Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro
Abstract
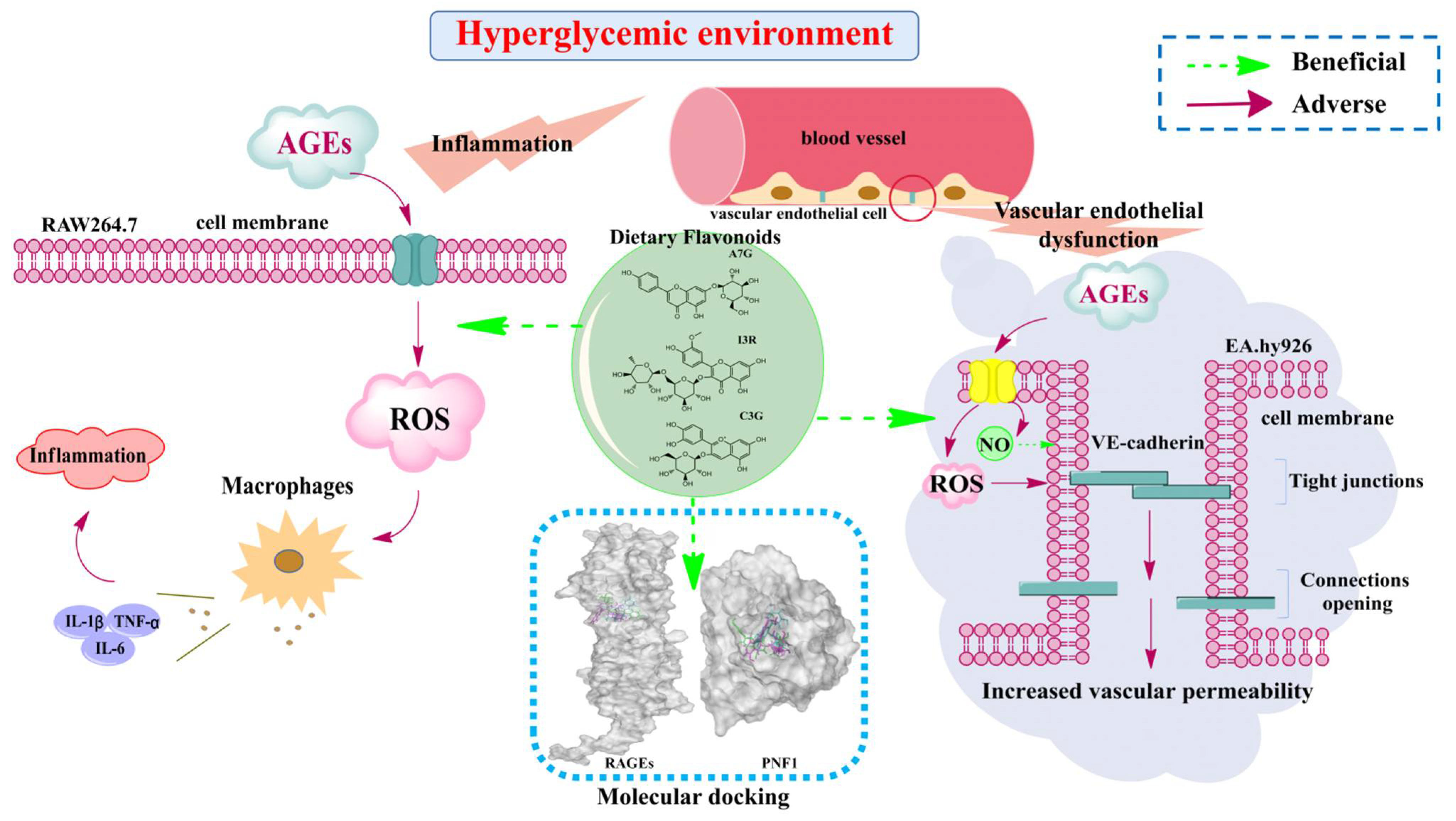
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of AGEs
2.3. Effects of Dietary Flavonoids on AGEs-Induced ROS Formation in RAW264.7 and EA.hy926 Cells
2.4. Effects of Dietary Flavonoids on the Secretion of Inflammatory Cytokines in AGEs-Induced RAW264.7 Cells
2.5. Effects of Dietary Flavonoids on NO Production in AGEs-Induced EA.hy926 Cells
2.6. Immunofluorescence Analysis
2.7. Transepithelial Electrical Resistance (TEER) Measurement
2.8. Molecular Docking Analysis
2.9. Statistical Analysis
3. Results and Discussion
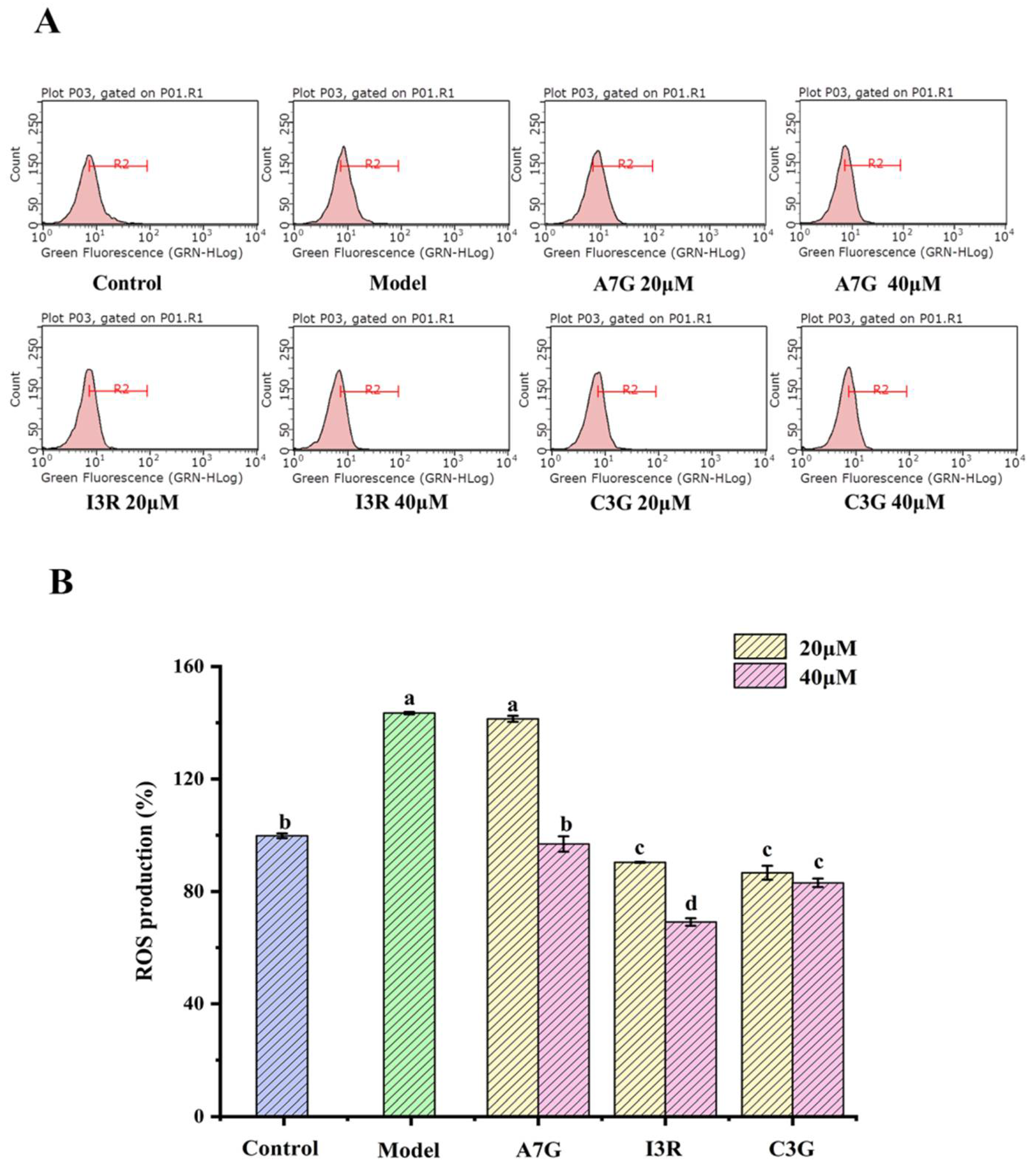
3.1. Dietary Flavonoids Suppressed AGEs-Induced ROS Production
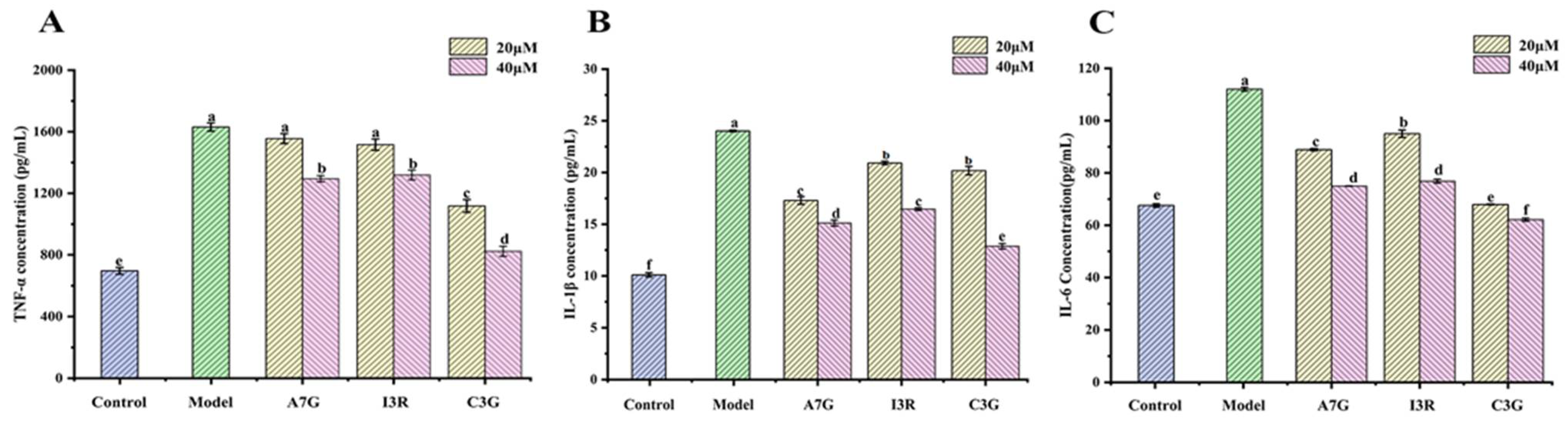
3.2. Dietary Flavonoids Reduced Pro-Inflammatory Cytokines Secretion in AGEs-Induced RAW264.7 Cells
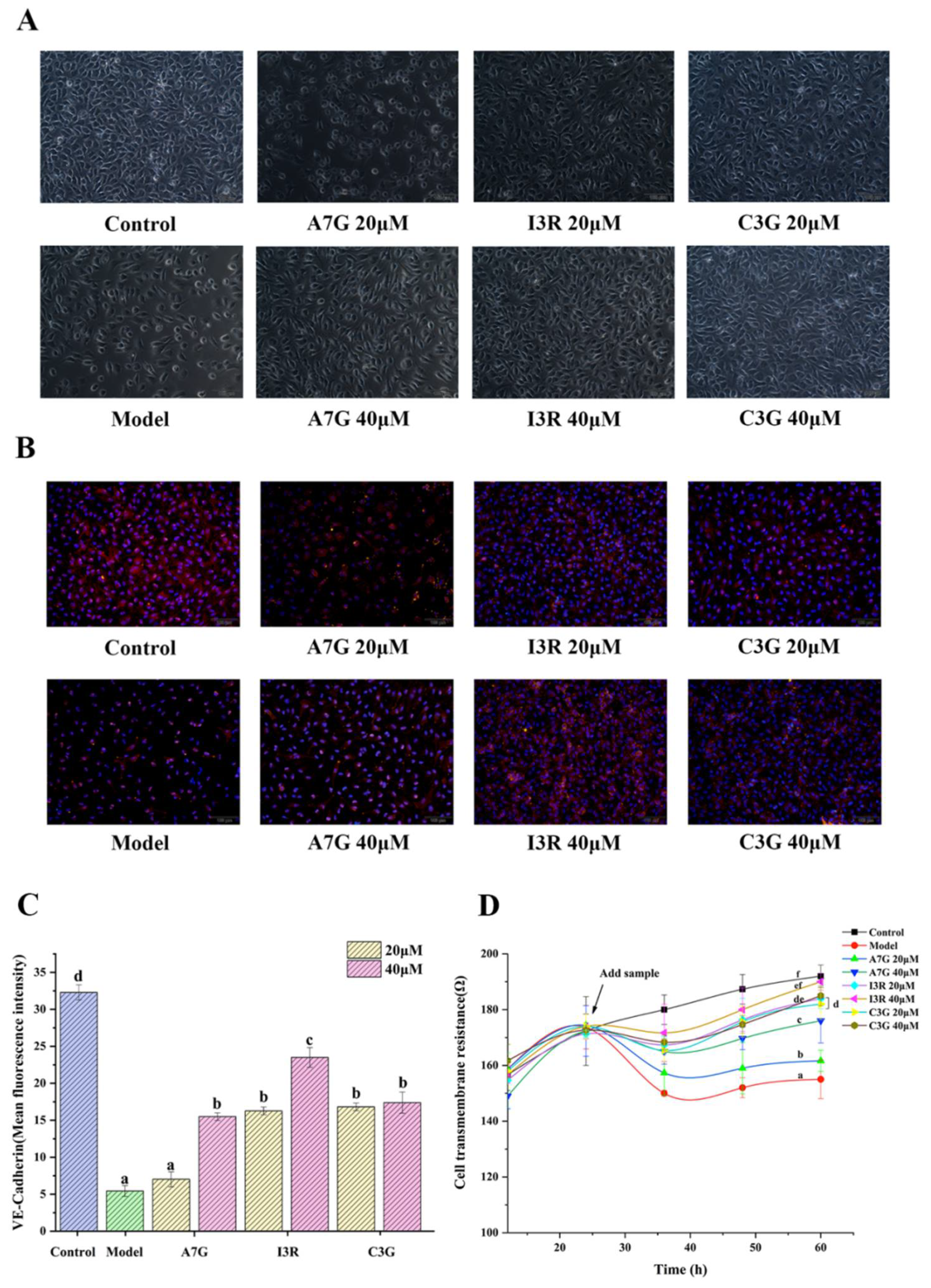
3.3. Protective Effects of Dietary Flavonoids on Endothelial Barrier Function in AGEs-Induced EA.hy926 Cells
3.4. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobi, A.; Bravo, B.S.; Veeren, B.; Paradela-Dobarro, B.; Álvarez, E.; Meilhac, O.; Viranaicken, W.; Baret, P.; Devin, A.; Rondeau, P. Advanced glycation end-products disrupt human endothelial cells redox homeostasis: New insights into reactive oxygen species production. Free Radic. Res. 2019, 53, 150–169. [Google Scholar] [CrossRef]
