Soy Formula Is Not Estrogenic and Does Not Result in Reproductive Toxicity in Male Piglets: Results from a Controlled Feeding Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Serum Soy Isoflavones
2.3. Serum Hormones
2.4. Testis Morphology and Cellularity
3. Gene Expression Analysis by mRNAseq
4. mRNAseq Confirmation by Real-Time RT-PCR
5. Statistical Analysis
6. Results
6.1. Serum Soy Isoflavone Concentrations Detected in the Soy- and M + G-Fed Piglets
6.2. Effects of Formula Feeding, Genistein and E2 on Body and Organ Weights
6.3. Effects of Formula, Genistein, and E2 on Endocrine Parameters in Neonatal Male Piglets
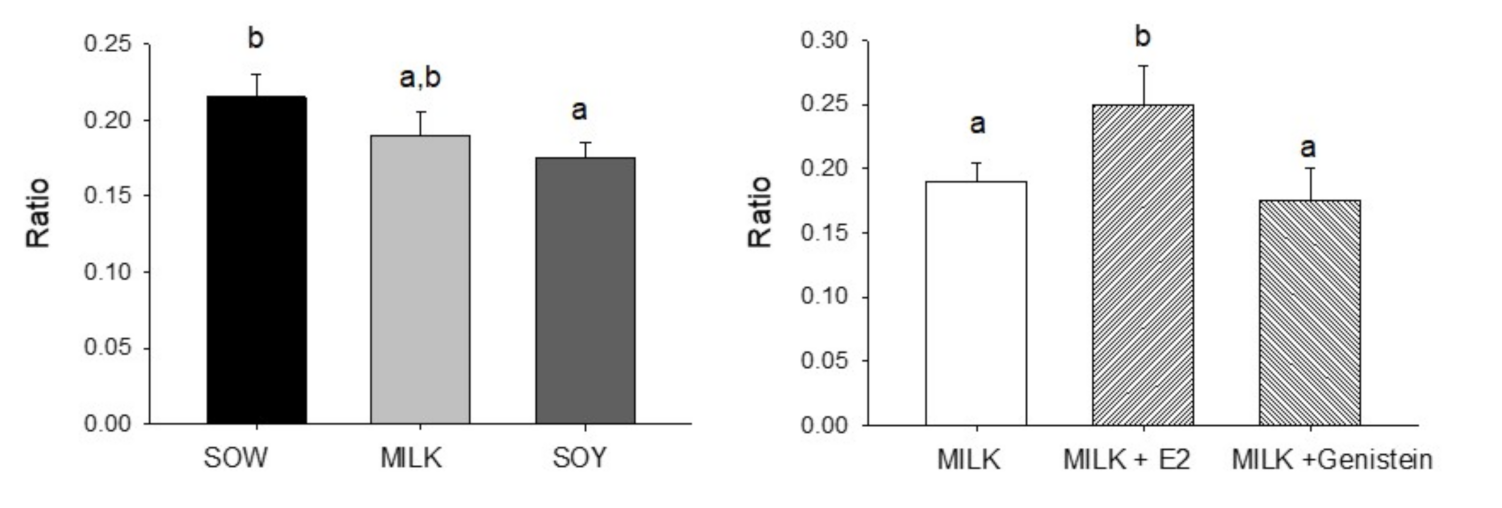
6.4. Effects of Formula Feeding, Genistein, and E2 on Testicular Morphology and Cellularity in Neonatal Male Piglets
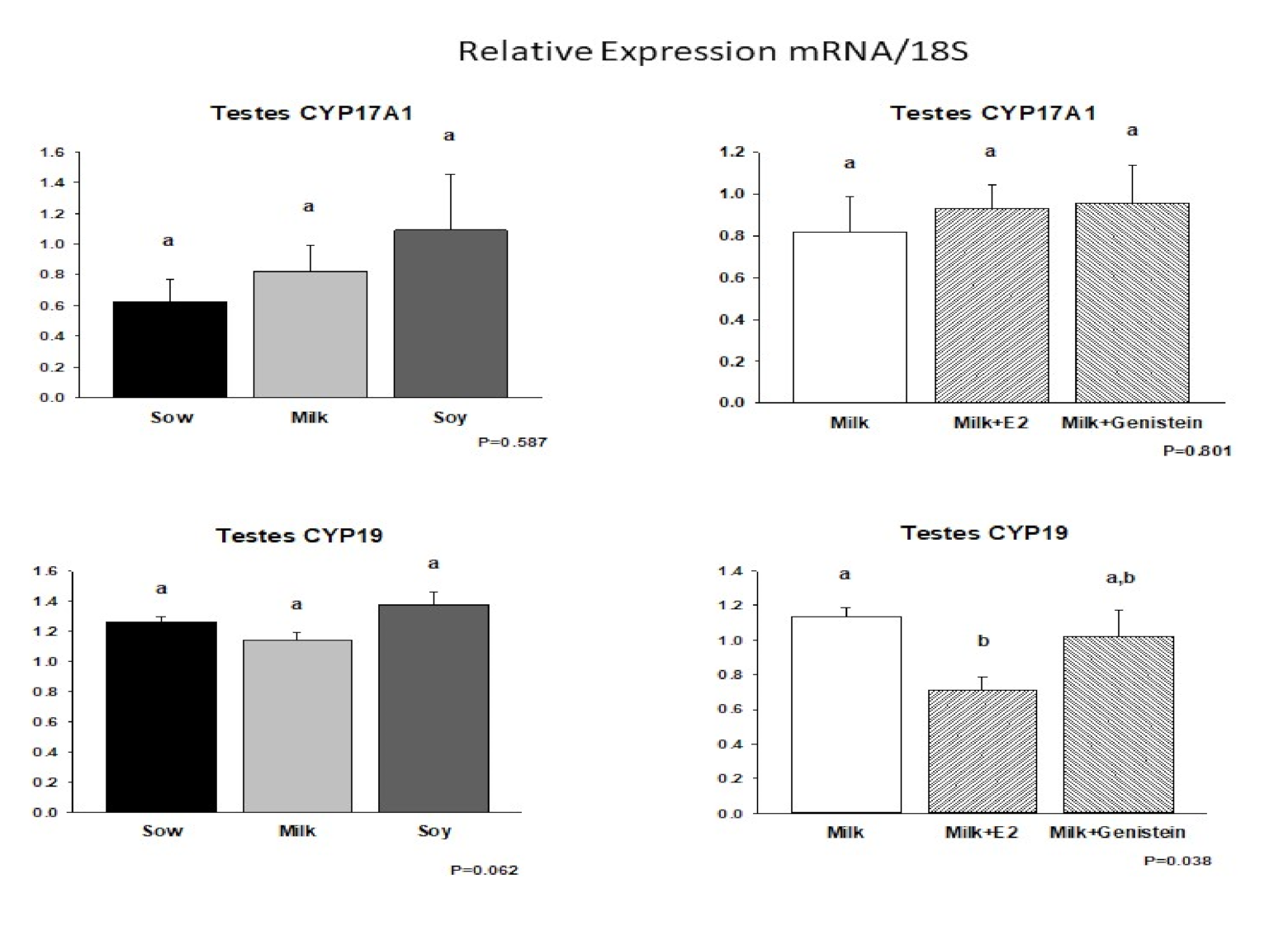
6.5. Effects of Formula Feeding, Genistein, and E2 on Expression of mRNAs Encoding Steroid Biosynthetic Enzymes in Neonatal Male Piglet Testis
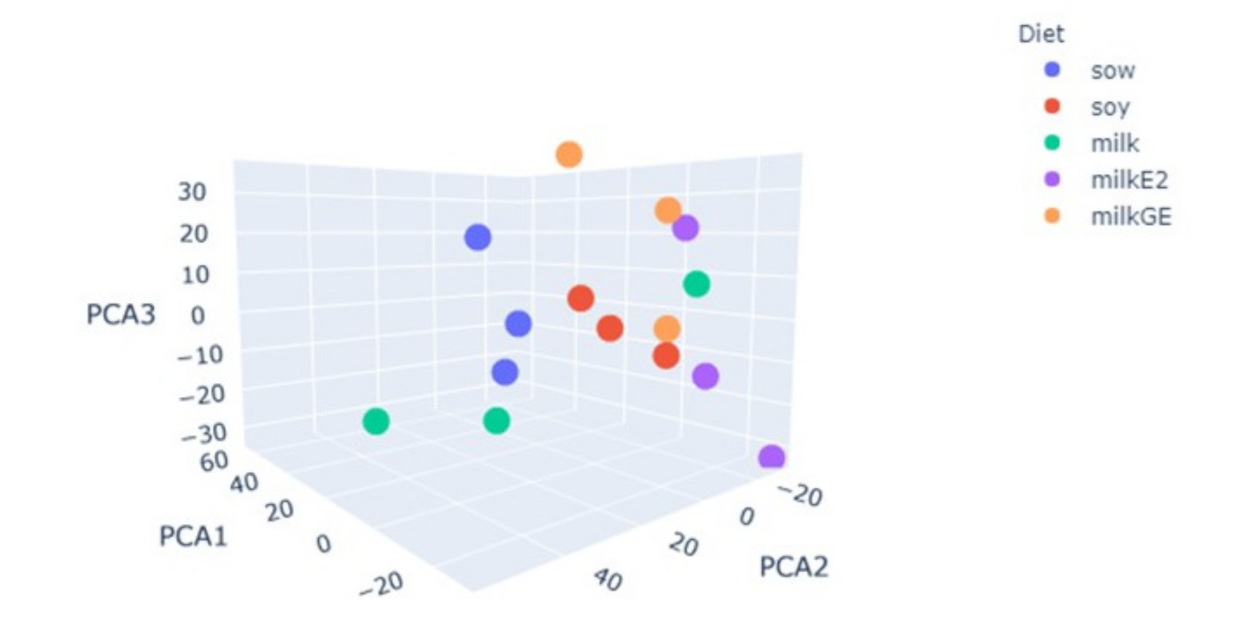
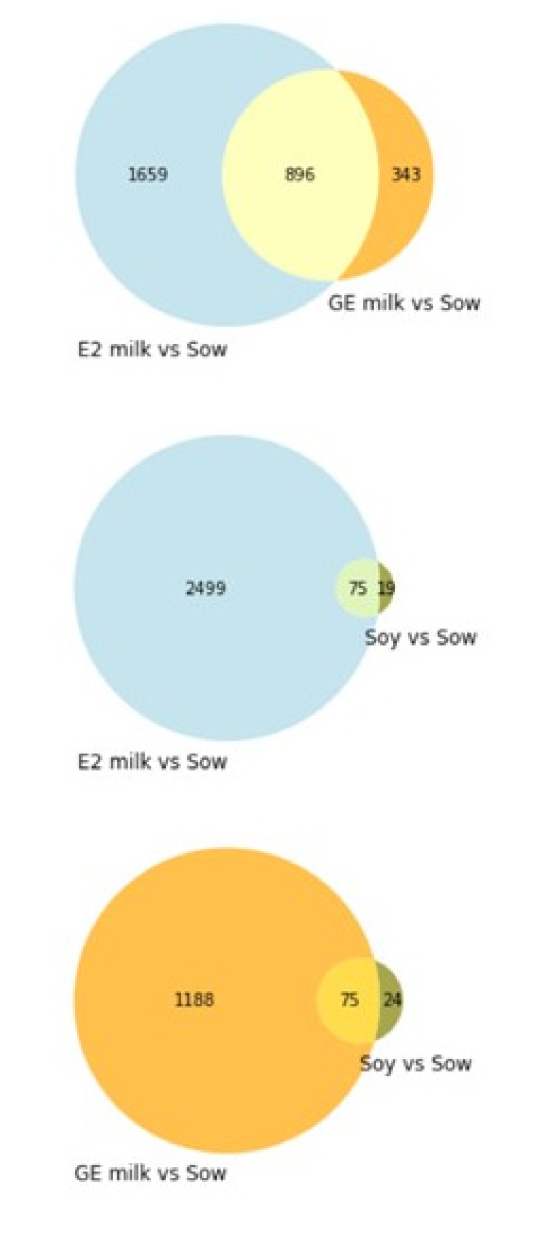
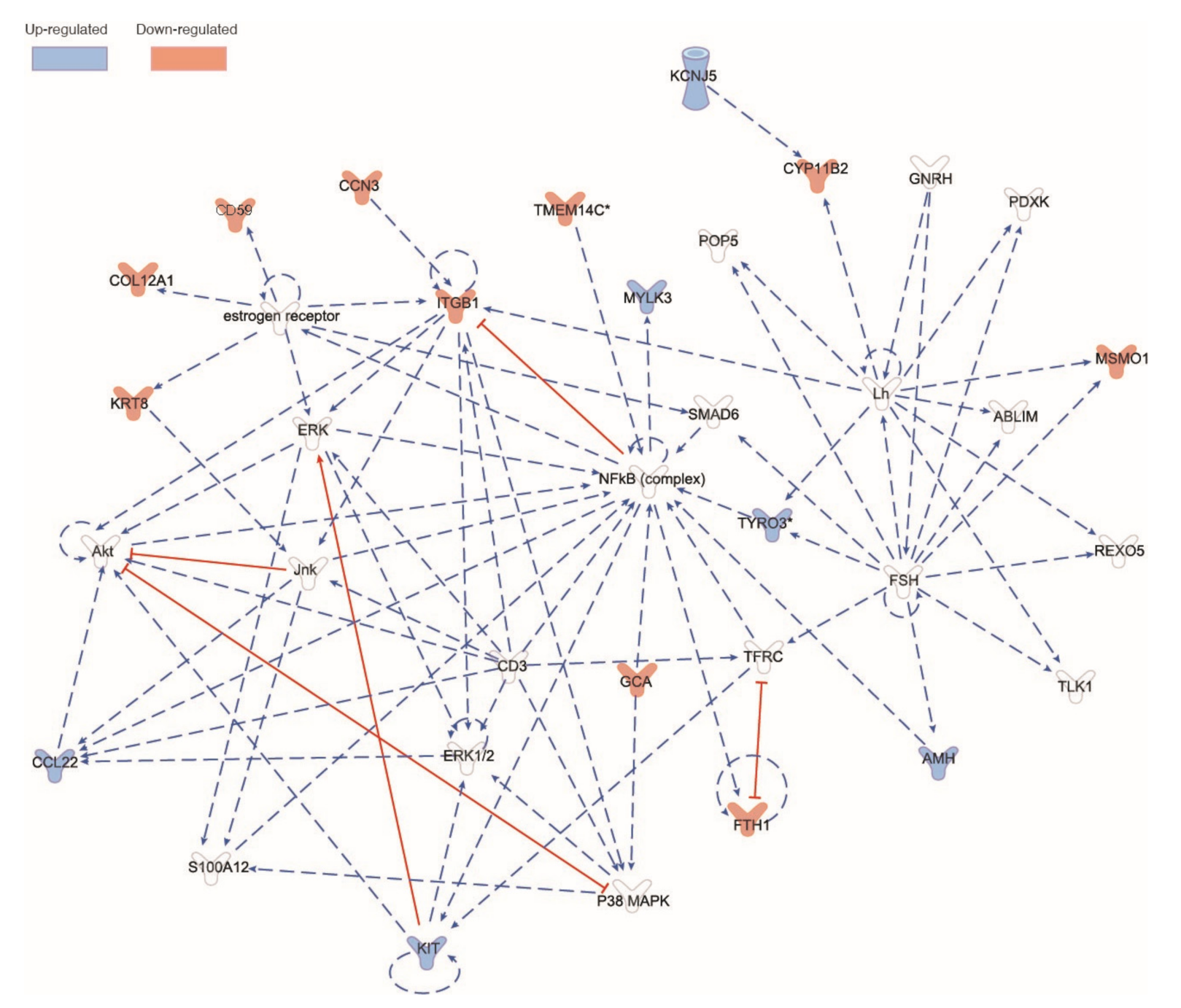
6.6. RNAseq Analysis of Male Piglet Testis
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Academy of Pediatrics. Promotion of breast feeding: Recommendations of the Councils of the Society for Pediatric Research (SPR) and American Pediatric Society (APS), and of the American Academy of Pediatrics (AAP). Pediatr. Res. 1982, 16, 264–265. [Google Scholar] [CrossRef][Green Version]
- Sayres, S.; Visentin, L. Breastfeeding: Uncovering barriers and offering solutions. Curr. Opin. Pediatr. 2018, 30, 591–596. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Merritt, R.J.; Jenks, B.H. Safety of soy-based infant formulas containing isoflavones: The clinical evidence. J. Nutr. 2004, 134, 1220S–1224S. [Google Scholar] [CrossRef]
- Badger, T.M.; Gilchrist, J.M.; Pivik, R.T.; Andres, A.; Shankar, K.; Chen, J.R.; Ronis, M.J. The health implications of soy infant formula. Am. J. Clin. Nutr. 2009, 89, 1668S–1672S. [Google Scholar] [CrossRef]
- Gilchrist, J.M.; Moore, M.B.; Andres, A.; Estroff, J.A.; Badger, T.M. Ultrasonographic patterns of reproductive organs in infants fed soy formula: Comparisons to infants fed breast milk and milk formula. J. Pediatr. 2010, 156, 215–220. [Google Scholar] [CrossRef]
