Maternal Amino Acid Status in Severe Preeclampsia: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Nutrient Intake Assessment
2.3. Sample Preparation and Amino Acid Measurements
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Subjects
3.2. Nutrient Intake within Subjects
3.3. Amino Acids Levels in Normal Pregnancy and Preeclampsia
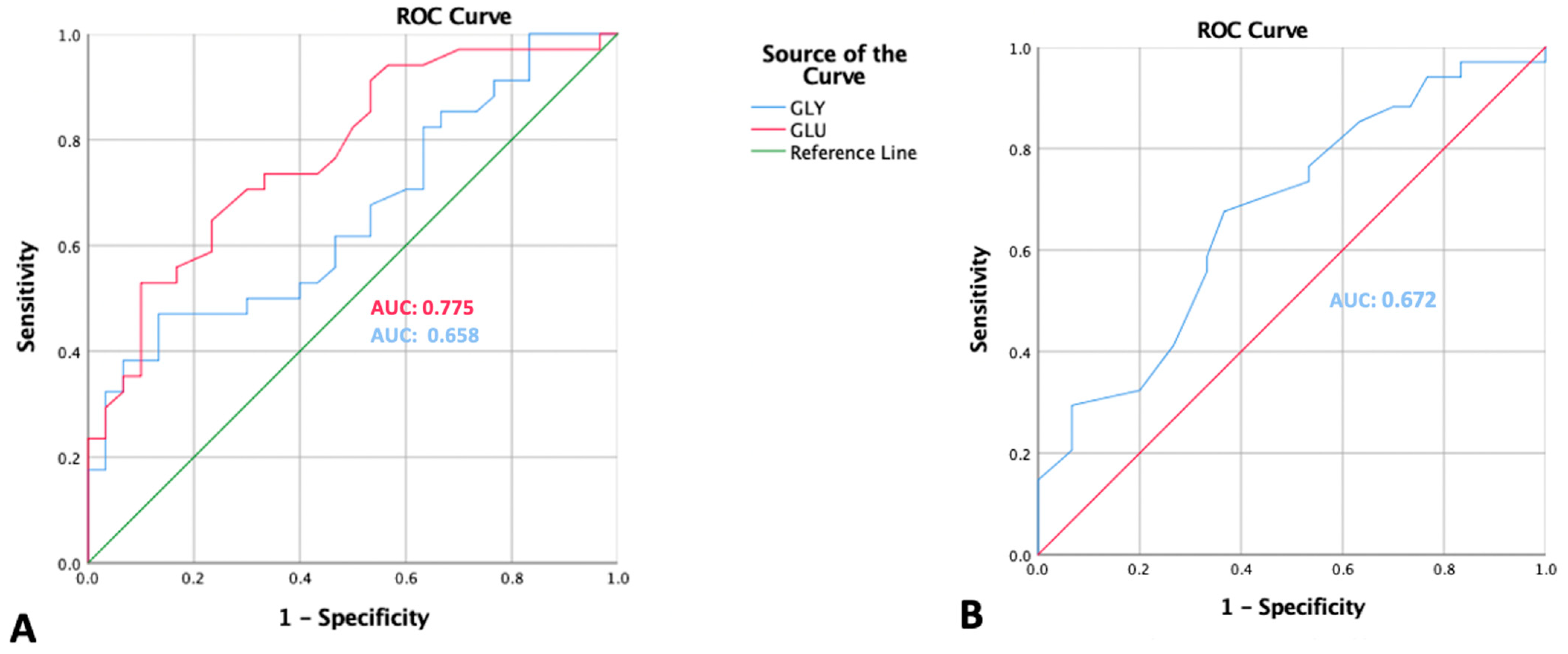
3.4. ROC Curve Analysis
3.5. Bivariate and Multivariate Analysis of the Cut-Off Points
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Renzo, G.C. The Great Obstetrical Syndromes. J. Matern. Neonatal Med. 2009, 22, 633–635. [Google Scholar] [CrossRef]
- Bahinipati, J. Ischemia Modified Albumin as a Marker of Oxidative Stress in Normal Pregnancy. J. Clin. Diagn. Res. 2016. Available online: http://jcdr.net/article_fulltext.asp?issn=0973-709x&year=2016&volume=10&issue=9&page=BC15&issn=0973-709x&id=8454 (accessed on 28 February 2021). [CrossRef]
- Fantone, S.; Mazzucchelli, R.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D.; Tossetta, G. AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem. Cell Biol. 2020, 154, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular Trends in the Rates of Preeclampsia, Eclampsia, and Gestational Hypertension, United States, 1987-2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Jeyabalan, A. Epidemiology of preeclampsia: Impact of obesity. Nutr. Rev. 2013, 71, S18–S25. [Google Scholar] [CrossRef]
- Al Fattah, A.N.; Putri, A.K.; Prakoso, R.; Irwinda, R.; Wibowo, N.; Santoso, B.I. Delivery Outcome Among Pregnant Women with Hypertension. J. Hypertens. 2015, 33, e13. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Bahado-Singh, R.O.; Syngelaki, A.; Mandal, R.; Graham, S.F.; Akolekar, R.; Han, B.; Bjondahl, T.C.; Dong, E.; Bauer, S.; Alpay-Savasan, Z.; et al. Metabolomic determination of pathogenesis of late-onset preeclampsia. J. Matern. Neonatal Med. 2016, 30, 658–664. [Google Scholar] [CrossRef]
- Wibowo, N.; Bardosono, S.; Irwinda, R.; Syafitri, I.; Putri, A.S.; Prameswari, N. Assesment of the nutrient intake an micronutrient status in the first trimester of pregnant women in Jakarta. Med. J. Indones. 2017, 26, 109–115. [Google Scholar] [CrossRef]
