Assessing the Causal Effects of Adipokines on Uric Acid and Gout: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasource and Selection of Instruments for MR
2.1.1. Outcome Datasource
2.1.2. Selection of Instruments for MR
2.1.3. Statistics Power and F-Statistics
2.2. Statistics Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rock, K.L.; Kataoka, H.; Lai, J.J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 2013, 9, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Butler, F.; Alghubayshi, A.; Roman, Y. The Epidemiology and Genetics of Hyperuricemia and Gout across Major Racial Groups: A Literature Review and Population Genetics Secondary Database Analysis. J. Pers. Med. 2021, 11, 231. [Google Scholar] [CrossRef]
- Chedid, R.; Zoghbi, F.; Halaby, G.; Gannage-Yared, M.H. Serum uric acid in relation with the metabolic syndrome components and adiponectin levels in Lebanese University students. J. Endocrinol. Investig. 2011, 34, e153–e157. [Google Scholar] [CrossRef]
- Tsioufis, C.; Kyvelou, S.; Dimitriadis, K.; Syrseloudis, D.; Sideris, S.; Skiadas, I.; Katsi, V.; Stefanadi, E.; Lalos, S.; Mihas, C.; et al. The diverse associations of uric acid with low-grade inflammation, adiponectin and arterial stiffness in never-treated hypertensives. J. Hum. Hypertens. 2011, 25, 554–559. [Google Scholar] [CrossRef][Green Version]
- Sirbu, A.E.; Buburuzan, L.; Kevorkian, S.; Martin, S.; Barbu, C.; Copaescu, C.; Smeu, B.; Fica, S. Adiponectin expression in visceral adiposity is an important determinant of insulin resistance in morbid obesity. Endokrynol. Pol. 2018, 69, 252–258. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.; Fu, S.; Xue, Y.; Liang, M.; Xuan, D.; Zhu, X.; Wan, W.; Lv, L.; Zou, H. Leptin Promotes Monosodium Urate Crystal-Induced Inflammation in Human and Murine Models of Gout. J. Immunol. 2019, 202, 2728–2736. [Google Scholar] [CrossRef]
