Association of Infants Small for Gestational Age with Anemia under Five Years Old in Two Large Longitudinal Chinese Birth Cohorts
Abstract
1. Introduction
2. Methods
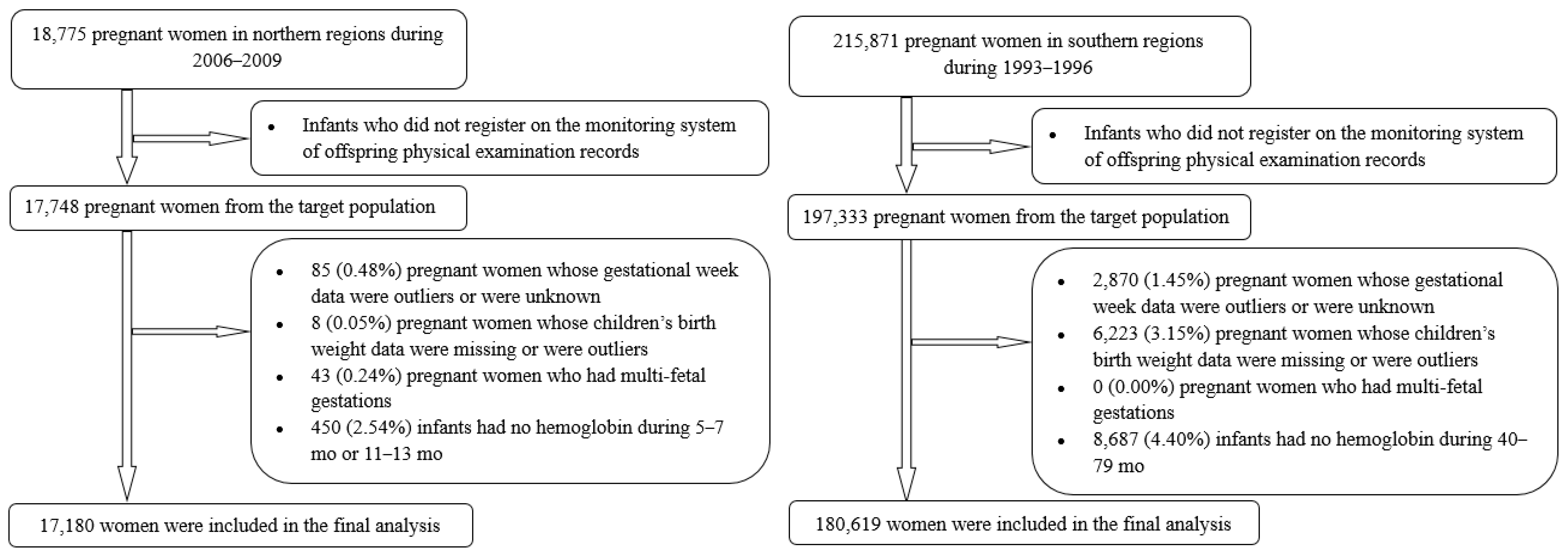
2.1. Background and Subjects for Current Study
2.2. Definition of SGA Status
2.3. Hemoglobin Measurement
2.4. Covariates’ Collection
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oudgenoeg-Paz, O.; Mulder, H.; Jongmans, M.J.; van der Ham, I.J.M.; Van der Stigchel, S. The link between motor and cognitive development in children born preterm and/or with low birth weight: A review of current evidence. Neurosci. Biobehav. Rev. 2017, 80, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Savvidou, M.D.; Kanu, C.; Evangelou, E.; Tzoulaki, I. Birth weight in relation to health and disease in later life: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2016, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Z.; Ye, R.; Liu, J.; Ren, A. Impact of Periconceptional Folic Acid Supplementation on Low Birth Weight and Small-for-Gestational-Age Infants in China: A Large Prospective Cohort Study. J Pediatr. 2017, 187, 105–110. [Google Scholar] [CrossRef]
- Zheng, J.S.; Guan, Y.; Zhao, Y.; Zhao, W.; Tang, X.; Chen, H.; Xu, M.; Wu, L.; Zhu, S.; Liu, H.; et al. Pre-conceptional intake of folic acid supplements is inversely associated with risk of preterm birth and small-for-gestational-age birth: A prospective cohort study. Br. J. Nutr. 2016, 115, 509–516. [Google Scholar] [CrossRef][Green Version]
- Figueiredo, A.; Gomes-Filho, I.S.; Silva, R.B.; Pereira, P.P.S.; Mata, F.; Lyrio, A.O.; Souza, E.S.; Cruz, S.S.; Pereira, M.G. Maternal Anemia and Low Birth Weight: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Delpishe, A.; Azami, M.; Hafezi Ahmadi, M.R.; Sayehmiri, K. Maternal Anemia during pregnancy and infant low birth weight: A systematic review and Meta-analysis. Int. J. Reprod. Biomed. 2017, 15, 125–134. [Google Scholar] [CrossRef]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Pena-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef]
- Dallman, P.R.; Siimes, M.A.; Stekel, A. Iron deficiency in infancy and childhood. Am. J. Clin. Nutr. 1980, 33, 86–118. [Google Scholar] [CrossRef] [PubMed]
- De Pee, S.; Bloem, M.W.; Sari, M.; Kiess, L.; Yip, R.; Kosen, S. The high prevalence of low hemoglobin concentration among Indonesian infants aged 3-5 months is related to maternal anemia. J. Nutr. 2002, 132, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.Q.; Chen, B.W.; Yin, D.L.; Xiao, F.; Li, R.L.; Yin, T.; Yang, H.M.; Zheng, X.G.; Wang, L.H. Prevalence of Anemia and its Risk Factors among Children under 36 Months Old in China. J. Trop. Pediatrics 2017, 63, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, J.; Yang, W. Association of Iron-Deficiency Anemia and Non-Iron-Deficiency Anemia with Neurobehavioral Development in Children Aged 6-24 Months. Nutrients 2021, 13, 3423. [Google Scholar] [CrossRef] [PubMed]
