Fasting-Mimicking Diet Reduces Trimethylamine N-Oxide Levels and Improves Serum Biochemical Parameters in Healthy Volunteers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. Study Design
2.3. Determination of Biochemical Measurements
2.4. Measurement of TMAO Levels by UPLC/MS/MS
2.5. Data Analysis
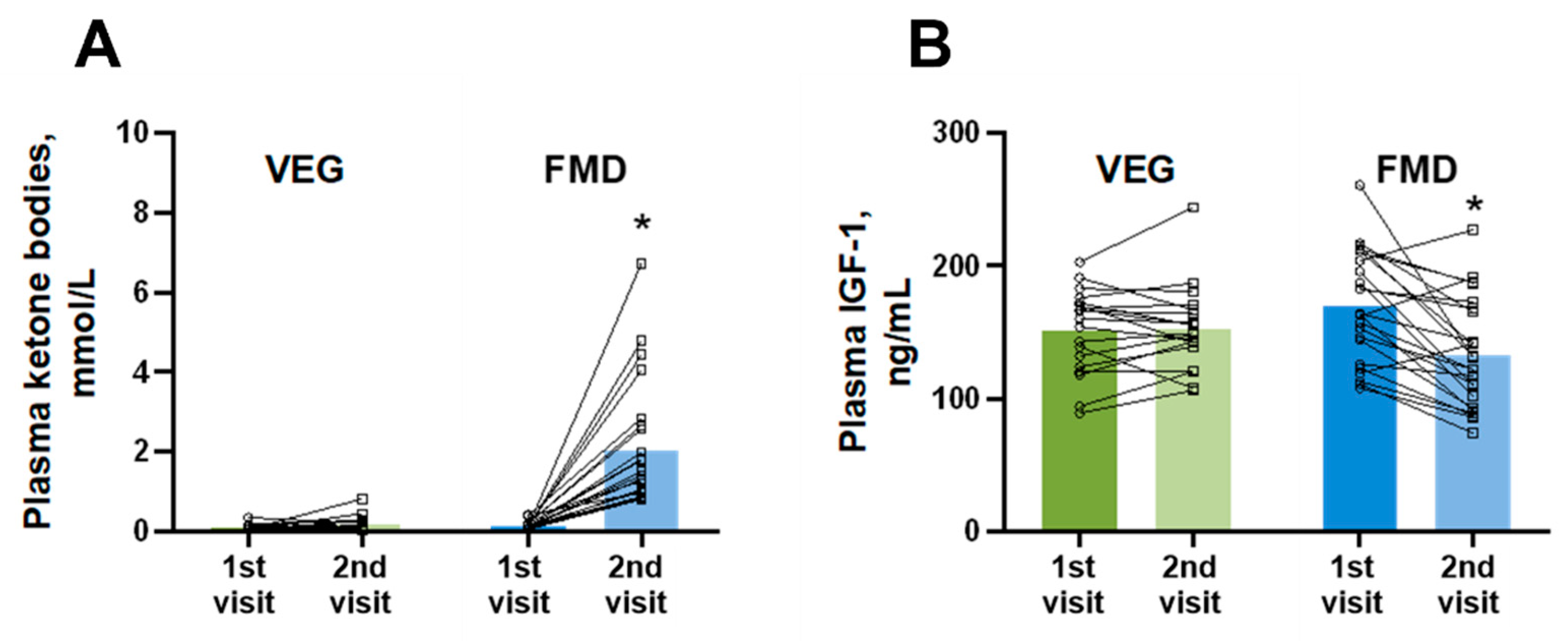
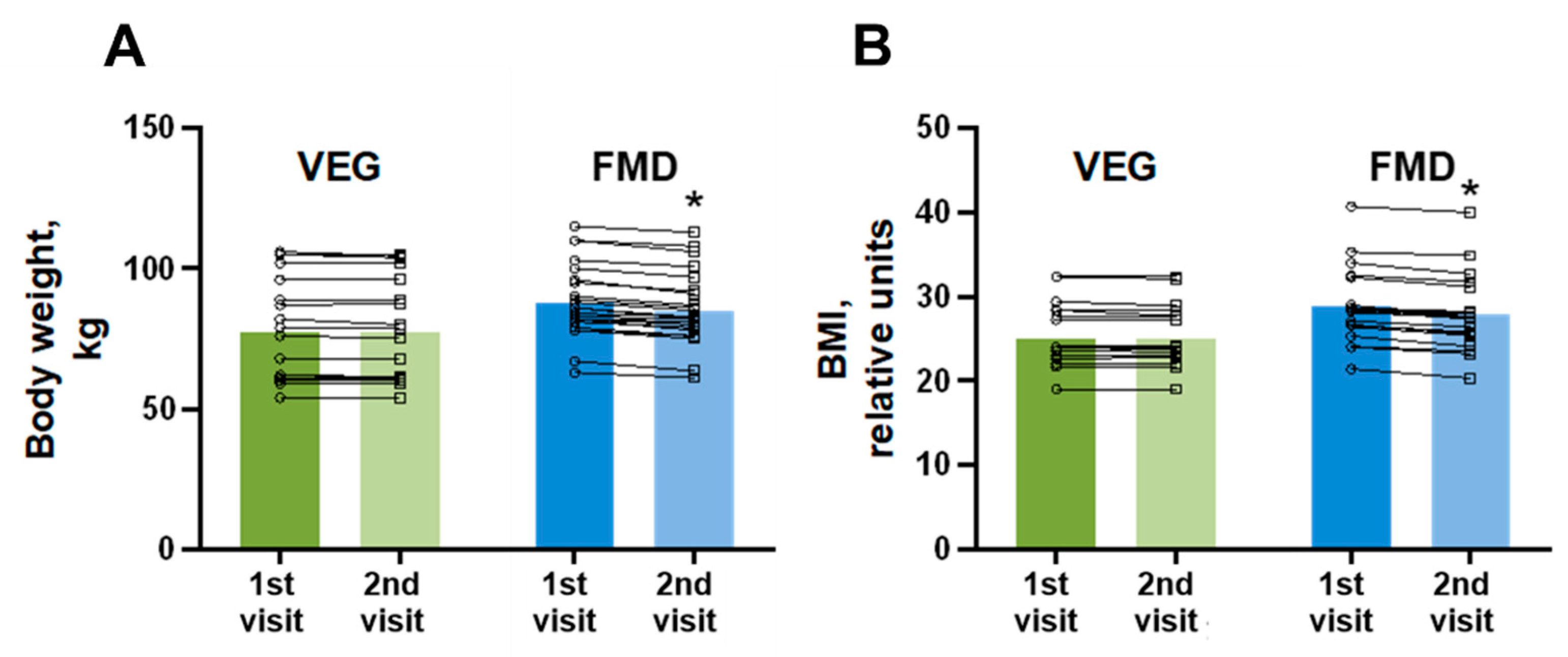
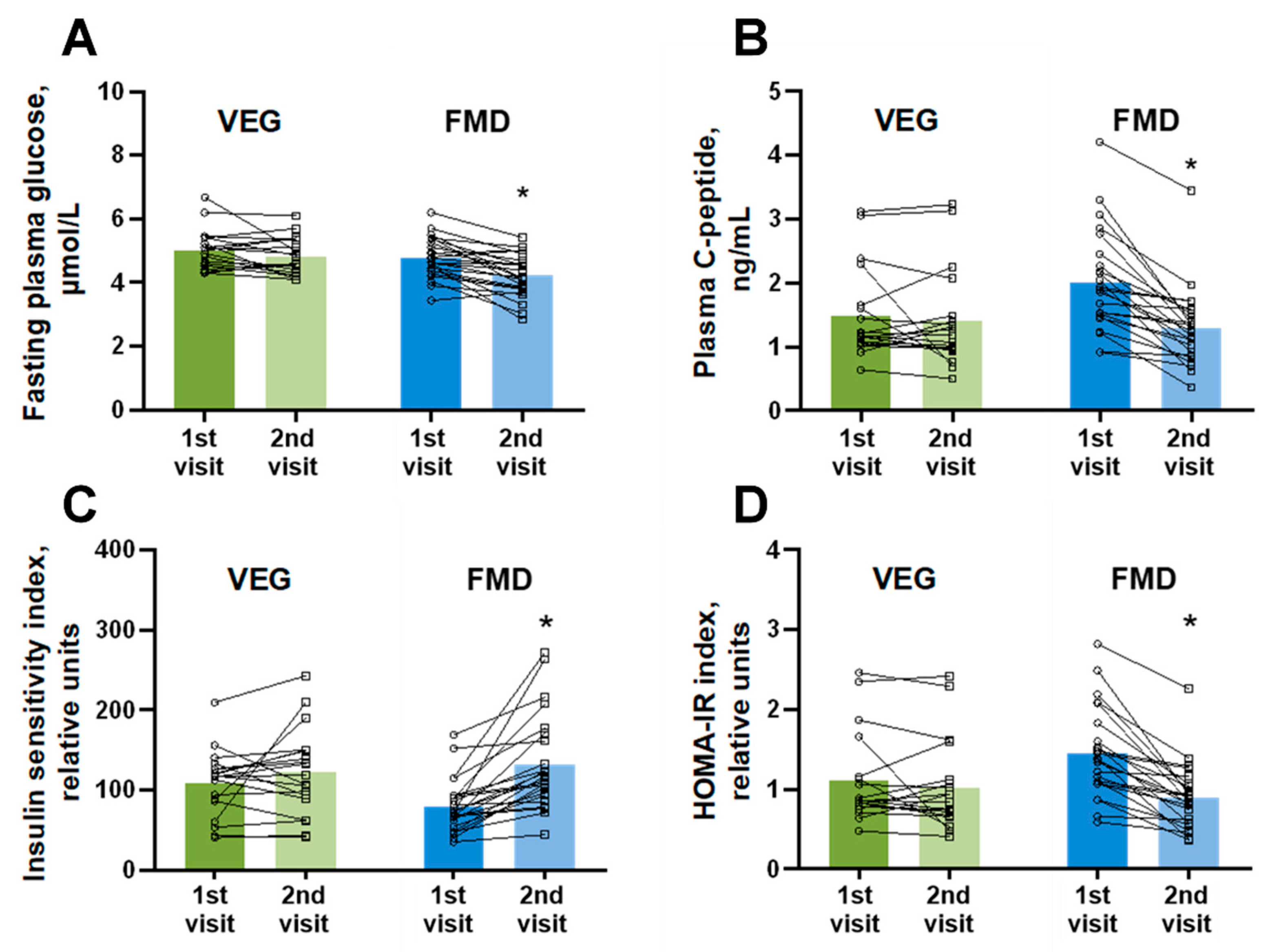
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Geng, J.; Yang, C.; Wang, B.; Zhang, X.; Hu, T.; Gu, Y.; Li, J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed. Pharmacother. 2018, 97, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediators Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.H.; Pan, B.; Chen, Y.; Guo, C.X.; Zhao, M.M.; Zheng, L.M.; Chen, B.X. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut microbiota-dependent metabolite Trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front. Physiol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Ufnal, M.; Jazwiec, R.; Dadlez, M.; Drapala, A.; Sikora, M.; Skrzypecki, J. Trimethylamine-N-Oxide: A Carnitine-Derived Metabolite That Prolongs the Hypertensive Effect of Angiotensin II in Rats. Can. J. Cardiol. 2014, 30, 1700–1705. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Liang, Z.; Wang, X.; Liu, W.; Zhao, L.; Wang, S.; Hai, X.; Yu, K. The correlation between plasma trimethylamine N-oxide level and heart failure classification in northern Chinese patients. Ann. Palliat. Med. 2020, 9, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Dambrova, M.; Latkovskis, G.; Kuka, J.; Strele, I.; Konrade, I.; Grinberga, S.; Hartmane, D.; Pugovics, O.; Erglis, A.; Liepinsh, E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp. Clin. Endocrinol. Diabetes 2016, 124, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lever, M.; George, P.M.; Slow, S.; Bellamy, D.; Young, J.M.; Ho, M.; McEntyre, C.J.; Elmslie, J.L.; Atkinson, W.; Molyneux, S.L.; et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: An observational study. PLoS ONE 2014, 9, e0114969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef]
- Sun, G.; Yin, Z.; Liu, N.; Bian, X.; Yu, R.; Su, X.; Zhang, B.; Wang, Y. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochem. Biophys. Res. Commun. 2017, 493, 964–970. [Google Scholar] [CrossRef]
- Boutagy, N.E.; Neilson, A.P.; Osterberg, K.L.; Smithson, A.T.; Englund, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity 2015, 23, 2357–2363. [Google Scholar] [CrossRef]
- Kalagi, N.A.; Abbott, K.A.; Alburikan, K.A.; Alkofide, H.A.; Stojanovski, E.; Garg, M.L. Modulation of Circulating Trimethylamine N-Oxide Concentrations by Dietary Supplements and Pharmacological Agents: A Systematic Review. Adv. Nutr. 2019, 10, 876–887. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of trimethylamine n-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Redman, L.M.; Ravussin, E. Caloric restriction in humans: Impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox Signal. 2011, 14, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Del Corral, P.; Chandler-Laney, P.C.; Casazza, K.; Gower, B.A.; Hunter, G.R. Effect of dietary adherence with or without exercise on weight loss: A mechanistic approach to a global problem. J. Clin. Endocrinol. Metab. 2009, 94, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.L.; Das, S.K.; Racette, S.B.; Apolzan, J.W.; Zhang, D.; Pieper, C.F.; Martin, C.K. Changes in body weight, adherence, and appetite during 2 years of calorie restriction: The CALERIE 2 randomized clinical trial. Eur. J. Clin. Nutr. 2020, 74, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Karfopoulou, E.; Yannakoulia, M. Weight regaining: From statistics and behaviors to physiology and metabolism. Metabolism. 2015, 64, 1395–1407. [Google Scholar] [CrossRef]