- Orlova, I.V.; Stanislavchuk, M.A.; Gunko, I.P. Dysadipokinemia in patients with gout and its association with the disease activity. Wiadomości Lek. 2018, 71, 289–294. [Google Scholar]
- Oikonen, M.; Wendelin-Saarenhovi, M.; Lyytikainen, L.P.; Siitonen, N.; Loo, B.M.; Jula, A.; Seppala, I.; Saarikoski, L.; Lehtimaki, T.; Hutri-Kahonen, N.; et al. Associations between serum uric acid and markers of subclinical atherosclerosis in young adults. The cardiovascular risk in Young Finns study. Atherosclerosis 2012, 223, 497–503. [Google Scholar] [CrossRef]
- Smith, G.D.; Ebrahim, S. “Mendelian randomization”: Can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.; Davey Smith, G.; Windmeijer, F.; Bowden, J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int. J. Epidemiol. 2019, 48, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Huffman, J.E.; Albrecht, E.; Teumer, A.; Mangino, M.; Kapur, K.; Johnson, T.; Kutalik, Z.; Pirastu, N.; Pistis, G.; Lopez, L.M.; et al. Modulation of genetic associations with serum urate levels by body-mass-index in humans. PLoS ONE 2015, 10, e0119752. [Google Scholar] [CrossRef] [PubMed]
- Kottgen, A.; Albrecht, E.; Teumer, A.; Vitart, V.; Krumsiek, J.; Hundertmark, C.; Pistis, G.; Ruggiero, D.; O’Seaghdha, C.M.; Haller, T.; et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 2013, 45, 145–154. [Google Scholar] [CrossRef]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Dastani, Z.; Hivert, M.F.; Timpson, N.; Perry, J.R.; Yuan, X.; Scott, R.A.; Henneman, P.; Heid, I.M.; Kizer, J.R.; Lyytikainen, L.P.; et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: A multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012, 8, e1002607. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, 7. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Cheng, L.; Zhuang, H.; Ju, H.; Yang, S.; Han, J.; Tan, R.; Hu, Y. Exposing the Causal Effect of Body Mass Index on the Risk of Type 2 Diabetes Mellitus: A Mendelian Randomization Study. Front. Genet. 2019, 10, 94. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Zhao, Q.; Lawlor, D.A.; Sheehan, N.A.; Thompson, J.; Davey Smith, G. Improving the accuracy of two-sample summary-data Mendelian randomization: Moving beyond the NOME assumption. Int. J. Epidemiol. 2019, 48, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Richette, P.; Poitou, C.; Manivet, P.; Denis, J.; Bouillot, J.L.; Clement, K.; Oppert, J.M.; Bardin, T. Weight Loss, Xanthine Oxidase, and Serum Urate Levels: A Prospective Longitudinal Study of Obese Patients. Arthritis Care Res. 2016, 68, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Lyngdoh, T.; Vuistiner, P.; Marques-Vidal, P.; Rousson, V.; Waeber, G.; Vollenweider, P.; Bochud, M. Serum uric acid and adiposity: Deciphering causality using a bidirectional Mendelian randomization approach. PLoS ONE 2012, 7, e39321. [Google Scholar] [CrossRef]
- Larsson, S.C.; Burgess, S.; Michaelsson, K. Genetic association between adiposity and gout: A Mendelian randomization study. Rheumatology 2018, 57, 2145–2148. [Google Scholar] [CrossRef]
- Inokuchi, T.; Tsutsumi, Z.; Takahashi, S.; Ka, T.; Moriwaki, Y.; Yamamoto, T. Increased frequency of metabolic syndrome and its individual metabolic abnormalities in Japanese patients with primary gout. J. Clin. Rheumatol. 2010, 16, 109–112. [Google Scholar] [CrossRef]