- Andres, A.; Moore, M.B.; Linam, L.E.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Compared with feeding infants breast milk or cow-milk formula, soy formula feeding does not affect subsequent reproductive organ size at 5 years of age. J. Nutr. 2015, 145, 871–875. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Devreker, T.; Hauser, B. Soy infant formula: Is it that bad? Acta Paediatr. 2011, 100, 162–166. [Google Scholar] [CrossRef]
- Harlid, S.; Adgent, M.; Jefferson, W.N.; Panduri, V.; Umbach, D.M.; Xu, Z.; Stallings, V.A.; Williams, C.J.; Rogan, W.J.; Taylor, J.A. Soy Formula and Epigenetic Modifications: Analysis of Vaginal Epithelial Cells from Infant Girls in the IFED Study. Environ. Health Perspect. 2017, 125, 447–452. [Google Scholar] [CrossRef]
- Adgent, M.A.; Umbach, D.M.; Zemel, B.S.; Kelly, A.; Schall, J.I.; Ford, E.G.; James, K.; Darge, K.; Botelho, J.C.; Vesper, H.W.; et al. A Longitudinal Study of Estrogen-Responsive Tissues and Hormone Concentrations in Infants Fed Soy Formula. J. Clin. Endocrinol. Metab. 2018, 103, 1899–1909. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Martin, B.; Morris, K.; Greig, I.; McKinnell, C.; McNeilly, A.S.; Walker, M. Infant feeding with soy formula milk: Effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum. Reprod. 2002, 17, 1692–1703. [Google Scholar] [CrossRef]
- Tan, K.A.; Walker, M.; Morris, K.; Greig, I.; Mason, J.I.; Sharpe, R.M. Infant feeding with soy formula milk: Effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Hum. Reprod. 2006, 21, 896–904. [Google Scholar] [CrossRef]
- McCarver, G.; Bhatia, J.; Chambers, C.; Clarke, R.; Etzel, R.; Foster, W.; Hoyer, P.; Leeder, J.S.; Peters, J.M.; Rissman, E.; et al. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 421–468. [Google Scholar] [CrossRef]
- Messina, M.J.; Persky, V.; Setchell, K.D.; Barnes, S. Soy intake and cancer risk: A review of the in vitro and in vivo data. Nutr. Cancer 1994, 21, 113–131. [Google Scholar] [CrossRef]