- Hamed, E.A.; Zakary, M.M.; Abdelal, R.M.; Abdel Moneim, E.M. Vasculopathy in type 2 diabetes mellitus: Role of specific angiogenic modulators. J. Pysiol. Biochem. 2011, 67, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Nowotn, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; He, Y.; Wang, S.; He, Y.; Wang, W.; Li, Q.; Cao, X. Ferulic acid inhibits advanced glycation end products (AGEs) formation and mitigates the AGEs-induced inflammatory response in HUVEC cells. J. Funct. Foods 2018, 48, 19–26. [Google Scholar] [CrossRef]
- Thomas, M.C.; Baynes, J.W.; Thorpe, S.R.; Cooper, M.E. The role of AGEs and AGE inhibitors in diabetic cardiovascular disease. Curr. Drug Targets 2005, 6, 453–474. [Google Scholar] [CrossRef] [PubMed]
- DeLoach, A.; Cozart, M.; Kiaei, A.; Kiaei, M. retrospective review of the progress in amyotrophic lateral sclerosis drug discovery over the last decade and a look at the latest strategies. Expert Opin. Drug Dis. 2015, 10, 1099–1118. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The protective effects of wine pomace products on the vascular endothelial barrier function. Food Funct. 2020, 11, 7878–7891. [Google Scholar] [CrossRef]
- Kanti, B.P.; Syed, I.R. Protective effect of resveratrol on formation of membrane protein carbonyls and lipid peroxidation in erythrocytes subjected to oxidative stress. Appl. Physiol. Nutr. Metab. 2009, 34, 1093–1097. [Google Scholar] [CrossRef]
- Teng, J.; Li, Y.; Yu, W.; Zhao, Y.; Hu, X.; Tao, N.; Wang, M. Naringenin, a common flavanone, inhibits the formation of AGEs in bread and attenuates AGEs-induced oxidative stress and inflammation in RAW264.7 cells. Food Chem. 2018, 269, 35–42. [Google Scholar] [CrossRef]
