Food Addiction in Eating Disorders: A Cluster Analysis Approach and Treatment Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedure
2.2. Assessment
2.3. Treatment
Treatment Outcome Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Strength and Limits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Schulte, E.M.; Avena, N.M.; Gearhardt, A.N. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE 2015, 10, e0117959. [Google Scholar] [CrossRef] [PubMed]
- Cope, E.C.; Gould, E. New Evidence Linking Obesity and Food Addiction. Biol. Psychiatry 2017, 81, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.R. Food Addiction and Obesity. Neuropsychopharmacology 2017, 42, 361–362. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Boswell, R.G.; White, M.A. The association of “food addiction” with disordered eating and body mass index. Eat. Behav. 2014, 15, 427–433. [Google Scholar] [CrossRef] [Green Version]
- De Vries, S.-K.; Meule, A. Food Addiction and Bulimia Nervosa: New Data Based on the Yale Food Addiction Scale 2.0. Eur. Eat. Disord. Rev. 2016, 24, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Meule, A.; Von Rezori, V.; Blechert, J. Food addiction and bulimia nervosa. Eur. Eat. Disord. Rev. 2014, 22, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; White, M.A.; Masheb, R.M.; Morgan, P.T.; Crosby, R.D.; Grilo, C.M. An examination of the food addiction construct in obese patients with binge eating disorder. Int. J. Eat. Disord. 2012, 45, 657–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granero, R.; Jiménez-Murcia, S.; Gearhardt, A.N.; Agüera, Z.; Aymamí, N.; Gómez-Peña, M.; Lozano-Madrid, M.; Mallorquí-Bagué, N.; Mestre-Bach, G.; Neto-Antao, M.I.; et al. Validation of the Spanish version of the Yale Food Addiction Scale 2.0 (YFAS 2.0) and clinical correlates in a sample of eating disorder, gambling disorder, and healthy control participants. Front. Psychiatry 2018, 9, 321. [Google Scholar] [CrossRef]
- Hauck, C.; Cook, B.; Ellrott, T. Food addiction, eating addiction and eating disorders. Proc. Nutr. Soc. 2020, 79, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Meule, A.; Gearhardt, A.N. Food addiction in the light of DSM-5. Nutrients 2014, 6, 3653–3671. [Google Scholar] [CrossRef]
- Wolz, I.; Granero, R.; Fernández-Aranda, F. A comprehensive model of food addiction in patients with binge-eating symptomatology: The essential role of negative urgency. Compr. Psychiatry 2017, 74, 118–124. [Google Scholar] [CrossRef]
- Fielding-Singh, P.; Patel, M.L.; King, A.C.; Gardner, C.D. Baseline Psychosocial and Demographic Factors Associated with Study Attrition and 12-Month Weight Gain in the DIETFITS Trial. Obesity 2019, 27, 1997–2004. [Google Scholar] [CrossRef]
- Romero, X.; Agüera, Z.; Granero, R.; Sánchez, I.; Riesco, N.; Jiménez-Murcia, S.; Gisbert-Rodriguez, M.; Sánchez-González, J.; Casalé, G.; Baenas, I.; et al. Is food addiction a predictor of treatment outcome among patients with eating disorder? Eur. Eat. Disord. Rev. 2019, 27, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Brunault, P.; Ducluzeau, P.-H.; Courtois, R.; Bourbao-Tournois, C.; Delbachian, I.; Réveillère, C.; Ballon, N. Food Addiction is Associated with Higher Neuroticism, Lower Conscientiousness, Higher Impulsivity, but Lower Extraversion in Obese Patient Candidates for Bariatric Surgery. Subst. Use Misuse 2018, 53, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Kay-Lambkin, F.; Pursey, K.; Skinner, J.; Dayas, C. Food addiction and associations with mental health symptoms: A systematic review with meta-analysis. J. Hum. Nutr. Diet. 2018, 31, 544–572. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Skinner, J.; McKenna, R.; Rollo, M. Food addiction, binge eating disorder, and obesity: Is there a relationship? Behav. Sci. 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Murcia, S.; Agüera, Z.; Paslakis, G.; Munguia, L.; Granero, R.; Sánchez-González, J.; Sánchez, I.; Riesco, N.; Gearhardt, A.N.; Dieguez, C.; et al. Food addiction in eating disorders and obesity: Analysis of clusters and implications for treatment. Nutrients 2019, 11, 2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; Text Revision (DSM-IV-TR®) No Title; American Psychiatric Association Publishing: Washington, DC, USA, 2010. [Google Scholar]
- Garner, D.M. Inventario de Trastornos de la Conducta Alimentaria (EDI-2)—Manual; TEA: Madrid, Spain, 1998.
