Altered Serum Acylcarnitines Profile after a Prolonged Stay in Intensive Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients—Data Sources
2.2. Serum Acylcarnitine Profiling
2.3. Ancillary Biochemical Parameters
2.4. Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Bonafe, L.; Berger, M.M.; Que, Y.A.; Mechanick, J.I. Carnitine deficiency in chronic critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Broman, M.; Forni, L.; Ostermann, M.; De Waele, E.; Wischmeyer, P.E. Nutrients and micronutrients at risk during renal replacement therapy: A scoping review. Curr. Opin. Crit. Care 2021, 27, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.; Hyltander, A.; Sjoberg, A.; Arfvidsson, B.; Sandstrom, R.; Wickstrom, I.; Lundholm, K. Prevalence of carnitine depletion in critically ill patients with undernutrition. Metabolism 1992, 41, 165–171. [Google Scholar] [CrossRef]
- Hatamkhani, S.; Karimzadeh, I.; Elyasi, S.; Farsaie, S.; Khalili, H. Carnitine and sepsis: A review of an old clinical dilemma. J. Pharm. Pharm. Sci. 2013, 16, 414–423. [Google Scholar] [CrossRef][Green Version]
- Moonen, H.; Van Zanten, A.R.H. Mitochondrial dysfunction in critical illness during acute metabolic stress and convalescence: Consequences for nutrition therapy. Curr. Opin. Crit. Care 2020, 26, 346–354. [Google Scholar] [CrossRef]
- Owen, A.M.; Patel, S.P.; Smith, J.D.; Balasuriya, B.K.; Mori, S.F.; Hawk, G.S.; Stromberg, A.J.; Kuriyama, N.; Kaneki, M.; Rabchevsky, A.G.; et al. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. Elife 2019, 8, e49920. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernandez-Fernandez, C.; Mourino-Bayolo, D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef]
- Carpentier, D.; Beduneau, G.; Girault, C. Prolonged Stay in Intensive Care Unit. Réanimation 2015, 24, 379–388. [Google Scholar] [CrossRef]