- Wilhelmi de Toledo, F.; Grundler, F.; Sirtori, C.R.; Ruscica, M. Unravelling the health effects of fasting: A long road from obesity treatment to healthy life span increase and improved cognition. Ann. Med. 2020, 52, 147–161. [Google Scholar] [CrossRef]
- Duregon, E.; Pomatto-Watson, L.C.D.D.; Bernier, M.; Price, N.L.; de Cabo, R. Intermittent fasting: From calories to time restriction. GeroScience 2021, 43, 1083–1092. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Crupi, A.N.; Haase, J.; Brandhorst, S.; Longo, V.D. Periodic and Intermittent Fasting in Diabetes and Cardiovascular Disease. Curr. Diab. Rep. 2020, 20, 83. [Google Scholar] [CrossRef]
- Erickson, M.L.; Malin, S.K.; Wang, Z.; Mark Brown, J.; Hazen, S.L.; Kirwan, J.P. Effects of lifestyle intervention on plasma trimethylamine N-oxide in obese adults. Nutrients 2019, 11, 179. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, T.; Zhang, L.; Ke, B.; Qin, J. Fasting Therapy Contributes to the Improvement of Endothelial Function and Decline in Vascular Injury-Related Markers in Overweight and Obese Individuals via Activating Autophagy of Endothelial Progenitor Cells. Evidence-Based Complement. Altern. Med. 2020, 2020, 3576030. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.L.; Cox, J.E.; Muhlestein, J.B.; May, H.T.; Carlquist, J.F.; Le, V.T.; Anderson, J.L.; Horne, B.D. Pilot study of novel intermittent fasting effects on metabolomic and trimethylamine N-oxide changes during 24-hour water-only fasting in the FEELGOOD trial. Nutrients 2019, 11, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafra, D.; Borges, N.A.; Cardozo, L.F.M. de