- Derogatis, L.R. SCL-90-R: Symptom Checklist-90-R. Administration, Scoring and Procedures Manuall—II for the Revised Version; Clinical Psychometric Research: Towson, MD, USA, 1994. [Google Scholar]
- Derogatis, L.R. SCL-90-R. Cuestionario de 90 Síntomas-Manual; TEA: Madrid, Spain, 2002.
- Cloninger, C.R. The Temperament and Character Inventory—Revised; Center for Psychobiology of Personality, Washington University: St Louis, MO, USA, 1999. [Google Scholar]
- Gutiérrez-Zotes, J.A.; Bayón, C.; Montserrat, C.; Valero, J.; Labad, A.; Cloninger, C.R.; Fernández-Aranda, F. Temperament and Character Inventory Revised (TCI-R). Standardization and normative data in a general population sample. Actas Esp. De Psiquiatr. 2004, 32, 8–15. [Google Scholar]
- Gearhardt, A.; Corbin, W.; Brownell, K. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav. 2016, 30, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Aranda, F.; Treasure, J.; Paslakis, G.; Agüera, Z.; Giménez, M.; Granero, R.; Sánchez, I.; Serrano-Troncoso, E.; Gorwood, P.; Herpertz-Dahlmann, B.; et al. The impact of duration of illness on treatment nonresponse and drop-out: Exploring the relevance of enduring eating disorder concept. Eur. Eat. Disord. Rev. 2021, 29, 499–513. [Google Scholar] [CrossRef]
- Agüera, Z.; Sánchez, I.; Granero, R.; Riesco, N.; Steward, T.; Martín-Romera, V.; Jiménez-Murcia, S.; Romero, X.; Caroleo, M.; Segura-García, C.; et al. Short-Term Treatment Outcomes and Dropout Risk in Men and Women with Eating Disorders. Eur. Eat. Disord. Rev. 2017, 25, 293–301. [Google Scholar] [CrossRef]
- Riesco, N.; Agüera, Z.; Granero, R.; Jiménez-Murcia, S.; Menchón, J.M.; Fernández-Aranda, F. Other Specified Feeding or Eating Disorders (OSFED): Clinical heterogeneity and cognitive-behavioral therapy outcome. Eur. Psychiatry 2018, 54, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stata-Corp. Stata Statistical Software: Release 17; Stata Press Publication (StataCorp LLC): College Station, TX, USA, 2021. [Google Scholar]