- Diaz-Torne, C.; Ortiz, M.A.; Garcia-Guillen, A.; Jeria-Navarro, S.; Sainz, L.; Fernandez-Sanchez, S.; Corominas, H.; Vidal, S. The inflammatory role of silent urate crystal deposition in intercritical gout. Rheumatology 2021, 60, 5463–5472. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Nishizawa, T.; Taniura, T.; Nomura, S. Effects of febuxostat on platelet-derived microparticles and adiponectin in patients with hyperuricemia. Blood Coagul. Fibrinolysis 2015, 26, 887–892. [Google Scholar] [CrossRef]
- Nakata, T.; Ikeda, S.; Koga, S.; Yonekura, T.; Tsuneto, A.; Doi, Y.; Fukae, S.; Minami, T.; Kawano, H.; Maemura, K. Randomized, Open-Label, Cross-Over Comparison of the Effects of Benzbromarone and Febuxostat on Endothelial Function in Patients with Hyperuricemia. Int. Heart J. 2020, 61, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J.; et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.J.; Munroe, P.B.; O’Neill, D.; Witkowska, K.; Charchar, F.J.; Doblado, M.; Evans, S.; Eyheramendy, S.; Onipinla, A.; Howard, P.; et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med. 2008, 5, e197. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sanna, S.; Maschio, A.; Busonero, F.; Usala, G.; Mulas, A.; Lai, S.; Dei, M.; Orru, M.; Albai, G.; et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007, 3, e194. [Google Scholar] [CrossRef]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef]
- Inokuchi, T.; Tsutsumi, Z.; Takahashi, S.; Ka, T.; Yamamoto, A.; Moriwaki, Y.; Masuzaki, H.; Yamamoto, T. Effects of benzbromarone and allopurinol on adiponectin in vivo and in vitro. Horm. Metab. Res. 2009, 41, 327–332. [Google Scholar] [CrossRef]