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997, 350, 23–27. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Messina, M.; Barnes, S.; Cassidy, A.; Duncan, A.; Kurzur, M.; Meija, S.B.; Ronis, M.; Rowland, I.; Sievenpiper, J. Neither Soyfoods nor isoflavones warrant classification as endocrine disruptors: A review of clinical and observational data. Crit. Rev. Food Sci. Nutr. 2021, 27, 1–57. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Morito, K.; Aomori, T.; Hirose, T.; Kinjo, J.; Hasegawa, J.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of phytoestrogens with estrogen receptors alpha and beta (II). Biol. Pharm. Bull. 2002, 25, 48–52. [Google Scholar] [CrossRef]
- Ye, H.; Shaw, I.C. Food flavonoid ligand structure/estrogen receptor-α affinity relationships—Toxicity or food functionality? Food Chem. Toxicol. 2019, 129, 328–336. [Google Scholar] [CrossRef]
- Cederroth, C.R.; Zimmermann, C.; Nef, S. Soy, phytoestrogens and their impact on reproductive health. Mol. Cell. Endocrinol. 2012, 355, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Akingbemi, B.T. Estrogen regulation of testicular function. Reprod. Biol. Endocrinol. 2005, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Bjornsdottir, S.; Hoyer, P.E.; Byskov, A.G. Ontogeny of oestrogen receptor alpha in gonads and sex ducts of fetal and newborn mice. J. Reprod. Fertil. 2001, 118, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Atanassova, N.; McKinnell, C.; Walker, M.; Turner, K.J.; Fisher, J.S.; Morley, M.; Millar, M.R.; Groome, N.P.; Sharpe, R.M. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology 1999, 140, 5364–5373. [Google Scholar] [CrossRef]
- Wisniewski, A.B.; Klein, S.L.; Lakshmanan, Y.; Gearhart, J.P. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J. Urol. 2003, 169, 1582–1586. [Google Scholar] [CrossRef]
- Meena, R.; Supriya, C.; Pratap Reddy, K.; Sreenivasula Reddy, P. Altered spermatogenesis, steroidogenesis and suppressed fertility in adult male rats exposed to genistein, a non-steroidal phytoestrogen during embryonic development. Food Chem. Toxicol. 2017, 99, 70–77. [Google Scholar] [CrossRef]
- Badger, T.M.; Ronis, M.J.; Hakkak, R. Developmental effects and health aspects of soy protein isolate, casein, and whey in male and female rats. Int. J. Toxicol. 2001, 20, 165–174. [Google Scholar] [CrossRef]