- Kelley, K.; Preacher, K.J. On effect size. Psychol. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Finner, H.; Roters, M. On the False Discovery Rate and Expected Type I Errors. J. Am. Stat. Assoc. 2001, 88, 920–923. [Google Scholar] [CrossRef]
- Hilker, I.; Sánchez, I.; Steward, T.; Jiménez-Murcia, S.; Granero, R.; Gearhardt, A.N.; Rodríguez-Muñoz, R.C.; Dieguez, C.; Crujeiras, A.B.; Tolosa-Sola, I.; et al. Food Addiction in Bulimia Nervosa: Clinical Correlates and Association with Response to a Brief Psychoeducational Intervention. Eur. Eat. Disord. Rev. 2016, 24, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-Gutiérrez, A.; Jiménez-Murcia, S.; Fernández-Aranda, F.; Agüera, Z.; Granero, R.; Hakansson, A.; Fagundo, A.B.; Bolao, F.; Valdepérez, A.; Mestre-Bach, G.; et al. The relevance of personality traits in impulsivity-related disorders: From substance use disorders and gambling disorder to bulimia nervosa. J. Behav. Addict. 2017, 6, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, K.L.; Byrne, S.M.; Oddy, W.H.; Crosby, R.D. Early onset binge eating and purging eating disorders: Course and outcome in a population-based study of adolescents. J. Abnorm. Child Psychol. 2013, 41, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.; White, M.; Potenza, M. Binge eating disorder and food addiction. Curr. Drug Abus. Rev. 2011, 4, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Van den Eynde, F.; Koskina, A.; Syrad, H.; Guillaume, S.; Broadbent, H.; Campbell, I.C.; Schmidt, U. State and trait food craving in people with bulimic eating disorders. Eat. Behav. 