- Xu, H.; Shatenstein, B.; Luo, Z.C.; Wei, S.; Fraser, W.N. Role of nutrition in the risk of preeclampsia. Nutr. Rev. 2009, 67, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Evans, R.W.; Powers, R.W.; Ness, R.B.; Cropcho, L.J.; Daftary, A.R.; Harger, G.F.; Vergona, R.; Finegold, D.N. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction 2003, 125, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Deng, W.; Cui, W.; Xie, Q.; Zhao, G.; Wu, X.; Dai, L.; Chen, D.; Yu, B. Analysis of amino acid and acyl carnitine profiles in maternal and fetal serum from preeclampsia patients. J. Matern. Neonatal Med. 2019, 33, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Practice Bulletin. Gestational Hypertension and Preeclampsia. Available online: https://pubmed.ncbi.nlm.nih.gov/32443079/ (accessed on 24 February 2022).
- Elango, R.; Ball, R. Protein and Amino Acid Requirements during Pregnancy. Adv. Nutr. Int. Rev. J. 2016, 7, 839S–844S. [Google Scholar] [CrossRef]
- Ermamilia, A.; Yonika, L.; Aulia, B.; Ganap, E.P. High Prepregnancy Body Mass Index and Excessive Gestational Weight Gain as Obesity-Related Risk Factors of Preeclampsia. Top. Clin. Nutr. 2020, 35, 299–308. [Google Scholar] [CrossRef]
- Amaral, L.M.; Pinheiro, L.C.; Guimaraes, D.A.; Palei, A.C.T.; Sertorio, J.T.; Portella, R.L.; Tanus-Santos, J.E. Anthypertensive effects of inducivle nitric oxide synthase inhibition in experimental pre-eclampsia. J. Cell. Mol. Med. 2013, 17, 1300–1307. [Google Scholar]
- Troen, A.M.; Lutgens, E.; Smith, D.E.; Rosenberg, I.H.; Selhub, J. The atherogenic effect of excess methionine intake. Proc. Natl. Acad. Sci. USA 2003, 100, 15089–15094. [Google Scholar] [CrossRef]
- Aye, I.L.M.H.; Aiken, C.E.; Charnock-Jones, D.S.; Smith, G.C.S. Placental energy metabolism in health and disease—Significance of development and implications for preeclampsia, American. J. Obstet. Gynecol. 2020, 226 (Suppl. 2), S928–S944. [Google Scholar] [CrossRef]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2008, 23, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Li, L.; Peng, X.; Wu, B. Effects of Methionine on the Immune Function in Animals. Health 2017, 09, 857–869. [Google Scholar] [CrossRef][Green Version]
- Kantorow, M.; Hawse, J.R.; Cowell, T.L.; Benhamed, S.; Pizarro, G.O.; Reddy, V.N.; Hejtmancik, J.F. Methionine sulfoxide reductase A is important for lens cell viability and resistance to oxidative stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9654–9659. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, P.G.; Kannan, R.; Yaung, J.; Spee, C.K.; Ryan, S.J.; Hinton, D.R. Protection from oxidative stress by methionine sulfoxide reductases in RPE cells. Biochem. Biophys. Res. Commun. 2005, 334, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Zaghari, M.; Lotfollahian, H.; Shivazad, M.; Moravaj, H. Reevaluation of Methionine Requirement Based on Performance and Immune Responses in Broiler Breeder Hens. J. Poult. Sci. 2012, 49, 26–33. [Google Scholar] [CrossRef]