- Singhal, R.; Shankar, K.; Badger, T.M.; Ronis, M.J. Hepatic gene expression following consumption of soy protein isolate in female Sprague Dawley rats differs from that produced by 17β-estradiol treatments. J. Endocrinol. 2009, 202, 141–152. [Google Scholar] [CrossRef]
- Zhang, J.; Lazarenko, O.P.; Wu, X.; Tong, Y.; Blackburn, M.L.; Gomez-Acevedo, H.; Shankar, K.; Badger, T.M.; Ronis, M.J.; Chen, J.R. Differential effects of short-term feeding of a soy protein isolate diet and estrogen treatment on bone in the pre-pubertal rat. PLoS ONE 2012, 7, e35736. [Google Scholar] [CrossRef]
- Miousse, I.R.; Sharma, N.; Blackburn, M.; Vantrease, J.; Gomez-Acevedo, H.; Hennings, L.; Shankar, K.; Cleves, M.A.; Badger, T.M.; Ronis, M.J. Feeding soy protein isolate and treatment with estradiol have different effects on mammary gland morphology and gene expression in weanling male and female rats. Physiol. Genom. 2013, 45, 1072–1083. [Google Scholar] [CrossRef][Green Version]
- Ronis, M.J.; Shankar, K.; Gomez-Acevedo, H.; Hennings, L.; Singhal, R.; Blackburn, M.L.; Badger, T.M. Mammary gland morphology and gene expression differ in female rats treated with 17β-estradiol or fed soy protein isolate. Endocrinology 2012, 153, 6021–6032. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Gomez-Acevedo, H.; Blackburn, M.L.; Cleves, M.A.; Singhal, R.; Badger, T.M. Uterine responses to feeding soy protein isolate and treatment with 17β-estradiol differ in ovariectomized female rats. Toxicol. Appl. Pharmacol. 2016, 297, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Gomez-Acevedo, H.; Shankar, K.; Sharma, N.; Blackburn, M.; Singhal, R.; Mercer, K.E.; Badger, T.M. Soy protein isolate feeding does not result in reproductive toxicity in the pre-pubertal rat testis. Exp. Biol. Med. (Maywood) 2018, 243, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Mercer, K.E.; Bhattacharyya, S.; Sharma, N.; Chaudhury, M.; Lin, H.; Yeruva, L.; Ronis, M.J. Infant Formula Feeding Changes the Proliferative Status in Piglet Neonatal Mammary Glands Independently of Estrogen Signaling. J. Nutr. 2020, 150, 730–738. [Google Scholar] [CrossRef]