- Fuglestad, A.J.; Georgieff, M.K.; Iverson, S.L.; Miller, B.S.; Petryk, A.; Johnson, D.E.; Kroupina, M.G. Iron deficiency after arrival is associated with general cognitive and behavioral impairment in post-institutionalized children adopted from Eastern Europe. Matern. Child Health J. 2013, 17, 1080–1087. [Google Scholar] [CrossRef]
- Allali, S.; Brousse, V.; Sacri, A.S.; Chalumeau, M.; de Montalembert, M. Anemia in children: Prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev. Hematol. 2017, 10, 1023–1028. [Google Scholar] [CrossRef]
- McCann, S.; Perapoch Amadó, M.; Moore, S.E. The Role of Iron in Brain Development: A Systematic Review. Nutrients 2020, 12, 2001. [Google Scholar] [CrossRef]
- Konstantyner, T.; Roma Oliveira, T.C.; de Aguiar Carrazedo Taddei, J.A. Risk Factors for Anemia among Brazilian Infants from the 2006 National Demographic Health Survey. Anemia 2012, 2012, 850681. [Google Scholar] [CrossRef]
- Chandran, V.; Kirby, R.S. An Analysis of Maternal, Social and Household Factors Associated with Childhood Anemia. Int. J. Environ. Res. Public Health 2021, 18, 3105. [Google Scholar] [CrossRef] [PubMed]
- Means, R.T. Iron Deficiency and Iron Deficiency Anemia: Implications and Impact in Pregnancy, Fetal Development, and Early Childhood Parameters. Nutrients 2020, 12, 447. [Google Scholar] [CrossRef]
- Liu, J.M.; Mei, Z.; Ye, R.; Serdula, M.K.; Ren, A.; Cogswell, M.E. Micronutrient supplementation and pregnancy outcomes: Double-blind randomized controlled trial in China. JAMA Intern. Med. 2013, 173, 276–282. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, R.; Zhang, S.; Shi, W.; Yan, W.; Wang, X.; Lyu, Q.; Liu, L.; Zhou, Q.; Qiu, Q.; et al. Chinese neonatal birth weight curve for different gestational age. Chin. J. Pediatrics 2015, 53, 97–103. [Google Scholar]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Gindler, J.; Liu, J.; Berry, R.; Li, Z.; Correa, A.; Wang, H.; Wang, Y. Growth of children whose mothers took folic acid during early pregnancy—Sino-US NTD Project. Paediatr. Perinat. Epidemiol. 2001, 15, A10. [Google Scholar] [CrossRef]
- Mi, J.; Lin, L.; Liu, Y.; Zhang, X.; Cao, L. A national sampling survey on birth weight in 1998 in China: Mean value and standard deviation. Zhonghua Yu Fang Yi Xue Za Zhi Chin. J. Prev. Med. 2002, 36, 154–157. [Google Scholar]
- WHO. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Available online: http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241548502/en/ (accessed on 18 February 2022).