- Capuzzo, M.; Volta, C.; Tassinati, T.; Moreno, R.; Valentin, A.; Guidet, B.; Iapichino, G.; Martin, C.; Perneger, T.; Combescure, C.; et al. Hospital mortality of adults admitted to Intensive Care Units in hospitals with and without Intermediate Care Units: A multicentre European cohort study. Crit. Care 2014, 18, 551. [Google Scholar] [CrossRef] [PubMed]
- Millington, D.S.; Kodo, N.; Norwood, D.L.; Roe, C.R. Tandem mass spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J. Inherit. Metab. Dis. 1990, 13, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, J.L.; Zhang, W.; Kahler, S.G.; Roe, C.R.; Chen, Y.T.; Terada, N.; Chace, D.H.; Iafolla, A.K.; Ding, J.H.; Millington, D.S. Medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: Diagnosis by acylcarnitine analysis in blood. Am. J. Hum. Genet. 1993, 52, 958–966. [Google Scholar]
- Millington, D.S.; Stevens, R.D. Acylcarnitines: Analysis in plasma and whole blood using tandem mass spectrometry. Metab. Profiling 2011, 708, 55–72. [Google Scholar]
- Rinaldo, P.; Cowan, T.M.; Matern, D. Acylcarnitine profile analysis. Genet. Med. 2008, 10, 151–156. [Google Scholar] [CrossRef]
- Boemer, F.; Schoos, R.; Deberg, M. Quantification of physiological aminoacids using aTRAQ((R)) kit: Evaluation and implementation of new markers. Ann. De Biol. Clin. 2015, 73, 427–442. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Molinger, J.; Pastva, A.M.; Whittle, J.; Wischmeyer, P.E. Novel approaches to metabolic assessment and structured exercise to promote recovery in ICU survivors. Curr. Opin. Crit. Care 2020, 26, 369–378. [Google Scholar] [CrossRef]
- Page, A.; Flower, L.; Prowle, J.; Puthucheary, Z. Novel methods to identify and measure catabolism. Curr. Opin. Crit. Care 2021, 27, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Jiroutkova, K.; Krajcova, A.; Ziak, J.; Fric, M.; Waldauf, P.; Dzupa, V.; Gojda, J.; Nemcova-Furstova, V.; Kovar, J.; Elkalaf, M.; et al. Mitochondrial function in skeletal muscle of patients with protracted critical illness and ICU-acquired weakness. Crit. Care 2015, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Wolf, A.; Weir, P.; Segar, P.; Stone, J.; Shield, J. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet 2001, 357, 606–607. [Google Scholar] [CrossRef]