- Chen, J.R.; Lazarenko, O.P.; Blackburn, M.; Badeaux, J.V.; Badger, T.M.; Ronis, M.J. Infant formula promotes bone growth in neonatal piglets by enhancing osteoblastogenesis through bone morphogenic protein signaling. J. Nutr. 2009, 139, 1839–1847. [Google Scholar] [CrossRef]
- Lanning, L.L.; Creasy, D.M.; Chapin, R.E.; Mann, P.C.; Barlow, N.J.; Regan, K.S.; Goodman, D.S. Recommended approaches for the evaluation of testicular and epididymal toxicity. Toxicol. Pathol. 2002, 30, 507–520. [Google Scholar] [CrossRef]
- Ronis, M.J.; Chen, Y.; Shankar, K.; Gomez-Acevedo, H.; Cleves, M.A.; Badeaux, J.; Blackburn, M.L.; Badger, T.M. Formula feeding alters hepatic gene expression signature, iron and cholesterol homeostasis in the neonatal pig. Physiol. Genom. 2011, 23, 1281–1293. [Google Scholar] [CrossRef]
- Chen, C.; Perry, T.L.l.; Chitko-McKown, C.G.; Smith, A.D.; Cheung, L.; Beshah, E.; Urban, J.F.; Dawson, H.D. The regulatory actions of retinoic acid on M2 polarization of porcine macrophages. Dev. Comp. Immunol. 2019, 98, 20–33. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Sugihara, M.; Morito, D.; Ainuki, S.; Hirano, Y.; Ogino, K.; Kitamura, A.; Hirata, H.; Nagata, K. The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets. J. Cell Biol. 2019, 218, 949–960. [Google Scholar] [CrossRef]
- Huang, S.; Ye, L.; Chen, H. Sex determination and maintenance: The role of DMRT1 and FOXL2. Asian J. Androl. 2017, 19, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.N.; Wang, C.Y.; Chen, C.F.; Sun, Z.; Lai, M.D.; Lin, Y.C. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kang, H.; Park, E.; Park, H.S.; Lee, K. The expression of CKLFSF2B is regulated by GATA1 and CREB in the Leydig cells, which modulates testicular steroidogenesis. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Dudem, S.; Large, R.J.; Kulkarni, S.; McClafferty, H.; Tikhonova, I.G.; Sergeant, G.P.; Thornbury, K.D.; Shipston, M.J.; Perrino, B.A.; Hollywood, M.A. LINGO1 is a regulatory subunit of large conductance, Ca 2+-activated potassium channels. Proc. Natl. Acad. Sci. USA 2020, 117, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Park, I.; Guililat, H.; Sang, S.; Talapatra, A.; Singhal, B.; Mills, N.C. Testosterone regulates granzyme K expression in rat testes. Endocr. Regul. 