2012, 13, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Beitscher-Campbell, H.; Blum, K.; Febo, M.; Madigan, M.A.; Giordano, J.; Badgaiyan, R.D.; Braverman, E.R.; Dushaj, K.; Li, M.; Gold, M.S. Pilot clinical observations between food and drug seeking derived from fifty cases attending an eating disorder clinic. J. Behav. Addict. 2016, 5, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.M.; Tweardy, S.; Geliebter, A.; Avena, N.M. A Longitudinal Preliminary Study of Addiction-Like Responses to Food and Alcohol Consumption Among Individuals Undergoing Weight Loss Surgery. Obes. Surg. 2019, 29, 2700–2703. [Google Scholar] [CrossRef]
- Schag, K.; Leehr, E.J.; Meneguzzo, P.; Martus, P.; Zipfel, S.; Giel, K.E. Food-related impulsivity assessed by longitudinal laboratory tasks is reduced in patients with binge eating disorder in a randomized controlled trial. Sci. Rep. 2021, 11, 8225. [Google Scholar] [CrossRef] [PubMed]
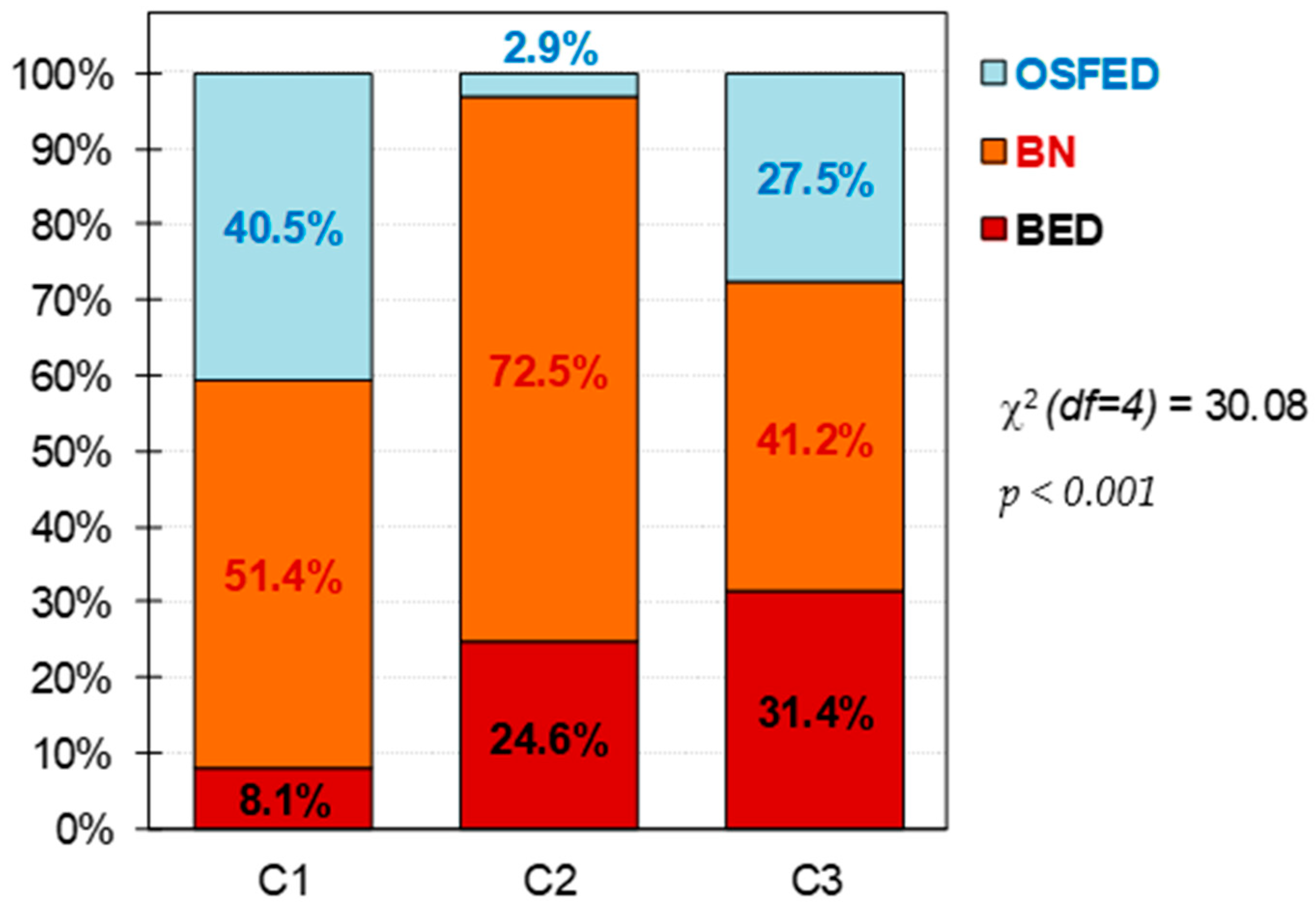
| BED (n = 36) | BN (n = 90) | OSFED (n = 31) | Cluster-1 (n = 37) | Cluster-2 (n = 69) | Cluster-3 (n = 51) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p | n | % | n | % | n | % | p | |
| Civil status | ||||||||||||||
| Single | 14 | 38.9% | 57 | 63.3% | 26 | 83.9% | 0.005 * | 29 | 78.4% | 41 | 59.4% | 27 | 52.9% | 0.144 |
| Married | 16 | 44.4% | 22 | 24.4% | 3 | 9.7% | 6 | 16.2% | 20 | 29.0% | 15 | 29.4% | ||
| Divorced | 6 | 16.7% | 11 | 12.2% | 2 | 6.5% | 2 | 5.4% | 8 | 11.6% | 9 | 17.6% | ||
| Education | ||||||||||||||
| Primary | 13 | 36.1% | 31 | 34.4% | 11 | 35.5% | 0.688 | 13 | 35.1% | 25 | 36.2% | 17 | 33.3% | 0.629 |
| Secondary | 14 | 38.9% | 44 | 48.9% | 16 | 51.6% | 20 | 54.1% | 32 | 46.4% | 22 | 43.1% | ||
| University | 9 | 25.0% | 15 | 16.7% | 4 | 12.9% | 4 | 10.8% | 12 | 17.4% | 12 | 23.5% | ||
| Mean | SD | Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | Mean | SD | p | |