- Romano, A.; Serviddio, G.; Calcagnini, S.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Linking lipid peroxidation and neuropsychiatric disorders: Focus on 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2017, 111, 281–293. [Google Scholar] [CrossRef]
- Salimi, S.; Saravani, M.; Yaghmaei, M.; Fazlali, Z.; Mokhtari, M.; Naghavi, A.; Mashhadi, F.F. The early-onset preeclampsia is associated with MTHFR and FVL polymorphisms. Arch. Gynecol. Obstet. 2014, 291, 1303–1312. [Google Scholar] [CrossRef]
- Mazloomi, S.; Alimohammadi, S.; Khodadadi, I.; Ghiasvand, T.; Shafiee, G. Evaluation of methylenetetrahydrofolate reductase (MTHFR) activity and the levels of homocysteine and malondialdehyde (MDA) in the serum of women with preeclampsia. Clin. Exp. Hypertens. 2020, 42, 590–594. [Google Scholar] [CrossRef]
- Lee, S.-G.; Yim, Y.S.; Lee, Y.-H.; Lee, B.-W.; Kim, H.-S.; Kim, K.-S.; Kim, J.-H. Fasting serum amino acids concentration is associated with insulin resistance and pro-inflammatory cytokines. Diabetes Res. Clin. Pract. 2018, 140, 107–117. [Google Scholar] [CrossRef]
| Variables | Control (n = 30) | Preeclampsia (n = 34) | p |
|---|---|---|---|
| Maternal age (years) | 26.9 ± 5.9 | 31.7 ± 7.3 * | 0.01 |
| Education level (n/%) | |||
| Low education level | 23 (76.7%) | 31 (91.2%) | 0.111 |
| High education | 7 (23.3%) | 3 (8.8%) | |
| Gestational age at delivery (weeks) | 38.7 (1.03) | 35.56 (3.4) | 0.06 |
| Parity (n/%) | |||
| Nuliparity | 19 (70.4%) | 8 (29.6%) | 0.001 |
| Multiparity | 11 (29.7%) | 26 (70.3%) | |
| Body mass index (n/%) | |||
| Underweight | 4 (13.3%) | 2 (5.9%) | |
| Normal | 11 (36.7) | 7 (20.6) | 0.053 |
| Overweight and obese | 15 (50%) | 25 (73.5%) | |
| Mode of delivery | |||
| Vaginal delivery | 22 (73.3%) | 9 (26.5%) | <0.005 |
| Caesarean Section | 8 (26.7%) | 25 (73.5%) | |
| Fetal Weight (grams) | 3091.67 ± 395.4 | 2386.91 ± 828.1 | <0.005 |
| Daily Maternal Intake | Control | Preeclampsia | p |
|---|---|---|---|
| Energy (kcal) | 1809.367 ± 707.19 | 1792.207 ± 759.59 | 0.869 * |
| Carbohydrate (g) | 244.711 ± 80.81 | 219.100 ± 112.28 | 0.167 * |
| Protein (g) | 60.867 ± 30.81 | 130.539 ± 334.37 | 0.148 * |
| Fat (g) | 70.093 ± 37.17 | 76.582 ± 31.12 | 0.162 * |
| Variables | Control (N = 30) | Preeclampsia (N = 34) | p |