2017, 51, 193–204. [Google Scholar] [CrossRef][Green Version]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Lu, X.J.; Chen, J.; Yu, C.H.; Shi, Y.H.; He, Y.Q.; Zhang, R.C.; Huang, Z.A.; Lv, J.N.; Zhang, S.; Xu, L. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J. Exp. Med. 2013, 210, 5–13. [Google Scholar] [CrossRef]
- Moore, F.L.; Jaruzelska, J.; Fox, M.S.; Urano, J.; Firpo, M.T.; Ture, P.J.; Dorfman, D.M.; Reijo Pera, R.A. Human Pumilio-2 is expressed in embryonic stem cells and germ cells and interacts with DAZ (Deleted in Azoospermia) and DAZ-Like proteins. Proc. Natl. Acad. Sci. USA 2002, 100, 538–543. [Google Scholar] [CrossRef]
- Patil, A.A.; Cai, Y.; Sang, Y.; Blecha, F.; Zhang, G. Cross-species analysis of the mammalian beta-defensin gene family: Presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol. Genom. 2005, 23, 5–17. [Google Scholar] [CrossRef]
- Bremmer, F.; Strobel, P.; Jarry, H.; Strecker, J.; Gaisa, N.; Strauss, A.; Schweyer, S.; Radzun, H.J.; Behnes, C.L. CK19 is a sensitive marker for yolk sac tumours of the testis. Diagn. Pathol. 2015, 10, 7. [Google Scholar] [CrossRef]
- Ammirante, M.; Shalapour, S.; Kang, Y.; Jamieson, C.A.; Karin, M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc. Natl. Acad. Sci. USA 2014, 111, 14776–14781. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Yu, S.; Badger, T.M. Comprehensive phytochemical profile of soy protein isolate. J. Agric. Food Chem. 2004, 52, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; House, S.E.; Prior, R.L.; Fang, N.; Ronis, M.J.; Clarkson, T.B.; Wilson, M.E.; Badger, T.M. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J. Nutr. 2006, 136, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Galandi, S.L.; Summer, S.S.; Zhao, X.; Heubi, J.E.; King, E.C.; Setchell, K.D.R. S-(-) equol production is developmentally regulated and related to early diet composition. Nutr. Res. 2014, 34, 401–409. [Google Scholar] [CrossRef]
- Suen, A.A.; Kenan, A.C.; Williams, C.J. Developmental exposure to phytoestrogens in soy: New findings and clinical implications. Biochem. Pharmacol. 2022, 195, 114848. [Google Scholar] [CrossRef]