- Zampino, M.; Tanaka, T.; Ubaida-Mohien, C.; Fantoni, G.; Candia, J.; Semba, R.D.; Ferrucci, L. A Plasma Proteomic Signature of Skeletal Muscle Mitochondrial Function. Int. J. Mol. Sci. 2020, 21, 9540. [Google Scholar] [CrossRef]
- Dare, A.J.; Phillips, A.R.; Hickey, A.J.; Mittal, A.; Loveday, B.; Thompson, N.; Windsor, J.A. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic. Biol. Med. 2009, 47, 1517–1525. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M.; Chace, D.H.; Fornasini, G. Determination of the reference range of endogenous plasma carnitines in healthy adults. Ann. Clin. Biochem. 2008, 45 Pt 6, 585–592. [Google Scholar] [CrossRef]
- Jarrell, Z.R.; Smith, M.R.; Hu, X.; Orr, M.; Liu, K.H.; Quyyumi, A.A.; Jones, D.P.; Go, Y.M. Plasma acylcarnitine levels increase with healthy aging. Aging 2020, 12, 13555–13570. [Google Scholar] [CrossRef]
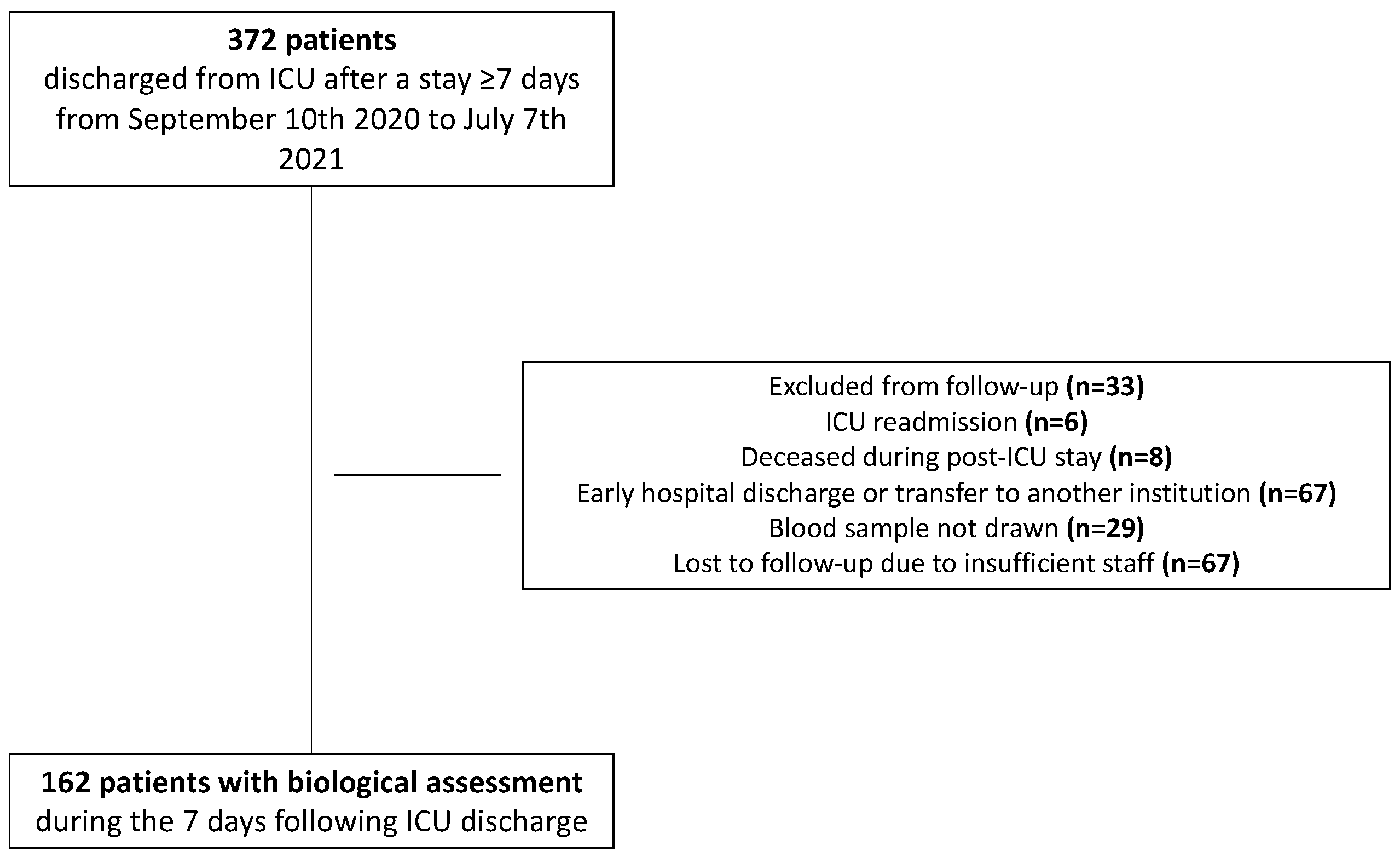
| Data | n = 162 | |
| Age, y | 67 (58.7–73) | |
| Male, n (%) | 106 (65.4) | |
| Weight, kg | 75.6 (64–90.5) | |
| Height, cm | 170 (163–177) | |
| BMI, kg/m2 | 26.5 (22.8–30.1) | |
| Comorbidities, n (%) | HIV | 1 (0.6) |
| Epilepsy treated by valproate | 3 (18.5) | |
| Admission category, n (%) | Medical | 83 (51.2) |
| Surgical | 79 (48.8) | |
| Primary failure, n (%) | Cardiovascular | 66 (40.7) |
| Pulmonary | 31 (19.1) | |
| Neurologic | 29 (17.9) | |
| Digestive | 8 (4.9) | |
| Hepatic | 4 (2.5) | |
| Polytrauma | 5 (3.1) | |
| Other | 19 (11.7) | |
| SAPS II | 50.5 (32–72) | |
| Mechanical ventilation >24 h, n (%) | 91 (56.2) | |
| Duration of mechanical ventilation, d | 6 (2–14) | |
| Renal replacement therapy, n (%) | 10 (6.2) | |
| Duration of renal replacement therapy, d | 8 (6.5–12.2) | |
| Extracorporeal membrane oxygenation, n (%) | 2 (1.2) | |
| Propofol-based sedation, n (%) | 113 (69.8) | |
| Duration of propofol infusion, d | 4 (2–8) | |