- Yu, W.; Hu, X.; Wang, M. Pterostilbene inhibited advanced glycation end products (AGEs)-induced oxidative stress and inflammation by regulation of RAGE/MAPK/NF-κB in RAW264.7 cells. J. Funct. Foods 2018, 40, 272–279. [Google Scholar] [CrossRef]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E–knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Ma, Y.; Cheng, G.; Zhang, Y.; Cai, S. Comparative study of dietary flavonoids with different structures as α-glucosidase inhibitors and insulin sensitizers. J. Agric. Food Chem. 2019, 67, 10521–10533. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Fu, Y.; Yi, J.; Gao, A.; Jia, Y.; Cai, S. Effects of Different Dietary Flavonoids on Dipeptidyl Peptidase-IV Activity and Expression: Insights into Structure–Activity Relationship. J. Agric. Food Chem. 2020, 68, 12141–12151. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Ding, H.; Hu, X.; Zhang, G.; Gong, D. Galangin inhibits α-glucosidase activity and formation of non-enzymatic glycation products. Food Chem. 2019, 271, 70–79. [Google Scholar] [CrossRef]
- Wu, C.; Lai, P.; Hsieh, S.; Cheng, C.; Hsieh, S. Suppression of carnosine on adhesion and extravasation of human colorectal cancer cells. Anticancer Res. 2019, 39, 6135–6144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. Free radicals, antioxidants and disease. Biol. Med. 2014, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Komarova, Y.; Kruse, K.; Mehta, D.; Malik, A. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Kondrashina, A.; Greco, L.; Gamon, L.; Lund, M.; Giblin, L.; Davies, M. Effects of protein-derived amino acid modification products present in infant formula on metabolic function, oxidative stress, and intestinal permeability in cell models. J. Agric. Food Chem. 2019, 67, 5634–5646. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tao, M.; Zhao, Y.; Hu, X.; Wang, M. 4′-Methoxyresveratrol alleviated AGE-induced inflammation via RAGE-mediated NF-κB and NLRP3 inflammasome pathway. Molecules 2018, 23, 1447. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Sidiq, T.; Joshi, P.; Jain, S.; Lawaniya, Y.; Kichlu, S.; Khajuria, A.; Vishwakarma, R.; Bharate, S. Anti-inflammatory and immunomodulatory flavones from Actinocarya tibetica Benth. Nat. Prod. Res. 2013, 27, 2227–2230. [Google Scholar] [CrossRef]
- Mirza, R.; Fang, M.; Ennis, W.; Koh, T. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.P.; Kim, H.G.; Hien, T.T.; Jeong, M.H.; Jeong, T.C.; Jeong, H.G. Puerarin activates endothelial nitric oxide synthase through estrogen receptor-dependent PI3-kinase and calcium-dependent AMP-activated protein kinase. Tocicol. Appl. Pharm. 2011, 257, 48–58. [Google Scholar] [CrossRef]
- Sun, L.; Dou, F.; Chen, J.; Chi, H.; Xing, S.; Liu, T.; Sun, S.; Chen, C. Salidroside slows the progression of EA.hy926 cell senescence by regulating the cell cycle in an atherosclerosis model. Mol. Med. Rep. 2018, 17, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pino-García, R.D.; Gerardi, G.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; García-Lomillo, J.; Muñiz, P. Wine pomace seasoning attenuates hyperglycaemia-induced endothelial dysfunction and oxidative damage in endothelial cells. J. Funct. Foods 2016, 22, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Lena, C.W. Vascular permeability—the essentials. Upsala J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.L.