- Dominguez-Lopez, I.; Yago-Aragon, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef]



| Diet | Genistein (pg/mL) | Daidzein (pg/mL) | Equal (pg/mL) | 0-DMA (pg/mL) | Glycitein (pg/mL) |
|---|---|---|---|---|---|
| Sow Milk | 56 ± 8 a | 256 ± 7 a | 5 ± 1 | 0 a | 139 ± 87 a |
| Milk Formula | 23 ± 9 a | 2 ± 1 a | 4 ± 1 | 0 a | 0 a |
| Soy Formula | 1712 ± 212 b | 1673 ± 374 b | 3 ± 1 | 2995 ± 714 b | 4567 ± 867 b |
| Milk + Genistein | 1898 ± 515 b | 7 ± 1 a | 9 ± 3 | 0 a | 379 ± 241 a |
| Milk + E2 | 142 ± 1 a | 3 ± 2 a | 5 ± 1 | 2 ± 1 a | 9 ± 1 a |
| Body Weight | Testis Weight | %BW | Prostate Weight | %BW | |
|---|---|---|---|---|---|
| (kg) | (g) | (g) | |||
| Sow Milk | 7.30 ± 0.18 | 6.64 ± 0.76 a | 0.0090 ± 0.0009 | 0.38 ± 0.02 | 0.0005 ± 0.0001 |
| Milk Formula | 8.33 ± 0.27 | 7.93 ± 0.57 a,* | 0.0096 ± 0.0010 * | 0.37 ± 0.03 | 0.0005 ± 0.0001 |
| Soy Formula | 8.52 ± 0.38 | 10.54 ± 1.37 b | 0.0122 ± 0.0013 | 0.31 ± 0.04 | 0.0004 ± 0.0001 |
| Milk + Genistein | 7.88 ± 0.42 | 9.46 ± 1.50 a,* | 0.0122 ± 0.0014 * | 0.36 ± 0.05 | 0.0005 ± 0.0001 |
| Milk + E2 | 8.27 ± 0.47 | 4.31 ± 0.5 a,# | 0.0052 ± 0.0006 # | 0.44 ± 0.05 | 0.0005 ± 0.0001 |
| Diet | FSH (mIU/mL) | LH (mIU/mL) | Androsterone (ng/mL) | Testosterone (ng/mL) | DHT (ng/mL) |
|---|---|---|---|---|---|
| Sow Milk | 0.10 ± 0.05 a | 0.007 ± 0.007 | 76 ± 8 b | 0.52 ± 0.10 b | 1.65 ± 0.17 |
| Milk Formula | 0.13 ± 0.04 a,# | 0.047 ± 0.02 | 42 ± 6 a,# | 0.35 ± 0.02 b,* | 1.33 ± 0.17 |
| Soy Formula | 0.52 ± 0.04 b | 0.001 ± 0.001 | 86 ± 5 b | 0.50 ± 0.10 b | 1.46 ± 0.14 |
| Milk + Genistein | 0.34 ± 0.04 b,* | 0.052 ± 0.003 | 64 ± 8 b,* | 0.42 ± 0.08 b,* | 1.24 ± 0.20 |
| Milk + E2 | 0.07 ± 0.04 a,# | 0.040 ± 0.03 | 28 ± 6 a,# | 0.09 ± 0.02 a,# | 0.88 ± 0.26 |
| Diet | DHEA (ng/mL) | DHEA-S (ng/mL) | Progesterone (ng/mL) | Estradiol (pg/mL) | Estrone (pg/mL) |
|---|---|---|---|---|---|
| Sow Milk | 3.9 ± 0.2 | 29.7 ± 1.1 a | 4.20 ± 0.25 b | 115 ± 22 a | 354 ± 42 a |
| Milk Formula | 4.5 ± 0.2 | 30.5 ± 1.6 a | 0.59 ± 0.05 a | 78 ± 18 a,# | 240 ± 23 a,# |
| Soy Formula | 4.2 ± 0.2 | 36.7 ± 1.2 b | 1.12 ± 0.23 a | 113 ± 12 a | 335 ± 31 a |
| Milk + Genistein | 4.2 ± 0.4 | 32.1 ± 1.0 a,b | 0.54 ± 0.26 a | 108 ± 12 a | 284 ± 21 a,# |
