ||
| Tsugawa et al. (2019) [58] | Hep G2 | 1, 3.3, 10 mM | + | L-arginine increase IGF-1 level by stimulating of growth hormone secretion | ||
| Cho et al. (2020) [59] | NIT-1 + HEK293FT | 0.1, 0.2, 0.6, 1, 2 mM | + | L-arginine induces insulin secretion due to UGGT1 regulatory functions | ||
| Animal Testing | ||||||
| Study, Year | Duration of Experiment | Dose(s) of L-Arginine Tested | Control Group | Number of Animals per Group | Animal Model | Outcome |
| Smajilovic et al. (2013) [56] | 1 min | 0.05 g/kg bw intravenously + 1 g/kg bw orally | + | 6–10 | Gprc6a−/− mice | Increase in insulin secretion after intravenous injection and oral administration of L-arginine |
| Tsugawa et al. (2019) [58] | 120 min | 3 mg/kg bw orally | + | 4 | C57BL/6J mice | L-arginine induces secretion of growth hormone and IGF-1 |
| Cho et al. (2020) [59] | 120 min | 0.75, 1.5, 3 mg/g intraperitoneally | + | - | β cell-specific UGGT1-transgenic mice | UGGT1 mediated proinsulin management regulates insulin secretion |
| Kohli et al. (2004) [60] | 2 weeks | 0.64% in diet + 1.25% in water | + | 8 | Sprague-Dawley rats | L-arginine stimulates endothelial NO synthesis by increasing BH4 concentration, increased insulin concentration in the blood and reduced blood glucose level in diabetic rats |
| Fu et al. (2005) [61] | 10 weeks | 1.44% in diet + 1.25% in water | + | 6 | Zucker diabetic fatty rats | L-arginine increases NO synthesis, lower glucose level and reduce body weight in obese and type 2 diabetic rats |
| Clemmensen et al. (2013) [62] | 15/120 min | 1 g/kg bw orally | + | 7–17 | C57BL/6 mice + Glp1r−/− mice | L-arginine increases GLP-1 and insulin levels and improves glucose clearance in obese mice; effects depends on GLP-1R-signaling |
| El-Missiry et al. (2004) [63] | 1 week | 100 mg/kg bw intragastrically | + | 6–8 | Wistar rats | L-arginine lowers serum glucose and oxidative stress in diabetic rats |
| Ortiz et al. (2013) [64] | 4 days | 622 mg/kg bw/day in water | + | 5 | Wistar rats | L-arginine ameliorates oxidative stress and the decrease in NO production in diabetic rats |
| Pai et al. (2010) [65] | 8 weeks | 1.5 g/kg bw/day orally | + | 6–13 | Wistar rats | L-arginine has no effect on plasma glucose levels, but decreases advanced glycation endproducts in diabetic rats |
| Human Research | ||||||
| Study, Year | Duration of Experiment | Dose(s) of L-Arginine Tested | Control Group | Number of Subjects per Group | Outcome | |
| Wascher et al. (1997) [66] | - | 0.52 mg/kg−1 bw/ min−1 (concomitant infusion) | + | 7–9 | L-arginine improves insulin sensitivity and restores vasodilatation (insulin-mediated) in obese and non-insulin-dependent diabetic patients; no effects was observed on insulin or IGF-1 levels | |
| Piatti et al. (2001) [67] | 3 months (1 month of intervention) | 3 × 3 g/day orally | + | 12–40 | L-arginine normalizes cGMP levels, improves glucose disposal and systolic blood pressure; the treatment attenuates insulin resistance in type 2 diabetic patients | |
| Lucotti et al. (2006) [68] | 3 weeks | 8.3 g/day orally | + | 16–17 | L-arginine positively affects glucose metabolism and insulin sensitivity, improves endothelial function, oxidative stress, and adipokine release in obese type 2 diabetic patients | |
| Lucotti et al. (2009) [69] | 6 months | 6.4 g/day orally | + | 32 | L-arginine regulates endothelial dysfunction, improves insulin sensitivity and reduces inflammation | |
| Bogdański et al. (2012) [70] | 3 months | 3 × 9 g/day orally | + | 20 | L-arginine decreases insulin level and improves insulin sensitivity; TNF-alpha plays role in the pathogenesis of insulin resistance in patients with obesity | |
| Jabłecka et al. (2012) [71] | 2 months | 3 × 2 g/day orally | + | 12–38 | L-arginine does not affect fasting glucose and HbA1 level in diabetic patients with atherosclerotic peripheral arterial disease, but increases NO and TAS levels | |
