Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Anthropometric Measurements
2.3. Dietary Intervention
2.4. Ethical Considerations
2.5. Determination of Biochemical Variables in Peripheral Blood
2.6. Bacterial DNA Extraction from Stool Samples
2.7. V3 16S rDNA Libraries Preparation
2.8. Massive Semiconductor Sequencing
2.9. Analysis of Sequenced Data for Microbial Diversity
2.10. Determination of Microbiota Relative Abundance, Diversity, and Significant Enrichment
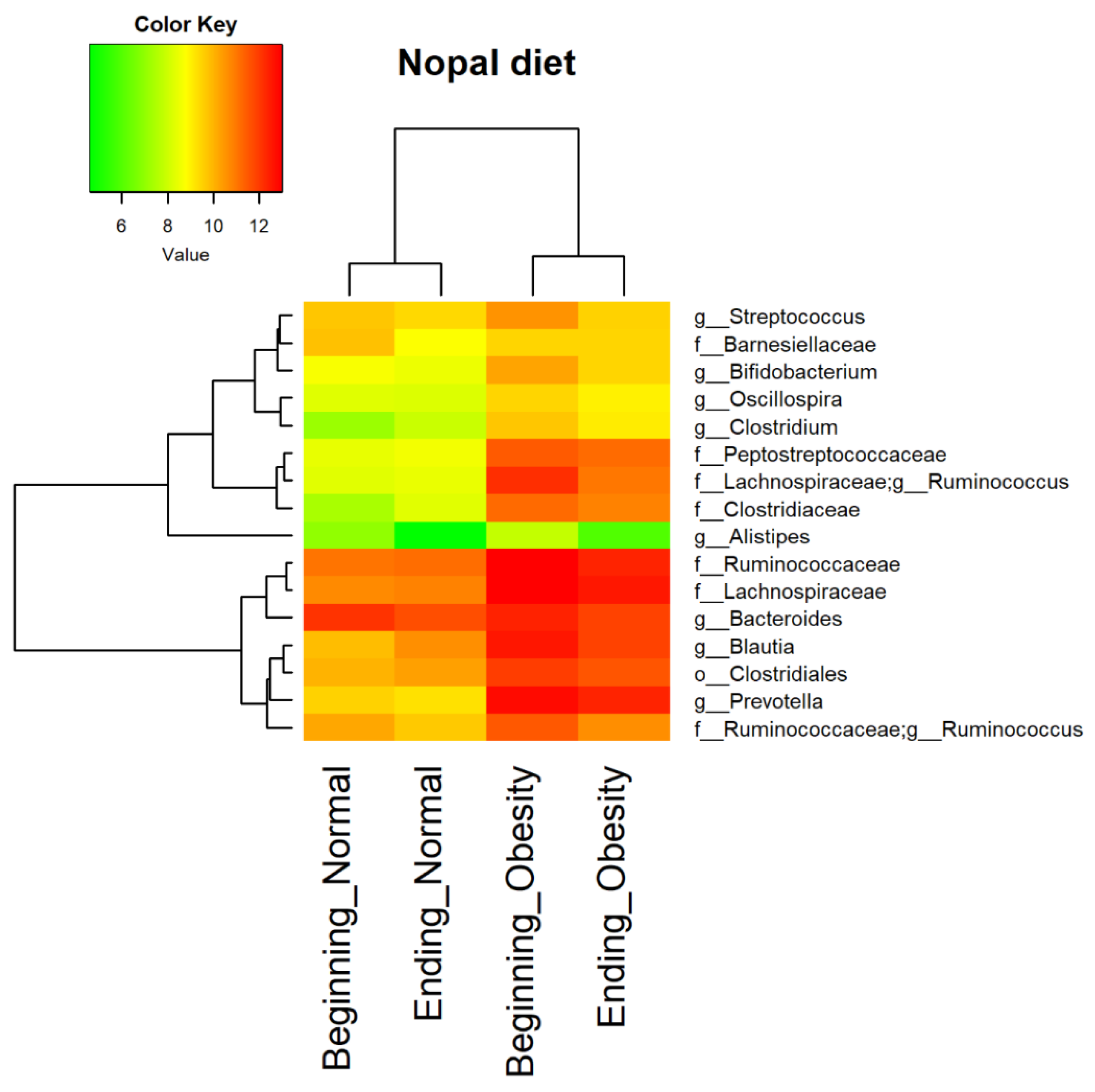
2.11. Core Microbiota Determination and Heat Map
2.12. Multivariate Association Analysis
2.13. Predicted Metabolic Pathways of the Gut Microbiota
2.14. Spearman Correlation Analysis
2.15. Statistical Analyses
2.16. Sequence Accession Numbers