|---|---|---|---|
| Essential Amino Acids | |||
| Arginine | 159.33 (44.5) | 172.70 (39.9) | 0.885 |
| Histidine | 66.6 (18.8) | 74.02 (18.7) | 0.330 |
| Isoleucine | 37.2 (12.7) | 40.4 (14.6) | 0.651 |
| Leucine | 84.03 (30.9) | 91.05 (34.8) | 0.726 |
| Lycine | 139.03 (62–307) | 150.44 (71–336) | 0.258 |
| Methionine | 16.3 (5.8) | 12.9 (5.7) | 0.022 * |
| Phenylalanine | 71.5 (20.5) | 85.5 (26.8) | 0.023 * |
| Threonine | 166.1 (55.66) | 193.588 (72.23) | 0.096 |
| Valine | 111.56 (38.1) | 111.8 (36.0) | 0.591 |
| Non-essential amino acids | |||
| Aspartate | 45.3 (15.50) | 47.5 (15.5) | 0.461 |
| Serine | 287.43 (85–398) | 331.55 (174–620) | 0.03 * |
| Glycine | 183.3 (59.2) | 234.35 (213.00) | 0.03 * |
| Cysteine | 4.36 (1.00–7.00) | 6.44 (1.00–45.00) | 0.788 |
| Alanine | 413.233 (196–822) | 417.64 (146.98) | 0.824 |
| Glutamate | 102.23 (29–183) | 160.70 (36–397) | 0.000 * |
| Proline | 114.1 (57.7) | 120.88 (56.1) | 0.483 |
| Tyrosine | 43.7 (12.1) | 44.7 (10.09) | 0.931 |
| Ornitine | 40.4 (15.15) | 47.1 (24.3) | 0.121 |
| Preeclampsia n (%) | Control n (%) | p Bivariate | OR (95%CI) | Adjusted OR (95%CI) * | |
|---|---|---|---|---|---|
| Glutamate | |||||
| 25 (73.5) | 10 (33.3) | 0.003 | 5.55 (1.89–16.28) | 5.89 (1.85–18.76) | |
| Low risk (<109) | 9 (26.5) | 20 (66.7) | 1.0 | 1.0 | |
| Glycine | |||||
| 21 (61.8) | 14 (46.7) | 0.33 | 31.84 (0.68–5.00) | ||
| Low risk (<187.5) | 13 (38.2) | 16 (53.3) | 1.0 | ||
| Methionine | |||||
| High risk (≤14.5) | 34 (67.6) | 11 (36.7) | 0.026 | 3.61 (1.28–10.1) | 3.75 (1.24–11.3) |
| Low risk (>14.5) | 11 (32.4) | 19 (63.3) | 1.0 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prameswari, N.; Irwinda, R.; Wibowo, N.; Saroyo, Y.B. Maternal Amino Acid Status in Severe Preeclampsia: A Cross-Sectional Study. Nutrients 2022, 14, 1019. https://doi.org/10.3390/nu14051019
Prameswari N, Irwinda R, Wibowo N, Saroyo YB. Maternal Amino Acid Status in Severe Preeclampsia: A Cross-Sectional Study. Nutrients. 2022; 14(5):1019. https://doi.org/10.3390/nu14051019
Chicago/Turabian StylePrameswari, Natasya, Rima Irwinda, Noroyono Wibowo, and Yudianto Budi Saroyo. 2022. "Maternal Amino Acid Status in Severe Preeclampsia: A Cross-Sectional Study" Nutrients 14, no. 5: 1019. https://doi.org/10.3390/nu14051019
APA StylePrameswari, N., Irwinda, R., Wibowo, N., & Saroyo, Y. B. (2022). Maternal Amino Acid Status in Severe Preeclampsia: A Cross-Sectional Study. Nutrients, 14(5), 1019. https://doi.org/10.3390/nu14051019