F.; Anjos, J.S.; Black, A.P.; Moraes, C.; Bergman, P.; Lindholm, B.; Stenvinkel, P. Red meat intake in chronic kidney disease patients: Two sides of the coin. Nutrition 2018, 46, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Cañueto, D.; Giardina, S.; Salas-Salvadó, J.; Cañellas, N.; Correig, X.; Bulló, M. Effect of pistachio consumption on the modulation of urinary gut microbiota-related metabolites in prediabetic subjects. J. Nutr. Biochem. 2017, 45, 48–53. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef]
- Zivin, J.A.; Snarr, J.F. An automated colorimetric method for the measurement of 3-hydroxybutyrate concentration. Anal. Biochem. 1973, 52, 456–461. [Google Scholar] [CrossRef]
- Dambrova, M.; Skapare-Makarova, E.; Konrade, I.; Pugovics, O.; Grinberga, S.; Tirzite, D.; Petrovska, R.; Kalvins, I.; Liepins, E. Meldonium decreases the diet-increased plasma levels of trimethylamine n-oxide, a metabolite associated with atherosclerosis. J. Clin. Pharmacol. 2013, 53, 1095–1098. [Google Scholar] [CrossRef]
- Grinberga, S.; Dambrova, M.; Latkovskis, G.; Strele, I.; Konrade, I.; Hartmane, D.; Sevostjanovs, E.; Liepinsh, E.; Pugovics, O. Determination of trimethylamine-N-oxide in combination with l-carnitine and γ-butyrobetaine in human plasma by UPLC/MS/MS. Biomed. Chromatogr. 2015, 29, 1670–1674. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
- Kühn, T.; Rohrmann, S.; Sookthai, D.; Johnson, T.; Katzke, V.; Kaaks, R.; Von Eckardstein, A.; Müller, D. Intra-individual variation of plasma trimethylamine-N-oxide (TMAO), betaine and choline over 1 year. Clin. Chem. Lab. Med. 2017, 55, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 9, 8700. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Gotto, A.M. HDL cholesterol and protective factors in atherosclerosis. Circulation 2004, 109, 8–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saris, W.H. Very-low-calorie diets and sustained weight loss. Obes. Res. 2001, 9 (Suppl. 4), 295S–301S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolland, C.; Broom, I. The effects of very-low-calorie diets on HDL: A review. Cholesterol 2011, 2011, 306278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamluk, L.; O’Doherty, M.G.; Orfanos, P.; Saitakis, G.; Woodside, J.V.; Liao, L.M.; Sinha, R.; Boffetta, P.; Trichopoulou, A.; Kee, F. Fruit and vegetable intake and risk of incident of type 2 diabetes: Results from the consortium on health and ageing network of cohorts in Europe and the United States (CHANCES). Eur. J. Clin. Nutr. 2017, 71, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Su, L.; Wang, J.; Duan, X.; Jiang, X. Fruit and vegetable consumption and risk of the metabolic syndrome: A meta-analysis. Public Health Nutr. 2018, 21, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Prevention of metabolic diseases: Fruits (including fruit sugars) vs. vegetables. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 286–293. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Meat consumption and risk of metabolic syndrome: Results from the Korean population and a meta-analysis of observational studies. Nutrients 2018, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Ding, J.; Liang, J.; Zhang, Y. Association of Red Meat and Poultry Consumption With the Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, C.; Mao, Z.; Zhou, W.; Zhang, L.; Fan, M.; Cui, S.; Li, L. Meat and fish intake and type 2 diabetes: Dose–response meta-analysis of prospective cohort studies. Diabetes Metab. 2020, 46, 345–352. [Google Scholar] [CrossRef]