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Camilla, C.; Ridley, A.J. Endothelial cell-cell adhesion and signaling. Exp. Cell Res. 2017, 358, 31–38. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsieh, S.L.; Leung, W.; Jeng, J.H.; Huang, G.; Lee, C.T.; Wu, C. 2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside suppresses human colorectal cancer cell metastasis through inhibiting NF-κB activation. Int. J. Oncol. 2016, 49, 629–638. [Google Scholar] [CrossRef]
- Suttitheptumrong, A.; Rawarak, N.; Reamtong, O.; Boonnak, K.; Pattanakitsaku, S. Plectin is Required for Trans-Endothelial Permeability: A Model of Plectin Dysfunction in Human Endothelial Cells After TNF-α Treatment and Dengue Virus Infection. Proteomics 2018, 18, 1800215. [Google Scholar] [CrossRef]
- Yamagishi, S.; Fukami, K.; Matsui, T. Crosstalk between advanced glycation end products (AGEs)-receptor RAGE axis and dipeptidyl peptidase-4-incretin system in diabetic vascular complications. Cardiovasc. Diabetol. 2015, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Cheng, K.; Xiao, J.; Wang, M. The multifunctional roles of flavonoids against the formation of advanced glycation end products (AGEs) and AGEs-induced harmful effects. Trends Food Sci. Technol. 2020, 103, 333–347. [Google Scholar] [CrossRef]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef] [PubMed]