| Bogdanski et al. (2013) [72] | 6 months | 3 × 9 g/day orally | + | 44 | L-arginine decreases plasminogen activator type 1, increases NO and TAS levels, and improves insulin sensitivity in obese patients | |
| Suliburska et al. (2014) [73] | 6 months | 3 × 9 g/day orally | + | 44 | L-arginine affects zinc serum concentrations in obese patients; positive correlation between the change in zinc and insulin sensitivity improvement was observed | |
| Monti et al. (2013) [74] | 6 weeks (2 weeks of intervention) | 6.6 g/day orally | cross-over study | 7–8/15 | L-arginine improves glucose metabolism, insulin secretion and insulin sensitivity; it enhances endothelial function in patients with impaired glucose tolerance and metabolic syndrome | |
| Monti et al. (2012) [75] | 18 months + 12-month follow-up period | 6.4 g/day orally | + | 72 | L-arginine improves β-cell function and insulin sensitivity, and increase probability to become normal glucose tolerant, but does not reduce the incidence of diabetes in patients with impaired glucose tolerance and metabolic syndrome | |
| Monti et al. (2018) [76] | 18 months + 90-month follow-up | 6.4 g/day orally | + | 45–47 | L-arginine delays the development of T2DM; the effect could be related to reduction in oxidative stress | |
| Cell Testing | ||||||
| Study (Year) | Cell Line | Dose(s) of L-Arginine Tested | Control Culture | Outcome | ||
| Tsao et al. (1994a) [79] | mononuclear cells of New Zealand White rabbits + WEHI 78/24 | 2.25% L-arginine HCl in water (animals) | + | Endothelial adhesiveness is attenuated by L-arginine; NO acts as an endogenous antiatherogenic agent; L-arginine normalizes NO-dependent vasodilation and inhibits atherogenesis in a hypercholesterolaemic rabbits | ||
| Zhang et al. (2020) [80] | aortic endothelial cells of Sprague-Dawley rats | 1 g/kg bw/day (injection to animals) + 5, 25, 50 mM (isolated cells) | + | L-arginine inhibits the expression of miR-221 and increases the expression of eNOS in cells; L-arginine exerts milder effects than simvastatin, but presumably has fewer side effects | ||
| Animal Testing | ||||||
| Study, Year | Duration of Experiment | Dose(s) of L-Arginine Tested | Control Group | Number of Animals per Group | Animal Model | Outcome |
| Cooke et al. (1992) [81] | 10 weeks | 2.25% L-arginine HCI in water | + | 16–20 | New Zealand White rabbits | L-arginine, as a endothelium-derived relaxing factor precursor, improves endothelium-dependent vasorelaxation |
| Tsao et al. (1994b) [82] | 10 weeks | 2.25% L-arginine HCI in water | + | 3 | New Zealand White rabbits | L-arginine has antiatherogenic properties and inhibits platelet aggregation in hypercholesterolaemic rabbits; the effect is presumably due to the increase in NO production |
| Nematbakhsh et al. (2008) [83] | 4 weeks | 3% L-arginine in water | + | 14–16 | white rabbits | L-arginine exerts no effect on T-C level, but increases nitrite concentration; L-arginine restores endothelial function in hypercholesterolaemic rabbits by the inhibition of apoptosis in endothelial cells |
| Méndez and Balderas (2001) [84] | 12 days | 10 mM/day (intraperitoneal injection) | + | 5–48 | Sprague-Dawley rats | L-arginine normalizes glycaemia and alleviate hyperlipidaemia by reducing TG, T-C and LDL-C levels in diabetic rats |
| El-Kirsh et al. (2011) [85] | 8 weeks | 100 mg/kg bw/day orally | + | 8 | albino rats | L-arginine has hypocholesterolaemic and hypolipidaemic effects; it regulates AST and ALT activities, urea level and lipid profile biomarkers; L-arginine, by promoting NO production, regulates biochemical disturbances and progression of aortic diseases; in high-fat and high-cholesterol diet fed rats. |