- Miao, Z.; Yan, S.; Wang, J.; Wang, B.; Li, Y.; Xing, X.; Yuan, Y.; Meng, D.; Wang, L.; Gu, J.; et al. Insulin resistance acts as an independent risk factor exacerbating high-purine diet induced renal injury and knee joint gouty lesions. Inflamm. Res. 2009, 58, 659–668. [Google Scholar] [CrossRef]
- Doshi, M.; Takiue, Y.; Saito, H.; Hosoyamada, M. The increased protein level of URAT1 was observed in obesity/metabolic syndrome model mice. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1290–1294. [Google Scholar] [CrossRef]
- Lammert, A.; Kiess, W.; Bottner, A.; Glasow, A.; Kratzsch, J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem. Biophys. Res. Commun. 2001, 283, 982–988. [Google Scholar] [CrossRef]
- Hirose, H.; Saito, I.; Kawai, T.; Tsujioka, M.; Kawabe, H.; Saruta, T. Relationships between baseline serum leptin levels and 2-year changes in body mass index, blood pressure and metabolic parameters in Japanese male adolescents and middle-aged men. Clin. Sci. 2001, 100, 145–150. [Google Scholar] [CrossRef]
- Lin, J.D.; Chiou, W.K.; Chang, H.Y.; Liu, F.H.; Weng, H.F. Serum uric acid and leptin levels in metabolic syndrome: A quandary over the role of uric acid. Metabolism 2007, 56, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Lyoussi, B.; Ragala, M.A.; Mguil, M.; Chraibi, A.; Israili, Z.H. Gender-specific leptinemia and its relationship with some components of the metabolic syndrome in Moroccans. Clin. Exp. Hypertens 2005, 27, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Samara, A.; Herbeth, B.; Aubert, R.; Berrahmoune, H.; Fumeron, F.; Siest, G.; Visvikis-Siest, S. Sex-dependent associations of leptin with metabolic syndrome-related variables: The Stanislas