| Milk + E2 | 4.5 ± 0.4 | 32.7 ± 2.2 a,b | 0.85 ± 0.21 a | 1376 ± 418 b,* | 1615 ± 321 b,* |
| PATHWAY | p-Value | FDR |
|---|---|---|
| ssc05204: Chemical carcinogenesis | 0.027065737 | 0.481770112 |
| ssc04146: Peroxisome | 0.030864823 | 0.515056728 |
| ssc00980: Metabolism of xenobiotics by cytochrome P450 | 0.036679394 | 0.576082249 |
| ssc01212: Fatty acid metabolism | 0.051689275 | 0.766724247 |
| ssc00100: Steroid biosynthesis | 0.071473127 | 0.970909091 |
| ssc05205: Proteoglycans in cancer | 0.092373727 | 0.970909091 |
| ssc03018: RNA degradation | 0.097239374 | 0.970909091 |
| ssc04260: Cardiac muscle contraction | 0.097239374 | 0.970909091 |
| ssc00650: Butanoate metabolism | 0.0987639250 | 0.970909091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronis, M.J.J.; Gomez-Acevedo, H.; Shankar, K.; Hennings, L.; Sharma, N.; Blackburn, M.L.; Miousse, I.; Dawson, H.; Chen, C.; Mercer, K.E.; et al. Soy Formula Is Not Estrogenic and Does Not Result in Reproductive Toxicity in Male Piglets: Results from a Controlled Feeding Study. Nutrients 2022, 14, 1126. https://doi.org/10.3390/nu14051126
Ronis MJJ, Gomez-Acevedo H, Shankar K, Hennings L, Sharma N, Blackburn ML, Miousse I, Dawson H, Chen C, Mercer KE, et al. Soy Formula Is Not Estrogenic and Does Not Result in Reproductive Toxicity in Male Piglets: Results from a Controlled Feeding Study. Nutrients. 2022; 14(5):1126. https://doi.org/10.3390/nu14051126
Chicago/Turabian StyleRonis, Martin J. J., Horacio Gomez-Acevedo, Kartik Shankar, Leah Hennings, Neha Sharma, Michael L. Blackburn, Isabelle Miousse, Harry Dawson, Celine Chen, Kelly E. Mercer, and et al. 2022. "Soy Formula Is Not Estrogenic and Does Not Result in Reproductive Toxicity in Male Piglets: Results from a Controlled Feeding Study" Nutrients 14, no. 5: 1126. https://doi.org/10.3390/nu14051126
APA StyleRonis, M. J. J., Gomez-Acevedo, H., Shankar, K., Hennings, L., Sharma, N., Blackburn, M. L., Miousse, I., Dawson, H., Chen, C., Mercer, K. E., & Badger, T. M. (2022). Soy Formula Is Not Estrogenic and Does Not Result in Reproductive Toxicity in Male Piglets: Results from a Controlled Feeding Study. Nutrients, 14(5), 1126. https://doi.org/10.3390/nu14051126