| Age (years-old) | 42.28 | 12.30 | 30.92 | 10.39 | 29.13 | 10.41 | 0.001 * | 28.14 | 8.86 | 34.74 | 12.89 | 34.71 | 11.62 | 0.012 * |
| Onset (years-old) | 27.72 | 11.45 | 18.98 | 7.89 | 19.29 | 7.44 | 0.001 * | 17.84 | 6.41 | 22.57 | 11.38 | 21.32 | 7.81 | 0.046 * |
| Duration (years) | 14.46 | 9.16 | 12.13 | 9.00 | 10.03 | 8.84 | 0.134 | 10.32 | 7.49 | 12.80 | 9.84 | 12.91 | 8.99 | 0.334 |
| Cluster-1 (n = 37) | Cluster-2 (n = 69) | Cluster-3 (n = 51) | Cluster-1 vs. Cluster-2 | Cluster-1 vs. Cluster-3 | Cluster-2 vs. Cluster-3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α | Mean | SD | Mean | SD | Mean | SD | p | |d| | p | |d| | p | |d| | |
| BMI-FA | |||||||||||||
| BMI (kg/m2) | 25.96 | 7.44 | 29.42 | 8.54 | 30.77 | 10.15 | 0.057 | 0.43 | 0.013 * | 0.54 † | 0.411 | 0.14 | |
| YFAS total score | 0.939 | 8.46 | 2.38 | 9.48 | 1.99 | 7.53 | 2.72 | 0.034 * | 0.46 | 0.068 | 0.36 | 0.001 * | 0.82 † |
| EDI -2 Drive-thinness | 0.767 | 18.03 | 2.71 | 15.94 | 4.77 | 14.14 | 4.94 | 0.022 * | 0.54 † | 0.001 * | 0.98 † | 0.029 * | 0.37 |
| EDI-2 Body-dissatisfac. | 0.850 | 21.30 | 5.73 | 20.59 | 6.52 | 16.96 | 7.17 | 0.600 | 0.11 | 0.003 * | 0.67 † | 0.003 * | 0.53 † |
| EDI-2 Int-awareness | 0.798 | 18.22 | 5.67 | 15.46 | 5.36 | 8.00 | 5.71 | 0.016 * | 0.50 † | 0.001 * | 1.80 † | 0.001 * | 1.35 † |
| EDI-2 Bulimia | 0.726 | 8.54 | 5.78 | 11.52 | 3.91 | 7.33 | 4.89 | 0.002 * | 0.60 † | 0.239 | 0.23 | 0.001 * | 0.95 † |
| EDI-2 Interper-distrust | 0.813 | 9.08 | 5.24 | 6.97 | 4.65 | 3.49 | 3.60 | 0.022 * | 0.43 | 0.001 * | 1.24 † | 0.001 * | 0.84 † |
| EDI-2 Ineffectiveness. | 0.848 | 17.38 | 6.55 | 14.88 | 5.70 | 6.88 | 4.68 | 0.031 * | 0.41 | 0.001 * | 1.84 † | 0.001 * | 1.53 † |
| EDI-2 Maturity-fears | 0.752 | 12.27 | 5.03 | 9.17 | 5.32 | 6.51 | 5.17 | 0.004 * | 0.60 † | 0.001 * | 1.13 † | 0.006 * | 0.51 † |