| Aly et al. (2014) [86] | 8 weeks | 10 mM/kg bw/day orally | + | 15 | Sprague-Dawley rats | L-arginine increases insulin and HDL-C levels, and decreases glucose, LDL-C, T-C and TG levels; L-arginine attenuates insulin resistance in diabetic rats |
| Human Research | ||||||
| Study, Year | Duration of Experiment | Dose(s) of L-Arginine Tested | Control Group | Number of Subjects per Group | Outcome | |
| Hurson et al. (1995) [87] | 2 weeks | 17 g/day orally | + | 15–30 | L-arginine improves nitrogen balance, elevates serum IGF-1 concentrations, and reduces T-C and LDL-C levels in elderly humans; no adverse effects were observed | |
| Clarkson et al. (1996) [88] | 12 weeks (4 weeks of intervention) | 3 × 7 g/day orally | cross-over study | 27 | L-arginine has no effect on lipid profile (TG, T-C, HDL-C, LDL-C levels); L-arginine improves endothelium-dependent dilation in hypercholesterolaemic young adults, which might attenuate atherogenic processes | |
| Blum et al. (2000) [89] | 3 months (1 month of intervention) | 3 × 3 g/day orally | cross-over study | 10 | L-arginine increases growth hormone level, but does not affect insulin, catecholamines and lipid profile (TG, T-C, HDL-C, LDL-C, VLDL-C levels) in postmenopausal women | |
| Schulze et al. (2009) [90] | 18 weeks (6 weeks of intervention) | 2 × 1.5 g/day orally | + | 11–22 | L-arginine + simvastatin reduces TG level compared to placebo + simvastatin; L-arginine attenuates increases in AST and fibrinogen induced by simvastatin; L-arginine intensifies effects of simvastatin on lipid metabolism markers, but it has no effects when given alone in patients with hypertriglyceridaemia | |
| Nascimento et al. (2014) [91] | 3 weeks (1 week of intervention) | 3 × 2 g/day orally | cross-over study | 7 | No effects on TG, T-C and adiponectin levels were observed; L-arginine decreases LDL-C and non-esterified fatty acids levels; L-arginine can enhance effects of exercise inducing changes in lipid profile in overweight men | |
| Tripathi et al. (2012) [92] | 15 days | 3 g/day orally | + | 60–70 | L-arginine administration was found to improve the lipid profile in patients with acute myocardial infarction; L-arginine regulates modified cholesterol levels and increases HDL-C; L-arginine might be useful against precipitation of myocardial ischemia in elderly population | |
| Pahlavani et al. (2017) [93] | 45 days | 2 g/day orally | + | 28 | L-arginine improves glycaemia and lipid profile (TG, T-C, LDL-C, HDL-C), but has no effect on blood pressure in male athletes | |
| Dashtabi et al. (2016) [94] | 8 week | 3 × 3 or 6 g/day orally | + | 27–28 | L-arginine decreases, blood pressure, glycaemia, MDA, TG, T-C, LDL-C and levels and increases HDL-C level; L-arginine improves anthropometric parameters, blood pressure and blood biochemical indices in patients with obesity | |
| Schulman et al. (2006) [35] | 6 months | 3 × 3 g/day orally | + | 28–30 | L-arginine does not improve measurements related to vascular stiffness or ejection fraction; supplementary L-arginine might be associated with higher postinfarction mortality and should not be recommended for elderly patients after acute myocardial infarction | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlas, A.; Kurek, J.M.; Krejpcio, Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review. Nutrients 2022, 14, 961. https://doi.org/10.3390/nu14050961
Szlas A, Kurek JM, Krejpcio Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review. Nutrients. 2022; 14(5):961. https://doi.org/10.3390/nu14050961
Chicago/Turabian StyleSzlas, Aleksandra, Jakub Michał Kurek, and Zbigniew Krejpcio. 2022. "The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review" Nutrients 14, no. 5: 961. https://doi.org/10.3390/nu14050961
APA StyleSzlas, A., Kurek, J. M., & Krejpcio, Z. (2022). The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review. Nutrients, 14(5), 961. https://doi.org/10.3390/nu14050961