- Argyridou, S.; Davies, M.J.; Biddle, G.J.H.; Bernieh, D.; Suzuki, T.; Dawkins, N.P.; Rowlands, A.V.; Khunti, K.; Smith, A.C.; Yates, T. Evaluation of an 8-Week Vegan Diet on Plasma Trimethylamine-N-Oxide and Postchallenge Glucose in Adults with Dysglycemia or Obesity. J. Nutr. 2021, 151, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.J.; McGrievy, M.E.; Turner-McGrievy, G.M. Dietary adherence and acceptability of five different diets, including vegan and vegetarian diets, for weight loss: The New DIETs study. Eat. Behav. 2015, 19, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Fuca, G.; Ligorio, F.; Huber, V.; Vingiani, A.; Iannelli, F.; Raimondi, A.; Rinchai, D.; Frige, G.; Belfiore, A.; et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in cancer patients. Cancer Discov. 2021, 12, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaide, M.; Singh, R.; Datta, P.; Rewers-Felkins, K.; Salguero, M.; Al-Obaidi, I.; Kottapalli, K.; Vasylyeva, T. Gut Microbiota-Dependent Trimethylamine-N-oxide and Serum Biomarkers in Patients with T2DM and Advanced CKD. J. Clin. Med. 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuka, J.; Videja, M.; Kuka, M.M.; Liepins, J.; Grinberga, S.; Sevostjanovs, E.; Vilks, K.; Liepinsh, E.; Dambrova, M. Metformin decreases bacterial trimethylamine production and trimethylamine N-oxide levels in db/db mice. Sci. Rep. 2020, 10, 14555. [Google Scholar] [CrossRef]
- Turnbaugh, P.; Backhed, F.; Fulton, L.; Gordon, J. Marked alterations in the distal gut microbiome linked to diet-induced obesity. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Dávila, L.A. The Microbiome and the Epigenetics of Diabetes Mellitus. In Diabetes Food Plan; Pirela, V.B., Ed.; IntechOpen: Rijeka, Croatia, 2018; ISBN 978-1-78923-275-2. [Google Scholar]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Han, R.; Zhao, J.; Wang, S.; Huang, M.; Wang, Y.; Chen, Y. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr. Metab. 2018, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.K.; Chen, C.C.; Liu, P.Y.; Panyod, S.; Liao, B.Y.; Chen, P.C.; Kao, H.L.; Kuo, H.C.; Kuo, C.H.; Chiu, T.H.T.; et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut 2019, 68, 1439–1449. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Xun, J.; Koeth, R.A.; Lin, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. L-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef] [PubMed]
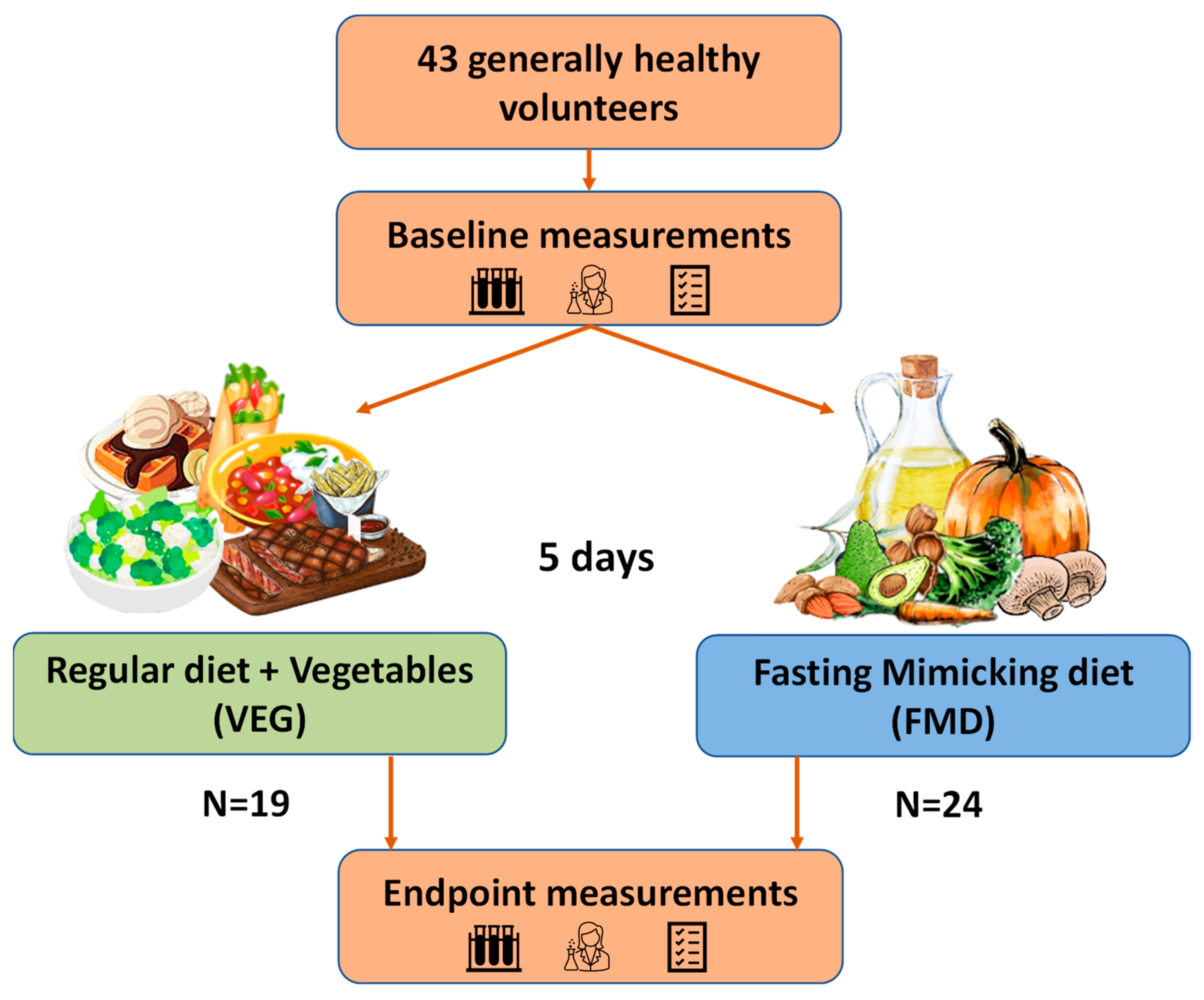

| Baseline Characteristics | VEG (n = 19) | FMD (n = 24) | p-Value |
|---|---|---|---|
| Age, years | 37 ± 3 | 39 ± 2 | 0.660 |
| Sex, n (%) | |||
| Men | 6 (31.6) | 9 (37.5) | |
| Women | 13 (68.4) | 15 (62.5) | |
| BMI, kg/m2 | 25.2 ± 0.9 | 28.8 ± 0.9 | 0.004 |
| Body type (regional fat distribution), n (%) | |||
| Abdominal | 8 (42.1) | 10 (41.7) | |
| Gluteofemoral | 11 (57.9) | 14 (58.3) | |
| Plasma biochemistry | |||
| Hemoglobin, g/L | 144.0 ± 3.5 | 150.3 ± 6.2 | 0.350 |
| Glucose, mmol/L | 4.99 ± 0.13 | 4.87 ± 0.11 | 0.470 |
| HDL cholesterol, mmol/L | 1.51 ± 0.07 | 1.49 ± 0.08 | 0.841 |
| LDL cholesterol, mmol/L | 3.33 ± 0.16 | 3.37 ± 0.19 | 0.857 |
| Triglycerides, mmol/L | 1.44 ± 0.22 | 1.30 ± 0.09 | 0.440 |
| Creatinine, μmol/L | 75.7 ± 3.8 | 75.2 ± 6.6 | 0.941 |
| eGFR, mL/min/1.73 m2 | 86.6 ± 5.9 | 92.7 ± 6.9 | 0.527 |
| ALT, U/L | 21.5 ± 2.7 | 24.7 ± 3.4 | 0.478 |
| Total bilirubin, μmol/L | 9.7 ± 1.1 | 10.3 ± 1.5 | 0.788 |
| Lipase, U/L | 41.6 ± 1.9 | 37.3 ± 1.8 | 0.149 |
| ESR, mm/h | 2.9 ± 0.9 | 2.3 ± 0.2 | 0.641 |
| CRP, mg/L | 1.26 ± 0.33 | 1.24 ± 0.22 | 0.964 |
| TMAO, μmol/L | 3.65 ± 0.68 | 6.22 ± 1.16 | 0.083 |
| Physical activity, n (%) | |||
| Low | 11 (57.9) | 15 (62.5) | |