study. Obesity 2010, 18, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Bajo, M.A.; Rebollo, F.; Diez, J.J.; Diaz, C.; Paiva, A.; Codoceo, R.; Selgas, R. Leptin as a marker of nutrition and cardiovascular risk in peritoneal dialysis patients. Adv. Perit. Dial. 2002, 18, 212–217. [Google Scholar]
- Fulda, S.; Linseisen, J.; Wolfram, G.; Himmerich, S.; Gedrich, K.; Pollmacher, T.; Himmerich, H. Leptin plasma levels in the general population: Influence of age, gender, body weight and medical history. Protein Pept. Lett. 2010, 17, 1436–1440. [Google Scholar] [CrossRef]
- Obeidat, A.A.; Ahmad, M.N.; Haddad, F.H.; Azzeh, F.S. Leptin and uric acid as predictors of metabolic syndrome in jordanian adults. Nutr. Res. Pract. 2016, 10, 411–417. [Google Scholar] [CrossRef]
- Ugur-Altun, B.; Altun, A. Circulating leptin and osteoprotegerin levels affect insulin resistance in healthy premenopausal obese women. Arch. Med. Res. 2007, 38, 891–896. [Google Scholar] [CrossRef]
- Matsubara, M.; Chiba, H.; Maruoka, S.; Katayose, S. Elevated serum leptin concentrations in women with hyperuricemia. J. Atheroscler. Thromb. 2002, 9, 28–34. [Google Scholar] [CrossRef][Green Version]
- Posadzy-Malaczynska, A.; Rajpold, K.; Woznicka-Leskiewicz, L.; Marcinkowska, J. Reversal of an unfavorable effect of hydrochlorothiazide compared to angiotensin converting enzyme inhibitor on serum uric acid and oxypurine levels by estrogen-progestin therapy in hypertensive postmenopausal women. Curr. Med. Res. Opin. 2019, 35, 1687–1697. [Google Scholar] [CrossRef]
- Koga, M.; Saito, H.; Mukai, M.; Kasayama, S.; Yamamoto, T. Factors contributing to increased serum urate in postmenopausal Japanese females. Climacteric 2009, 12, 146–152. [Google Scholar] [CrossRef]