- Li, H.; Ye, R.; Pei, L.; Ren, A.; Zheng, X.; Liu, J. Caesarean delivery, caesarean delivery on maternal request and childhood overweight: A Chinese birth cohort study of 181 380 children. Pediatric Obes. 2014, 9, 10–16. [Google Scholar] [CrossRef]
- Dewey, K.G.; Cohen, R.J.; Rivera, L.L.; Brown, K.H. Effects of age of introduction of complementary foods on iron status of breast-fed infants in Honduras. Am. J. Clin. Nutr. 1998, 67, 878–884. [Google Scholar] [CrossRef]
- Lartey, A.; Manu, A.; Brown, K.H.; Peerson, J.M.; Dewey, K.G. A randomized, community-based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. Am. J. Clin. Nutr. 1999, 70, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Emond, A.M.; Hawkins, N.; Pennock, C.; Golding, J. Haemoglobin and ferritin concentrations in infants at 8 months of age. Arch. Dis. Child. 1996, 74, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bo, Y.; Ren, H.; Zhou, C.; Lao, X.; Zhao, L.; Yu, D. Regional Differences in the Prevalence of Anaemia and Associated Risk Factors among Infants Aged 0–23 Months in China: China Nutrition and Health Surveillance. Nutrients 2021, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Uijterschout, L.; Vloemans, J.; Rövekamp-Abels, L.; Feitsma, H.; van Goudoever, J.B.; Brus, F. The influences of factors associated with decreased iron supply to the fetus during pregnancy on iron status in healthy children aged 0.5 to 3 years. J. Perinatol. 2014, 34, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Kaciroti, N.; Walter, T. Iron deficiency in infancy: Applying a physiologic framework for prediction. Am. J. Clin. Nutr. 2006, 84, 1412–1421. [Google Scholar] [CrossRef]
- Stoltzfus, R.J. Iron interventions for women and children in low-income countries. J. Nutr. 2011, 141, 756S–762S. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Miao, H.; Liang, Z.; Zhang, Y.; Jiang, W.; Deng, Z.; Tang, J.; Liu, G.; Luo, X. Prevalence of small for gestational age infants in 21 cities in China, 2014–2019. Sci. Rep. 2021, 11, 7500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, J.; Xia, H.; Ge, J.; Ye, X.; Guo, B.; Liu, M.; Dai, L.; Zhang, L.; Chen, L.; et al. Stillbirths in China: A nationwide survey. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 67–76. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cohort 1 | p | Cohort 2 | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SGA Group (N = 1214) | Non-SGA Group (N = 15,966) | SGA Group (N = 10,353) | Non-SGA Group (N = 170,266) | |||||||
| n | % | n | % | n | % | n | % | |||
| Mother | ||||||||||
| Age (years, mean [SD]) | 22.96 (2.56) | 23.41 (2.84) | <0.001 | 24.50 (3.54) | 24.92 (3.65) | <0.001 | ||||
| Body mass index (kg/m2, mean [SD]) | 21.53 (2.69) | 22.35 (2.86) | <0.001 | 20.11 (2.00) | 20.49 (2.02) | <0.001 | ||||
| Han ethnic group | 1201 | 98.93 | 15,774 | 98.80 | 0.684 | 10,253 | 99.24 | 169,012 | 99.42 | 0.020 |
| Education | 0.001 | <0.001 | ||||||||
| High school or higher | 205 | 16.89 | 2903 | 18.18 | 875 | 8.45 | 18,193 | 10.69 | ||
| Junior high school | 974 | 80.23 | 12,823 | 80.31 | 6041 | 58.35 | 101,454 | 59.59 | ||
| Primary school or lower, or unknown | 35 | 2.88 | 240 | 1.50 | 3437 | 33.20 | 50,619 | 29.73 | ||