| EDI-2 Perfectionism | 0.740 | 6.95 | 5.12 | 6.14 | 4.24 | 4.65 | 3.97 | 0.371 | 0.17 | 0.016 * | 0.50 † | 0.066 | 0.36 |
| EDI-2 Impulse-regulat. | 0.730 | 13.22 | 5.28 | 7.57 | 4.37 | 3.18 | 3.18 | 0.001 * | 1.17 † | 0.001 * | 2.30 † | 0.001 * | 1.15 † |
| EDI-2 Ascetic | 0.702 | 10.35 | 2.99 | 8.77 | 2.92 | 5.61 | 3.11 | 0.010 * | 0.54 † | 0.001 * | 1.56 † | 0.001 * | 1.05 † |
| EDI-2 Social Insecurity | 0.752 | 12.76 | 4.78 | 9.41 | 4.17 | 4.49 | 2.82 | 0.001 * | 0.75 † | 0.001 * | 2.11 † | 0.001 * | 1.38 † |
| EDI-2 Total score | 0.923 | 148.1 | 27.28 | 126.4 | 20.73 | 81.24 | 22.63 | 0.001 * | 0.89 † | 0.001 * | 2.67 † | 0.001 * | 2.08 † |
| SCL-90R GSI | 0.966 | 2.67 | 0.33 | 2.07 | 0.35 | 1.28 | 0.36 | 0.001 * | 1.80 † | 0.001 * | 4.03 † | 0.001 * | 2.22 † |
| SCL-90R PST | 0.966 | 81.81 | 6.10 | 72.46 | 7.78 | 55.98 | 11.90 | 0.001 * | 1.34 † | 0.001 * | 2.73 † | 0.001 * | 1.64 † |
| SCL-90R PSDI | 0.966 | 2.94 | 0.33 | 2.58 | 0.36 | 2.04 | 0.34 | 0.001 * | 1.05 † | 0.001 * | 2.70 † | 0.001 * | 1.54 † |
| TCI-R Novelty-seeking | 0.806 | 103.5 | 16.07 | 98.4 | 17.27 | 102.7 | 15.88 | 0.133 | 0.31 | 0.811 | 0.05 | 0.168 | 0.26 |
| TCI-R Harm-avoidance | 0.887 | 133.7 | 14.52 | 126.4 | 17.00 | 109.0 | 16.24 | 0.028 * | 0.46 | 0.001 * | 1.60 † | 0.001 * | 1.05 † |
| TCI-R Reward.depend. | 0.831 | 97.5 | 17.30 | 98.3 | 14.06 | 104.8 | 15.59 | 0.797 | 0.05 | 0.029 * | 0.44 | 0.023 * | 0.44 |
| TCI-R Persistence | 0.896 | 102.8 | 22.46 | 100.8 | 20.27 | 108.4 | 19.78 | 0.633 | 0.09 | 0.213 | 0.26 | 0.048 * | 0.38 |
| TCI-R Self-directed. | 0.840 | 96.9 | 14.94 | 102.9 | 13.17 | 125.3 | 16.89 | 0.053 | 0.42 | 0.001 * | 1.78 † | 0.001 * | 1.48 † |
| TCI-R Cooperativeness | 0.861 | 127.8 | 20.24 | 133.7 | 17.15 | 139.3 | 11.88 | 0.082 | 0.31 | 0.002 * | 0.69 † | 0.067 | 0.38 |