| Moderate | 6 (31.6) | 8 (33.3) | |
| High | 2 (10.5) | 1 (4.2) | |
| Meat consumption, n (%) | |||
| >5 servings per week | 10 (52.6) | 14 (58.3) | |
| 3–5 servings per week | 9 (47.4) | 9 (37.5) | |
| <3 servings per week | 0 (0.0) | 1 (4.2) |
| VEG | FMD | |||
|---|---|---|---|---|
| 1st Visit | 2nd Visit | 1st Visit | 2nd Visit | |
| High-density lipoprotein, μmol/L | 1.51 ± 0.07 | 1.51 ± 0.07 | 1.49 ± 0.08 | 1.30 ± 0.07 |
| Low-density lipoprotein, μmol/L | 3.33 ± 0.16 | 3.32 ± 0.15 | 3.38 ± 0.19 | 3.41 ± 0.20 |
| Triglycerides, μmol/L | 1.44 ± 0.22 | 1.22 ± 0.20 * | 1.30 ± 0.09 | 1.10 ± 0.07 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Videja, M.; Sevostjanovs, E.; Upmale-Engela, S.; Liepinsh, E.; Konrade, I.; Dambrova, M. Fasting-Mimicking Diet Reduces Trimethylamine N-Oxide Levels and Improves Serum Biochemical Parameters in Healthy Volunteers. Nutrients 2022, 14, 1093. https://doi.org/10.3390/nu14051093
Videja M, Sevostjanovs E, Upmale-Engela S, Liepinsh E, Konrade I, Dambrova M. Fasting-Mimicking Diet Reduces Trimethylamine N-Oxide Levels and Improves Serum Biochemical Parameters in Healthy Volunteers. Nutrients. 2022; 14(5):1093. https://doi.org/10.3390/nu14051093
Chicago/Turabian StyleVideja, Melita, Eduards Sevostjanovs, Sabine Upmale-Engela, Edgars Liepinsh, Ilze Konrade, and Maija Dambrova. 2022. "Fasting-Mimicking Diet Reduces Trimethylamine N-Oxide Levels and Improves Serum Biochemical Parameters in Healthy Volunteers" Nutrients 14, no. 5: 1093. https://doi.org/10.3390/nu14051093
APA StyleVideja, M., Sevostjanovs, E., Upmale-Engela, S., Liepinsh, E., Konrade, I., & Dambrova, M. (2022). Fasting-Mimicking Diet Reduces Trimethylamine N-Oxide Levels and Improves Serum Biochemical Parameters in Healthy Volunteers. Nutrients, 14(5), 1093. https://doi.org/10.3390/nu14051093