| Farmer occupation | 1112 | 91.60 | 14,517 | 90.92 | 0.430 | 6160 | 59.50 | 101,528 | 59.63 | 0.794 |
| Anemia during pregnancy | 89 | 7.33 | 986 | 6.18 | 0.094 | 6582 | 63.58 | 109,970 | 64.59 | 0.113 |
| Exclusive breastfeeding | 1141 | 94.00 | 15,277 | 95.68 | 0.006 | 8820 | 85.19 | 148,475 | 87.20 | <0.001 |
| Child | ||||||||||
| Age at follow-up visit, months | 6.25 (0.49) a | 6.26 (0.44) a | 0.785 a | 55.72 (8.31) | 55.46 (8.19) | 0.002 | ||||
| 12.25 (0.47) b | 12.27 (0.43) b | 0.124 b | ||||||||
| Children with Anemia | ||||||
|---|---|---|---|---|---|---|
| SGA Group | Non-SGA Group | |||||
| Mean Age at Follow-Up | n | % | n | % | Crude RR (95% CI) | Adjusted RR (95% CI) a |
| Cohort 1 | ||||||
| 6 months | 119 | 8.87 | 1048 | 6.64 | 1.55 (1.27, 1.89) | 1.52 (1.24, 1.86) |
| 12 months | 89 | 7.51 | 817 | 5.12 | 1.47 (1.17, 1.84) | 1.42 (1.13, 1.79) |
| Cohort 2 | ||||||
| 55 months | 1481 | 14.31 | 22,377 | 13.14 | 1.10 (1.04, 1.17) | 1.11 (1.05, 1.17) |
| SGA Group | Non-SGA Group | |||
|---|---|---|---|---|
| Mean Age at Follow-Up | Mean ± SD | Mean ± SD | Crude Mean Difference (95% CI) | Adjusted Mean Difference (95% CI) a |
| Cohort 1 | ||||
| 6 months | 120.12 ± 9.13 | 121.84 ± 8.62 | −1.72 (−2.23, −1.22) | −1.61 (−2.11, −1.11) |
| 12 months | 121.12 ± 8.53 | 122.14 ± 8.15 | −1.02 (−1.50, −0.54) | −0.92 (−1.40, −0.45) |
| Cohort 2 | ||||
| 55 months | 119.31 ± 10.31 | 119.52 ± 10.18 | −0.21 (−0.41, −0.01) | −0.26 (−0.46, −0.06) |
| Anemic Group | Non-Anemic Group | |||
|---|---|---|---|---|
| Mean Age at Follow-Up | Mean ± SD | Mean ± SD | Crude Mean Difference (95% CI) | Adjusted Mean Difference (95% CI) a |
| Cohort 1 | ||||
| 6 months | 3251.80 ± 441.10 | 3301.06 ± 380.68 | −0.008 (−0.012, −0.004) | −0.009 (−0.013, −0.005) |
| 12 months | 3257.92 ± 416.90 | 3299.93 ± 383.32 | −0.005 (−0.009, −0.002) | −0.006 (−0.009, −0.002) |
| Cohort 2 | ||||
| 55 months | 3291.08 ± 418.38 | 3303.00 ± 420.05 | −0.003 (−0.005, −0.002) | −0.004 (−0.006, −0.003) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; An, H.; Jin, M.; Li, Z.; Zhang, Y.; Zhang, L.; Liu, J.; Ye, R. Association of Infants Small for Gestational Age with Anemia under Five Years Old in Two Large Longitudinal Chinese Birth Cohorts. Nutrients 2022, 14, 1006. https://doi.org/10.3390/nu14051006
Li N, An H, Jin M, Li Z, Zhang Y, Zhang L, Liu J, Ye R. Association of Infants Small for Gestational Age with Anemia under Five Years Old in Two Large Longitudinal Chinese Birth Cohorts. Nutrients. 2022; 14(5):1006. https://doi.org/10.3390/nu14051006
Chicago/Turabian StyleLi, Nan, Hang An, Ming Jin, Zhiwen Li, Yali Zhang, Le Zhang, Jianmeng Liu, and Rongwei Ye. 2022. "Association of Infants Small for Gestational Age with Anemia under Five Years Old in Two Large Longitudinal Chinese Birth Cohorts" Nutrients 14, no. 5: 1006. https://doi.org/10.3390/nu14051006
APA StyleLi, N., An, H., Jin, M., Li, Z., Zhang, Y., Zhang, L., Liu, J., & Ye, R. (2022). Association of Infants Small for Gestational Age with Anemia under Five Years Old in Two Large Longitudinal Chinese Birth Cohorts. Nutrients, 14(5), 1006. https://doi.org/10.3390/nu14051006