- Witke, W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Sriramoju, S.; Goetz, K. Molecular Docking Interaction Between Carotenoids and Curcumin and RAGE Receptor Prevents Diabetic Retinopathy Progression (P06-044-19). Curr. Dev. Nutr. 2019, 3 (Suppl. S1), nzz031-P06. [Google Scholar] [CrossRef] [Green Version]
- KAY, A.M.; Rushing, B.J.; Simpson, C.L.; Stewart, J.A. AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification in Vascular Smooth Muscle Cells. FASEB J. 2017, 3, 1017-1. [Google Scholar] [CrossRef]
- Huang, S.; Wu, C.; Yen, G. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Singh, R. An allied approach for in vitro modulation of aldose reductase, sorbitol accumulation and advanced glycation end products by flavonoid rich extract of Coriandrum sativum L. seeds. Toxicol. Rep. 2018, 5, 800–807. [Google Scholar] [CrossRef]


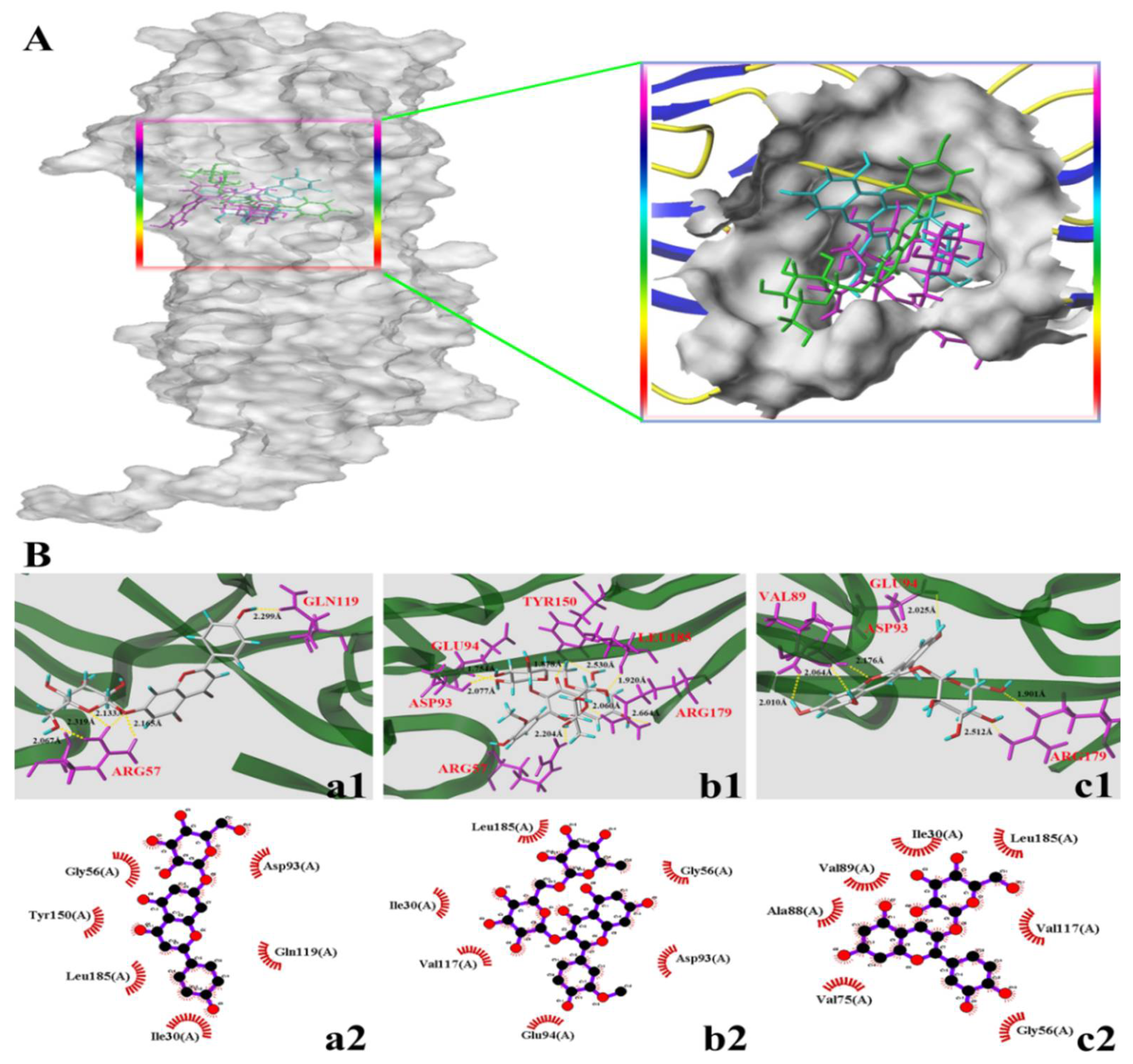

| A7G | I3R | C3G | |
|---|---|---|---|
| C-score | 4 | 4 | 4 |
| T-score | 5.0281 | 5.2227 | 7.349 |
| D-score | −90.4449 | −125.569 | −143.8509 |
| PMF-score | −46.7436 | −77.7488 | −51.0954 |
| G-score | −113.5269 | −114.4509 | −192.7999 |
| CHEM-score | −12.429 | −14.0453 | −22.6174 |
| Number of hydrogen bonds | 5 | 8 | 6 |
| Amino acid residues involved in hydrogen bonds | Arg57, Gln119 | Arg57, Asp93, Glu94, Tyr150, Arg179, Leu185 | Val89, Asp93, Glu94, Arg179 |
| Number of hydrophobic interactions | 6 | 6 | 7 |
| Amino acid residues involved in hydrophobic interactions | Ile30, Gly56, Asp93, Gln119, Tyr150, Leu185, | Ile30, Gly56, Asp93, Glu94, Val117, Leu185 | Ile30, Gly56, Val75, Ala88, Val89, Val117, Leu185, |
| Average distance (Å) | 2.1966 | 2.1359 | 2.220 |
| A7G | I3R | G3G | |
|---|---|---|---|
| C-score | 5 | 4 | 5 |