| Valproate treatment during ICU stay, n (%) | 4 (2.5) | |
| Oral nutrition, n (%) | 70 (43.2) | |
| Enteral nutrition, n (%) | 97 (59.9) | |
| Duration of enteral nutrition, d | 9 (6–17) | |
| Parenteral nutrition, n (%) | 23 (14.2) | |
| Duration of parenteral nutrition, d | 7 (4–9) | |
| ICU LOS, d | 9.7 (7.1–19.3) | |
| Hospital LOS, d | 35 (22–57) | |
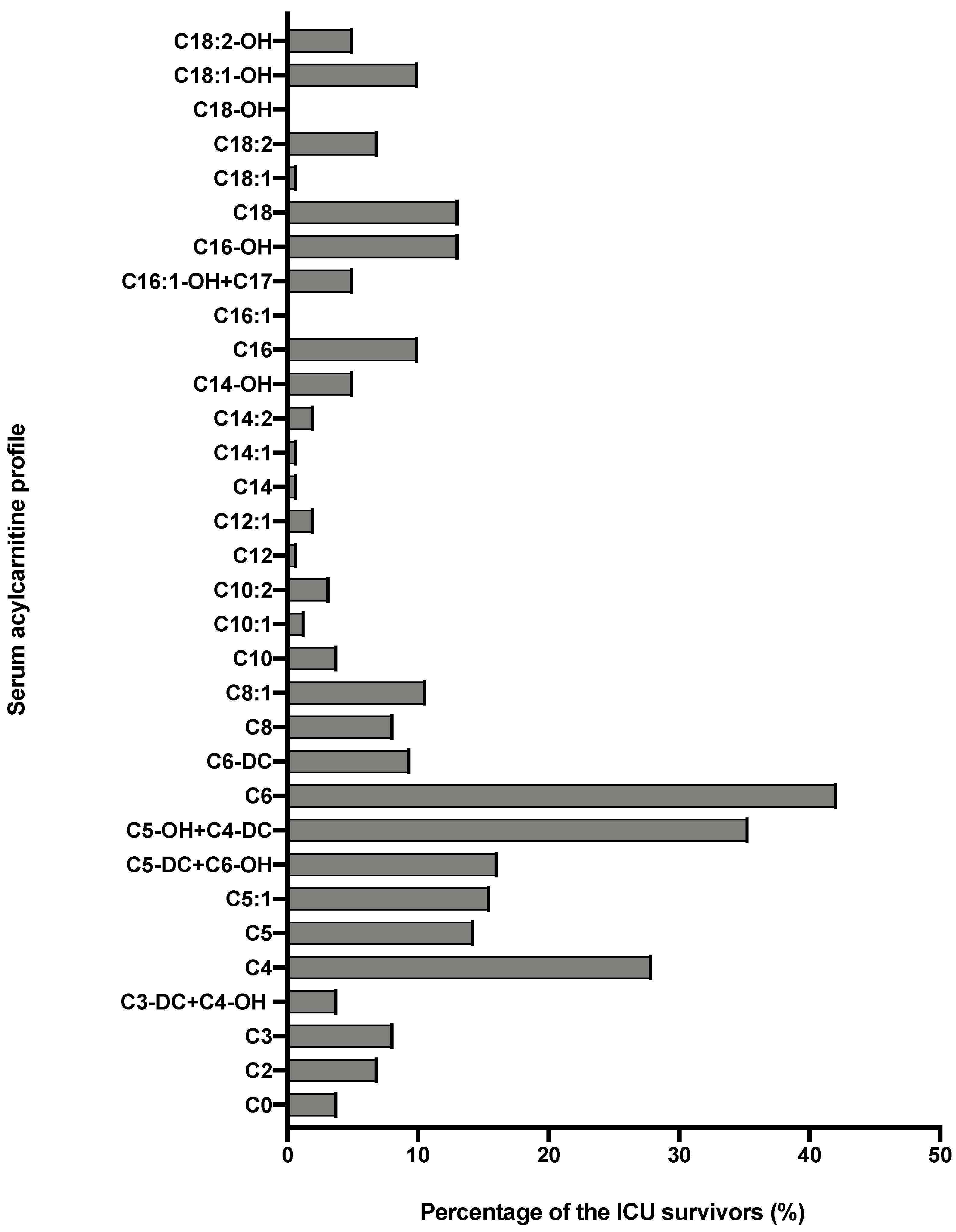
| Acylcarnitines (μmol/L) | ICU Survivors n = 162 | Reference Ranges, Based on 50 Serum Samples of Apparently Healthy Individuals | p Value |
|---|---|---|---|
| C0 | 46.06 (35.04–56.35) | 43.64 (36.43–52.96) | 0.549 |
| C2 | 9.92 (6.96–15.12) | 9.92 (5.45–11.46) | 0.058 |
| C3 | 0.81 (0.53–1.20) | 0.41 (0.30–0.48) | <0.001 |
| C3-DC + C4-OH | 0.04 (0.02–0.08) | 0.03 (0.02–0.05) | 0.005 |
| C4 | 0.28 (0.18–0.42) | 0.15 (0.12–0.22) | <0.001 |
| C5 | 0.11 (0.07–0.15) | 0.10 (0.07–0.13) | 0.353 |
| C5:1 | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | 0.590 |
| C5-DC + C6-OH | 0.14 (0.08–0.21) | 0.08 (0.06–0.11) | <0.001 |
| C5-OH + C4-DC | 0.04 (0.03–0.07) | 0.02 (0.02–0.03) | <0.001 |
| C6 | 0.12 (0.07–0.32) | 0.05 (0.04–0.07) | <0.001 |
| C6-DC | 0.08 (0.05–0.17) | 0.06 (0.04–0.09) | 0.031 |
| C8 | 0.11 (0.07–0.17) | 0.10 (0.07–0.14) | 0.315 |
| C8:1 | 0.15 (0.11–0.23) | 0.14 (0.09–0.21) | 0.044 |
| C10 | 0.14 (0.09–0.24) | 0.16 (0.10–0.23) | 0.459 |
| C10:1 | 0.08 (0.05–0.12) | 0.07 (0.05–0.10) | 0.528 |
| C10:2 | 0.02 (0.01–0.03) | 0.01 (0.01–0.02) | 0.037 |
| C12 | 0.05 (0.03–0.07) | 0.06 (0.04–0.09) | 0.022 |
| C12:1 | 0.07 (0.04–0.12) | 0.07 (0.04–0.12) | 0.708 |
| C14 | 0.03 (0.02–0.04) | 0.03 (0.02–0.05) | 0.474 |
| C14:1 | 0.08 (0.04–0.11) | 0.03 (0.05–0.14) | 0.412 |
| C14:2 | 0.02 (0.01–0.04) | 0.02 (0.02–0.04) | 0.458 |
| C14-OH | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.33 |
| C16 | 0.17 (0.12–0.23) | 0.13 (0.10–0.18) | 0.003 |
| C16:1 | 0.03 (0.02–0.05) | 0.03 (0.02–0.05) | 0.928 |
| C16-OH + C17 | 0.01 (0.01–0.01) P2.5 = 0.002 P97.5 = 0.030 | 0.01 (0.01–0.01) P2.5 = 0.002 P97.5 = 0.068 | 0.006 |