| TCI-R Self-transcend. | 0.862 | 77.1 | 12.09 | 62.1 | 14.38 | 63.2 | 16.37 | 0.001 * | 1.13 † | 0.001 * | 0.97 † | 0.672 | 0.07 |
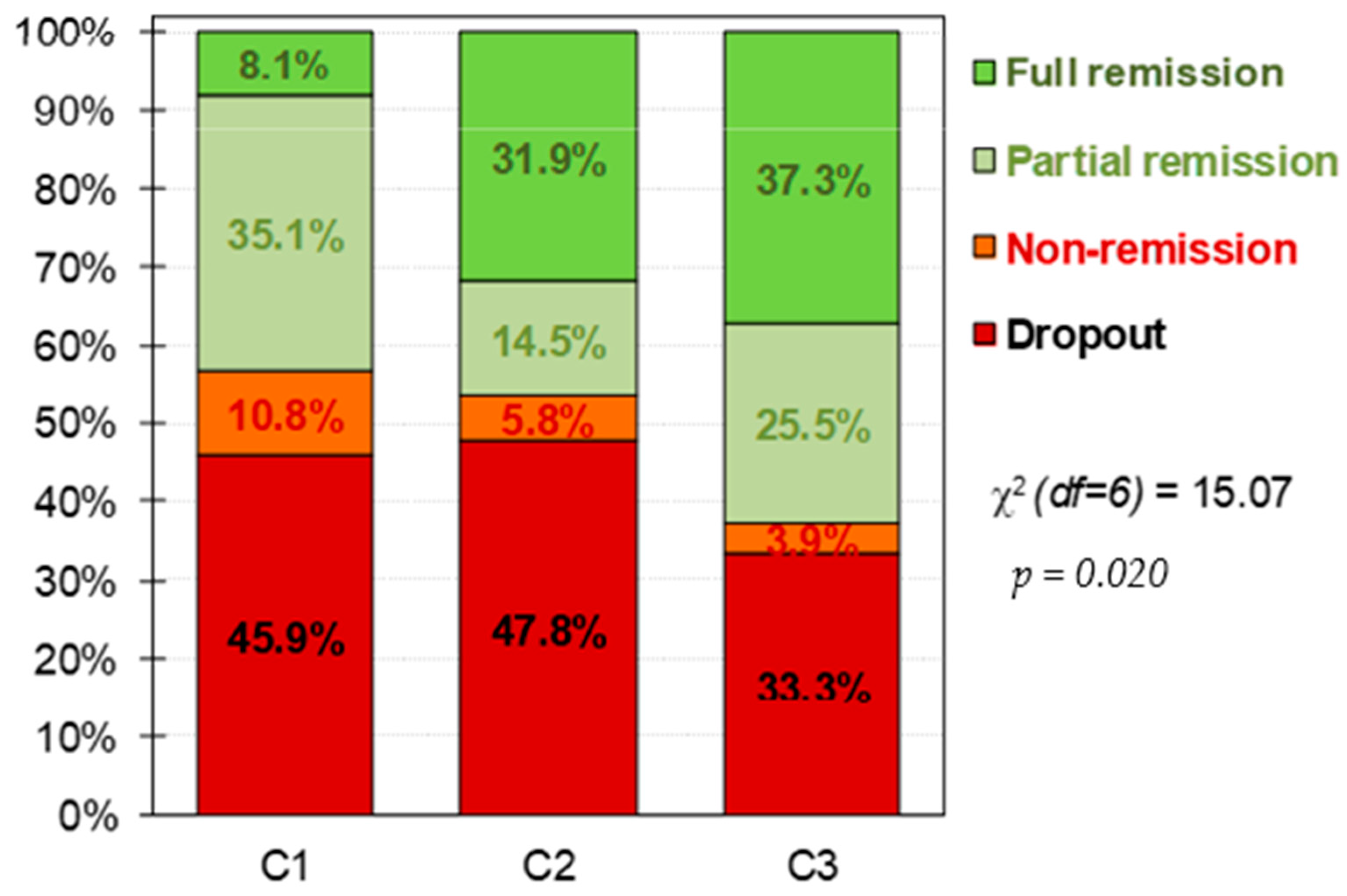
| CBT outcomes | n | % | n | % | n | % | p | |h| | p | |h| | p | |h| | |
| Dropout | 17 | 45.9% | 33 | 47.8% | 17 | 33.3% | 0.010 * | 0.04 | 0.016 * | 0.26 | 0.286 | 0.30 | |
| Non-remission | 4 | 10.8% | 4 | 5.8% | 2 | 3.9% | 0.18 | 0.27 | 0.09 | ||||
| Partial remission | 13 | 35.1% | 10 | 14.5% | 13 | 25.5% | 0.51 † | 0.21 | 0.28 | ||||
| Full remission | 3 | 8.1% | 22 | 31.9% | 19 | 37.3% | 0.62 † | 0.74 † | 0.11 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguía, L.; Gaspar-Pérez, A.; Jiménez-Murcia, S.; Granero, R.; Sánchez, I.; Vintró-Alcaraz, C.; Diéguez, C.; Gearhardt, A.N.; Fernández-Aranda, F. Food Addiction in Eating Disorders: A Cluster Analysis Approach and Treatment Outcome. Nutrients 2022, 14, 1084. https://doi.org/10.3390/nu14051084
Munguía L, Gaspar-Pérez A, Jiménez-Murcia S, Granero R, Sánchez I, Vintró-Alcaraz C, Diéguez C, Gearhardt AN, Fernández-Aranda F. Food Addiction in Eating Disorders: A Cluster Analysis Approach and Treatment Outcome. Nutrients. 2022; 14(5):1084. https://doi.org/10.3390/nu14051084
Chicago/Turabian StyleMunguía, Lucero, Anahí Gaspar-Pérez, Susana Jiménez-Murcia, Roser Granero, Isabel Sánchez, Cristina Vintró-Alcaraz, Carlos Diéguez, Ashley N. Gearhardt, and Fernando Fernández-Aranda. 2022. "Food Addiction in Eating Disorders: A Cluster Analysis Approach and Treatment Outcome" Nutrients 14, no. 5: 1084. https://doi.org/10.3390/nu14051084
APA StyleMunguía, L., Gaspar-Pérez, A., Jiménez-Murcia, S., Granero, R., Sánchez, I., Vintró-Alcaraz, C., Diéguez, C., Gearhardt, A. N., & Fernández-Aranda, F. (2022). Food Addiction in Eating Disorders: A Cluster Analysis Approach and Treatment Outcome. Nutrients, 14(5), 1084. https://doi.org/10.3390/nu14051084