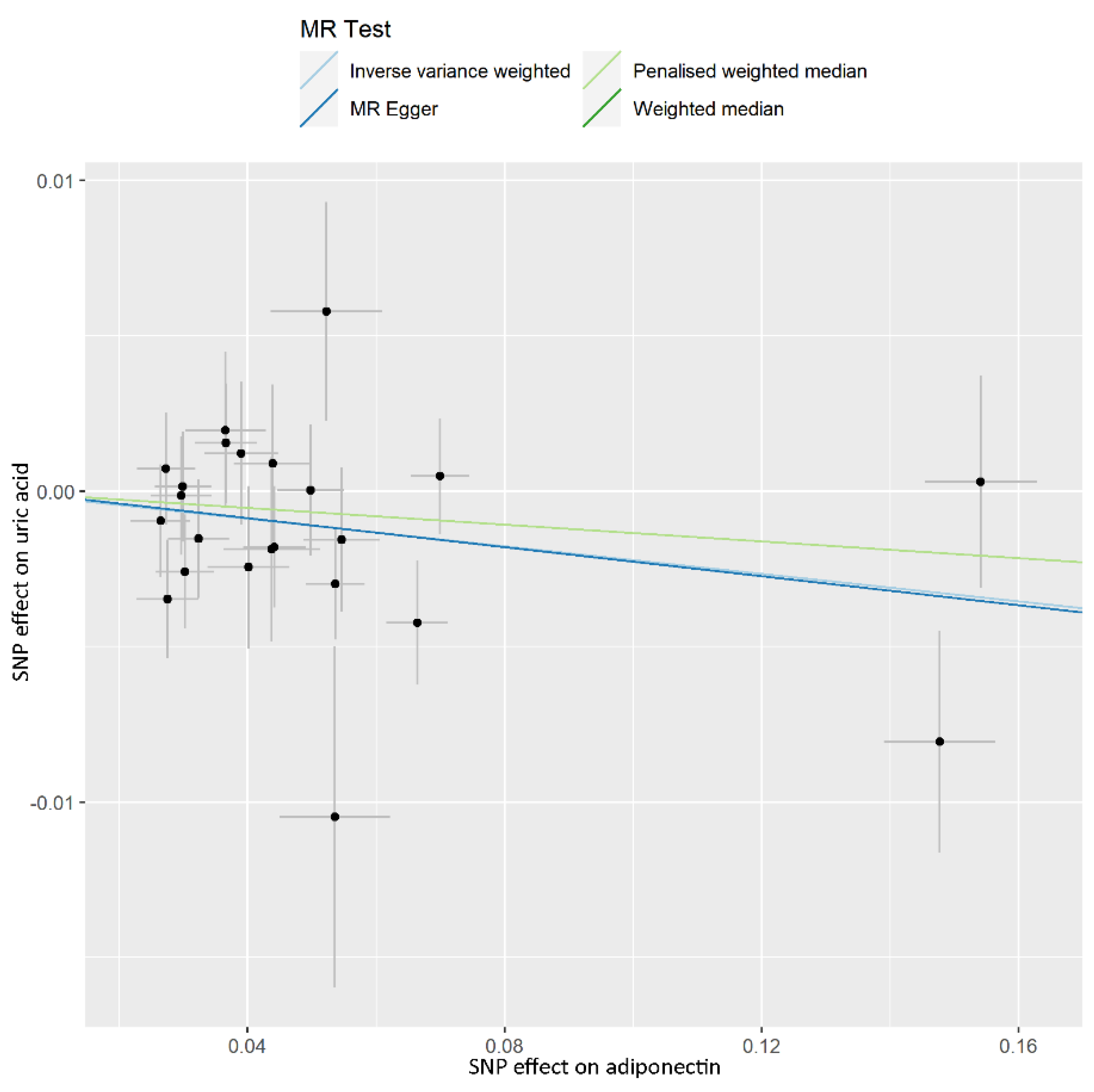
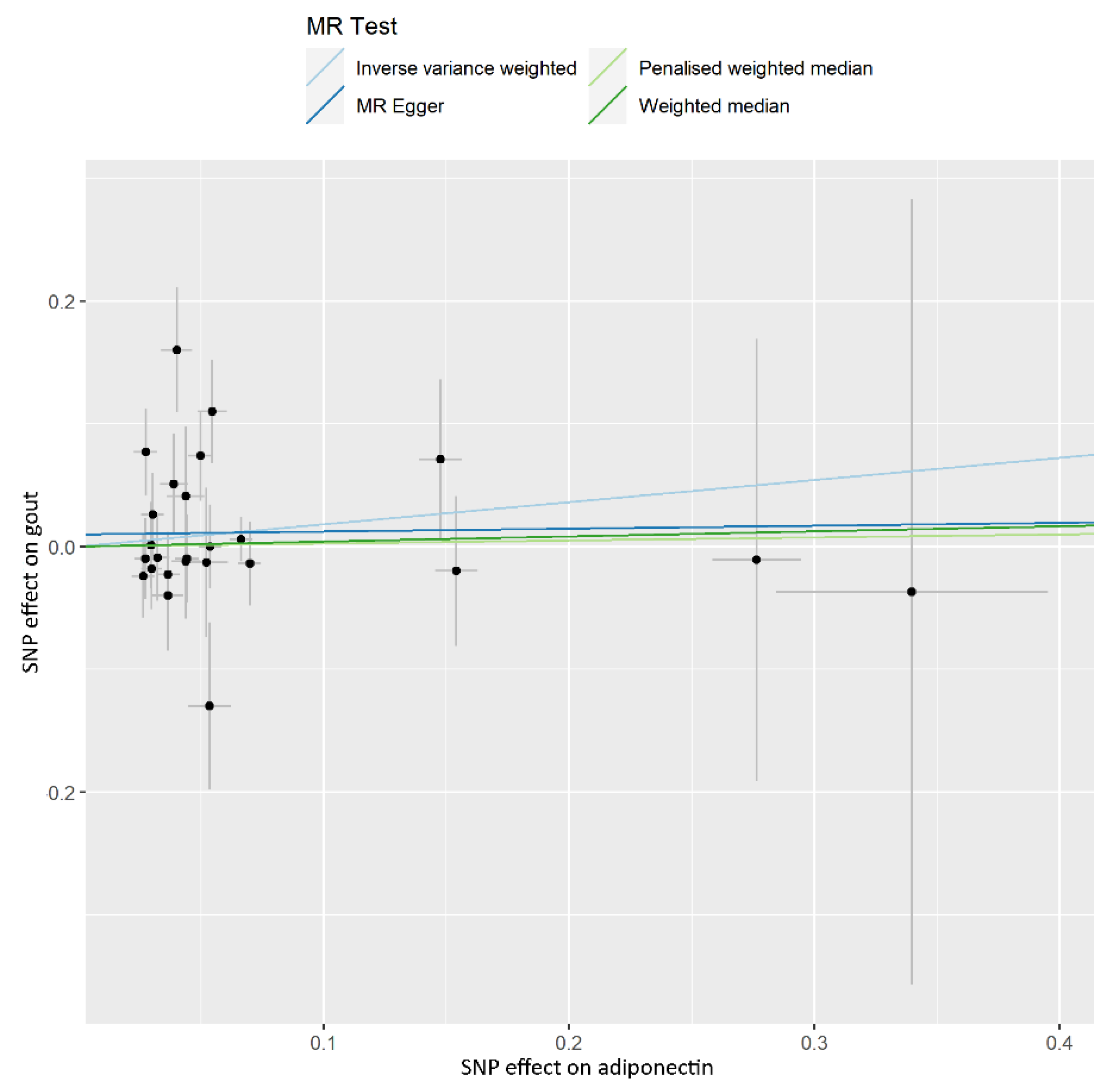
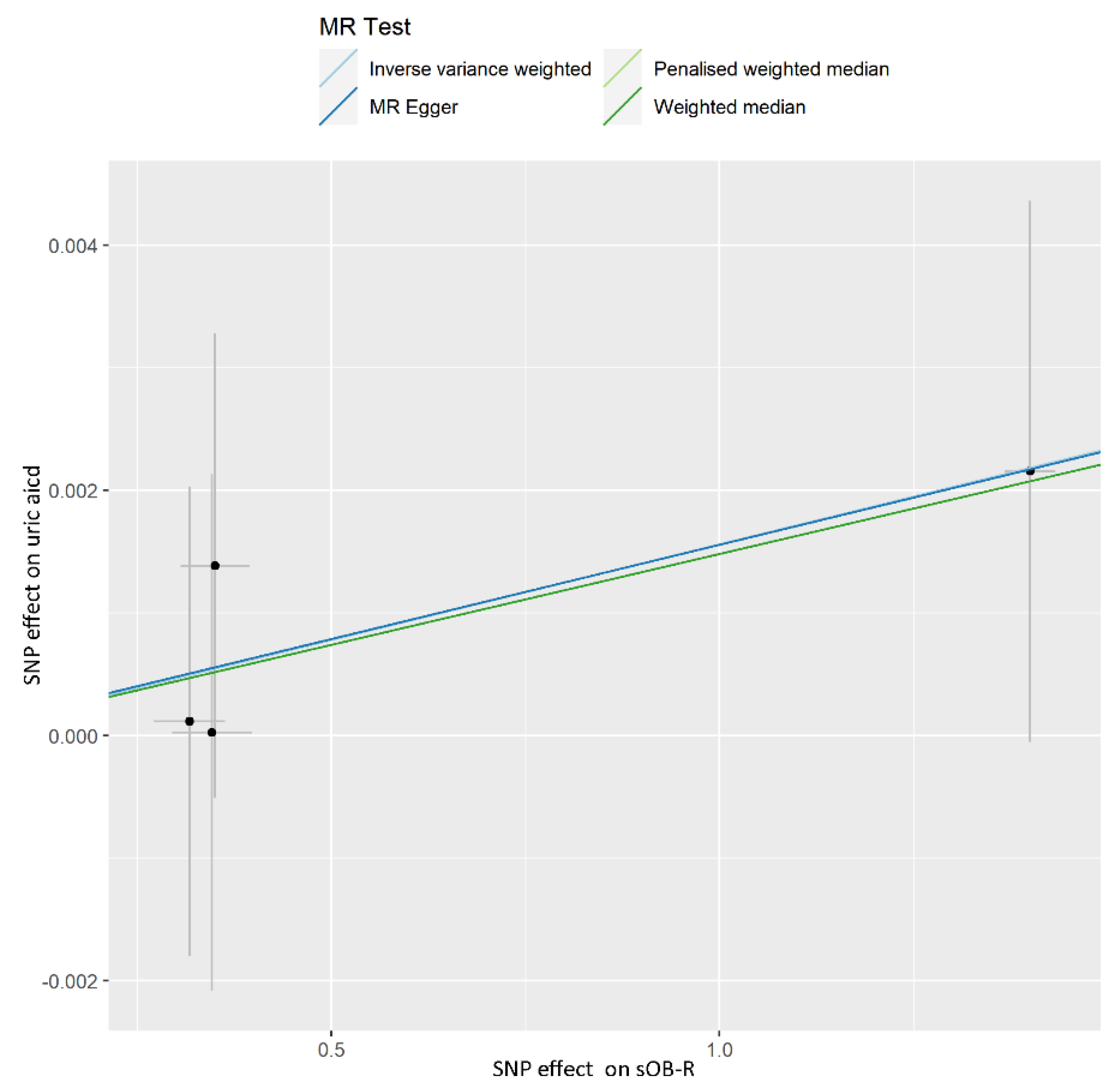
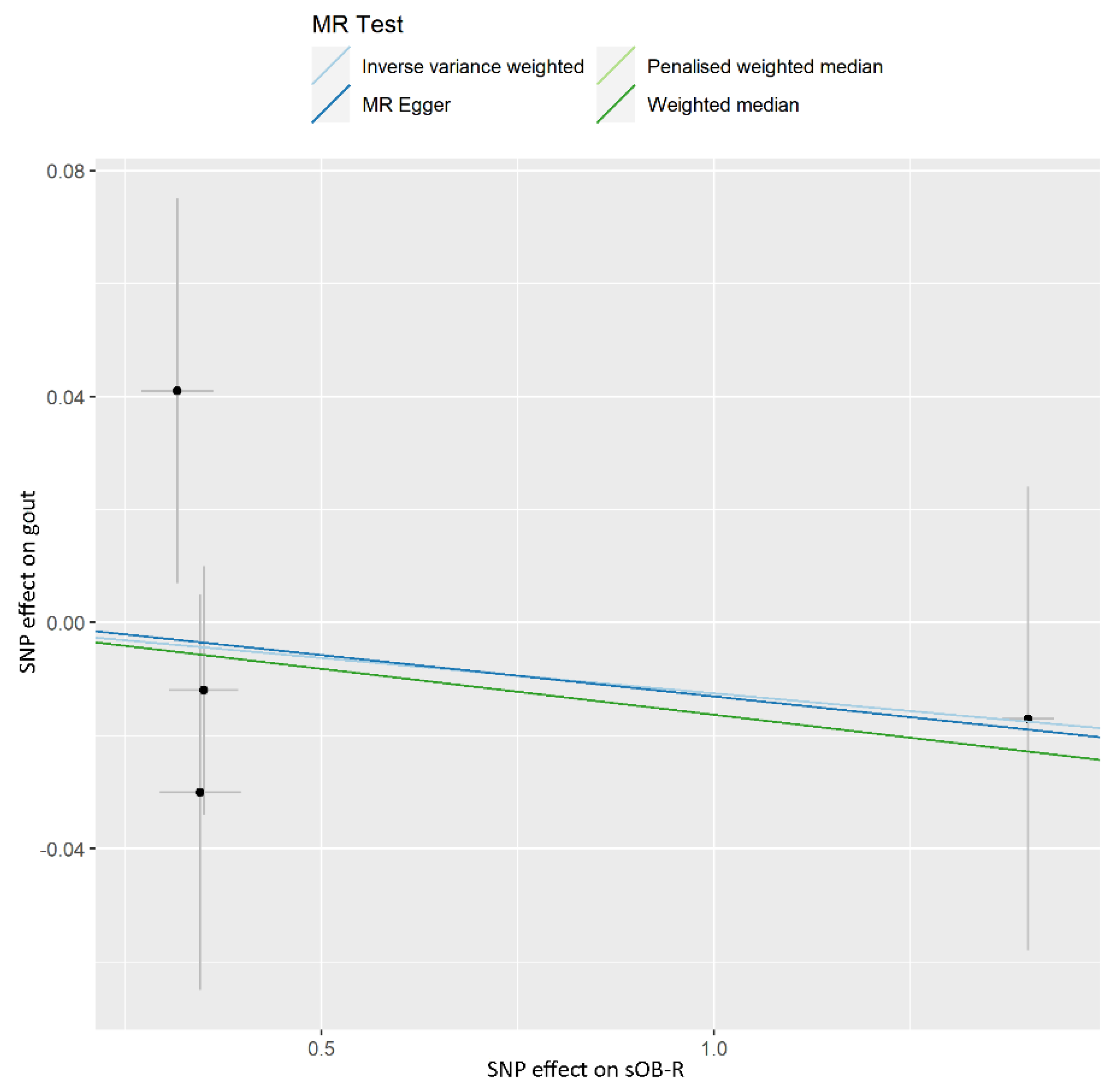
| Phenotype | Numbers of SNPs | OR (95% CI) | Beta (SE) | p | Q Statistic | F-Statistic |
|---|---|---|---|---|---|---|
| ADP vs. UA | 4349.6 | |||||
| IVW | 23 | 0.978 (0.961–0.996) | −0.022 (0.009) | 0.016 | 0.389 | |
| Weighted median | 23 | 0.987 (0.961–1.013) | −0.013 (0.014) | 0.324 | ||
| Penalised weighted median | 23 | 0.987 (0.961–1.013) | −0.013 (0.201) | 0.311 | ||
| MR-PRESSO | 23 | −0.017 (0.011) | 0.146 | |||
| global test | 0.438 | |||||
| MR-Egger | 23 | 0.977 (0.939–1.016) | −0.023 (0.020) | 0.256 | ||
| egger_intercept | 0.00007 (0.001) | 0.946 | ||||
| ADP vs. gout | 5751.4 | |||||
| IVW | 25 | 1.198 (0.865–1.659) | 0.181 (0.166) | 0.277 | 0.083 | |
| Weighted median | 25 | 1.043 (0.698–1.556) | 0.042 (0.204) | 0.839 | ||
| Penalised weighted median | 25 | 1.025 (0.692–1.519) | 0.025 (0.201) | 0.901 | ||
| MR-PRESSO | 25 | 0.181 (0.166) | 0.288 | |||
| global test | 0.116 | |||||
| MR-Egger | 25 | 1.024 (0.513–2.045) | 0.024 (0.353) | 0.947 | ||
| egger_intercept | 0.010 (0.019) | 0.618 | ||||
| Phenotype | Numbers of SNPs | OR (95% CI) | Beta (SE) | p | Q Statistic | F-Statistic |
|---|---|---|---|---|---|---|
| sOB-R vs. UA | 44.8 | |||||
| IVW | 4 | 1.002 (0.999–1.004) | 0.002 (0.001) | 0.274 | 0.961 | |
| Weighted median | 4 | 1.001 (0.999–1.004) | 0.001 (0.002) | 0.326 | ||
| Penalised weighted median | 4 | 1.001 (0.999–1.004) | 0.001 (0.002) | 0.325 | ||
| MR-PRESSO | 4 | 0.002 (0.0004) | 0.040 | |||
| global test | 0.969 | |||||
| MR-Egger | 4 | 1.002 (0.997–1.006) | 0.002 (0.002) | 0.578 | ||