| C16-OH | 0.01 (0.01–0.01) P2.5 = 0.000 P97.5 = 0.029 | 0 (0–0) P2.5 = 0.001 P97.5 = 0.023 | 0.013 |
| C18 | 0.04 (0.03–0.06) | 0.04 (0.03–0.06) | 0.436 |
| C18:1 | 0.17 (0.11–0.25) | 0.16 (0.10–0.23) | 0.384 |
| C18:2 | 0.05 (0.03–0.06) | 0.04 (0.03–0.06) | 0.486 |
| C18-OH | 0.01 (0.01–0.01) | 0 (0–0) | 0.945 |
| C18:1-OH | 0.01 (0.01–0.01) | 0 (0–0) | 0.971 |
| C18:2-OH | 0.01 (0.01–0.01) | 0 (0–0) | 0.245 |
| Biomarkers | ICU Survivors n = 162 | Reference Ranges Provided by the Manufacturers |
|---|---|---|
| C-reactive protein (mg/L) | 35.9 (15.1–82.2) | 0–5 |
| Triglycerides (mg/dL) | 136 (104.5–180) | <175 |
| Total cholesterol (mg/dL) | 139 (113.5–167.5) | <190 |
| Leucine (μmol/L) | 129.5 (106.5–167.5) | 73.5–228 |
| Isoleucine (μmol/L) | 80.3 (63.5–103.8) | 36.5–132 |
| Valine (μmol/L) | 211 (174–259.8) | 105–352 |
| Glutamine (μmol/L) | 486.5 (430–589.5) | 311–650 |
| Methionine (μmol/L) | 21.95 (17.48–29.13) | 13.1–34.1 |
| Phenylalanine (μmol/L) | 78.85 (61.15–101) | 41.3–130 |
| Tyrosine (μmol/L) | 55.2 (46.05–71.68) | 37.6–101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau, A.-F.; Schmitz, S.; Cavalier, E.; Misset, B.; Boemer, F. Altered Serum Acylcarnitines Profile after a Prolonged Stay in Intensive Care. Nutrients 2022, 14, 1122. https://doi.org/10.3390/nu14051122
Rousseau A-F, Schmitz S, Cavalier E, Misset B, Boemer F. Altered Serum Acylcarnitines Profile after a Prolonged Stay in Intensive Care. Nutrients. 2022; 14(5):1122. https://doi.org/10.3390/nu14051122
Chicago/Turabian StyleRousseau, Anne-Françoise, Sarah Schmitz, Etienne Cavalier, Benoit Misset, and François Boemer. 2022. "Altered Serum Acylcarnitines Profile after a Prolonged Stay in Intensive Care" Nutrients 14, no. 5: 1122. https://doi.org/10.3390/nu14051122
APA StyleRousseau, A.-F., Schmitz, S., Cavalier, E., Misset, B., & Boemer, F. (2022). Altered Serum Acylcarnitines Profile after a Prolonged Stay in Intensive Care. Nutrients, 14(5), 1122. https://doi.org/10.3390/nu14051122