| T-score | 3.9599 | 4.4235 | 3.6886 |
| D-score | −103.0791 | −107.758 | −109.7659 |
| PMF-score | −10.9616 | −2.636 | 17.8642 |
| G-score | −152.0601 | −105.9407 | −126.2047 |
| CHEM-score | −12.8453 | −17.9643 | −21.6537 |
| Number of hydrogen bonds | 3 | 7 | 8 |
| Amino acid residues involved in hydrogen bonds | Asp53, Gln79 | Asp53, Pro57, Gly58, Gln76, Gly77, Gln79 | Asp53, Pro57, Gly58, Val74, Ile75, Gly77, Arg84 |
| Number of hydrophobic interactions | 11 | 6 | 4 |
| Amino acid residues involved in hydrophobic interactions | Gly58, Pro57, Ala61, Val74, Gln76, Gly77, Glu78, Gln79, Val82, Ile83, Arg84 | Gly58, Ala61, Val74, Gln76, Glu78, Ile83 | Pro57, Ala61, Gln76, Ile83 |
| Average distance (Å) | 2.108 | 2.188 | 2.096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Jia, Y.; Sun, Y.; Liu, X.; Yi, J.; Cai, S. Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro. Nutrients 2022, 14, 1026. https://doi.org/10.3390/nu14051026
Fu Y, Jia Y, Sun Y, Liu X, Yi J, Cai S. Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro. Nutrients. 2022; 14(5):1026. https://doi.org/10.3390/nu14051026
Chicago/Turabian StyleFu, Yishan, Yijia Jia, Yilin Sun, Xiaojing Liu, Junjie Yi, and Shengbao Cai. 2022. "Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro" Nutrients 14, no. 5: 1026. https://doi.org/10.3390/nu14051026
APA StyleFu, Y., Jia, Y., Sun, Y., Liu, X., Yi, J., & Cai, S. (2022). Dietary Flavonoids Alleviate Inflammation and Vascular Endothelial Barrier Dysfunction Induced by Advanced Glycation End Products In Vitro. Nutrients, 14(5), 1026. https://doi.org/10.3390/nu14051026