| egger_intercept | 0.00002 (0.002) | 0.991 | ||||
| sOB-R vs. gout | 71.4 | |||||
| IVW | 4 | 0.988 (0.940–1.037) | −0.013 (0.025) | 0.616 | 0.492 | |
| Weighted median | 4 | 0.984 (0.933–1.037) | −0.016 (0.027) | 0.547 | ||
| Penalised weighted median | 4 | 0.984 (0.933–1.037) | −0.016 (0.027) | 0.544 | ||
| MR-PRESSO | 4 | −0.013 (0.022) | 0.615 | |||
| global test | 0.697 | |||||
| MR-Egger | 4 | 0.985 (0.901–1.078) | −0.015 (0.046) | 0.779 | ||
| egger_intercept | 0.002 (0.028) | 0.959 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, R.; Zhang, X.; Song, Z.; Chen, S.; Liu, G.; Liu, Y.; Pang, X.; Dong, F.; Xing, W.; Wang, Y.; et al. Assessing the Causal Effects of Adipokines on Uric Acid and Gout: A Two-Sample Mendelian Randomization Study. Nutrients 2022, 14, 1091. https://doi.org/10.3390/nu14051091
Cong R, Zhang X, Song Z, Chen S, Liu G, Liu Y, Pang X, Dong F, Xing W, Wang Y, et al. Assessing the Causal Effects of Adipokines on Uric Acid and Gout: A Two-Sample Mendelian Randomization Study. Nutrients. 2022; 14(5):1091. https://doi.org/10.3390/nu14051091
Chicago/Turabian StyleCong, Ruyi, Xiaoyu Zhang, Zihong Song, Shanshan Chen, Guanhua Liu, Yizhi Liu, Xiuyu Pang, Fang Dong, Weijia Xing, Youxin Wang, and et al. 2022. "Assessing the Causal Effects of Adipokines on Uric Acid and Gout: A Two-Sample Mendelian Randomization Study" Nutrients 14, no. 5: 1091. https://doi.org/10.3390/nu14051091
APA StyleCong, R., Zhang, X., Song, Z., Chen, S., Liu, G., Liu, Y., Pang, X., Dong, F., Xing, W., Wang, Y., & Xu, X. (2022). Assessing the Causal Effects of Adipokines on Uric Acid and Gout: A Two-Sample Mendelian Randomization Study. Nutrients, 14(5), 1091. https://doi.org/10.3390/nu14051091