3. Results
3.1. Anthropometric and Biochemical Parameters Evaluated in the Obesity and Normal Weight Groups
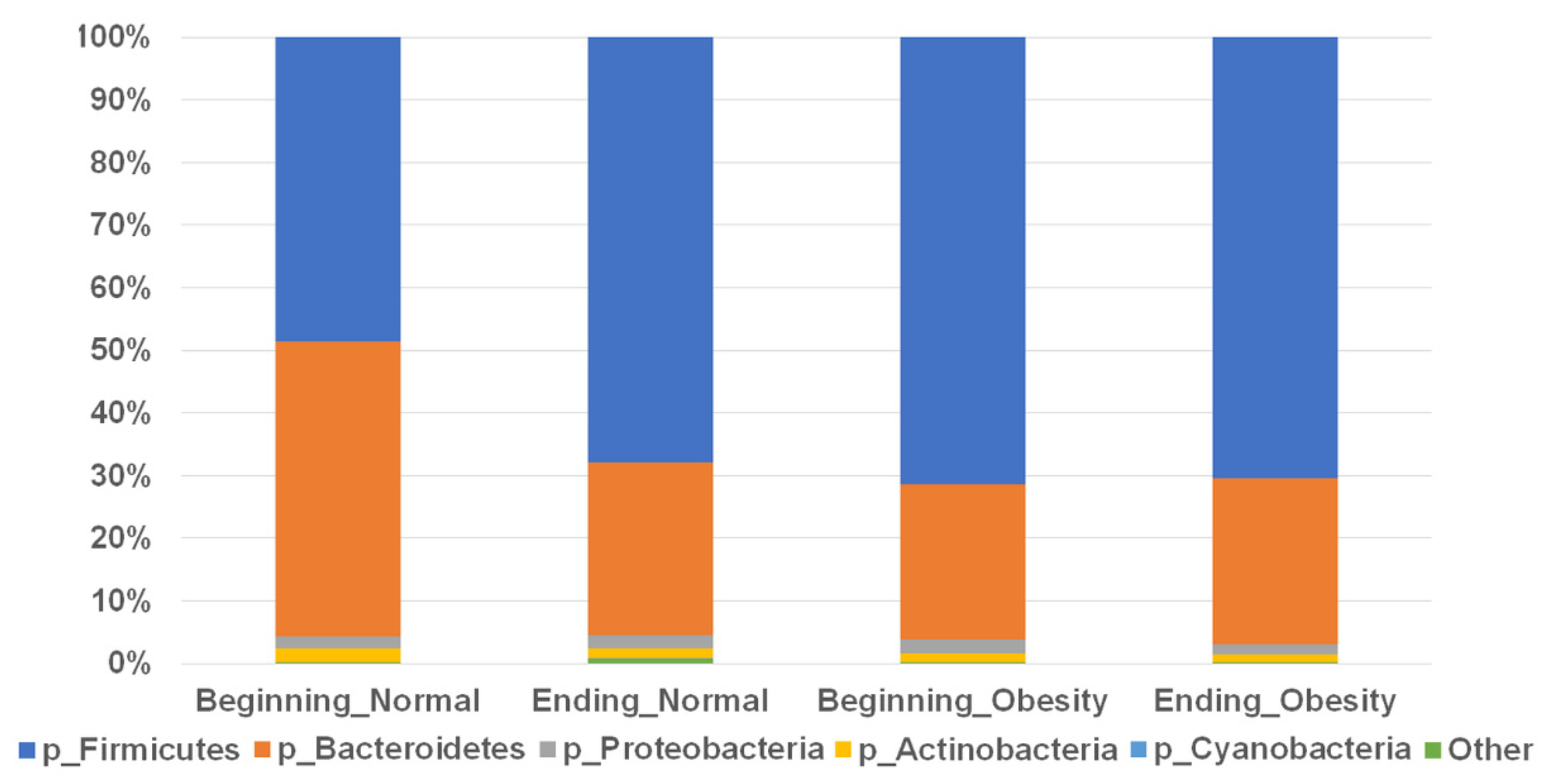
3.2. Composition of the Faecal Microbiota Is Not Associated with Nopal Diet Intervention
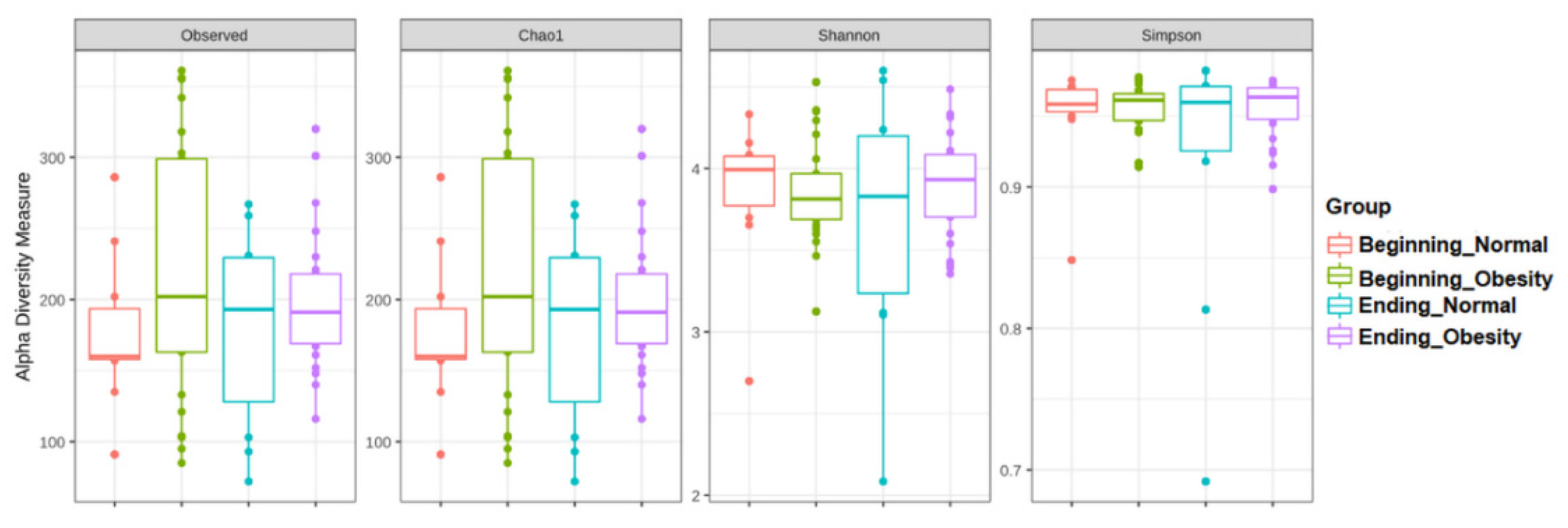
3.3. The Alpha and Beta Diversities of Faecal Microbiota Were Not Affected by the Intervention with Nopal
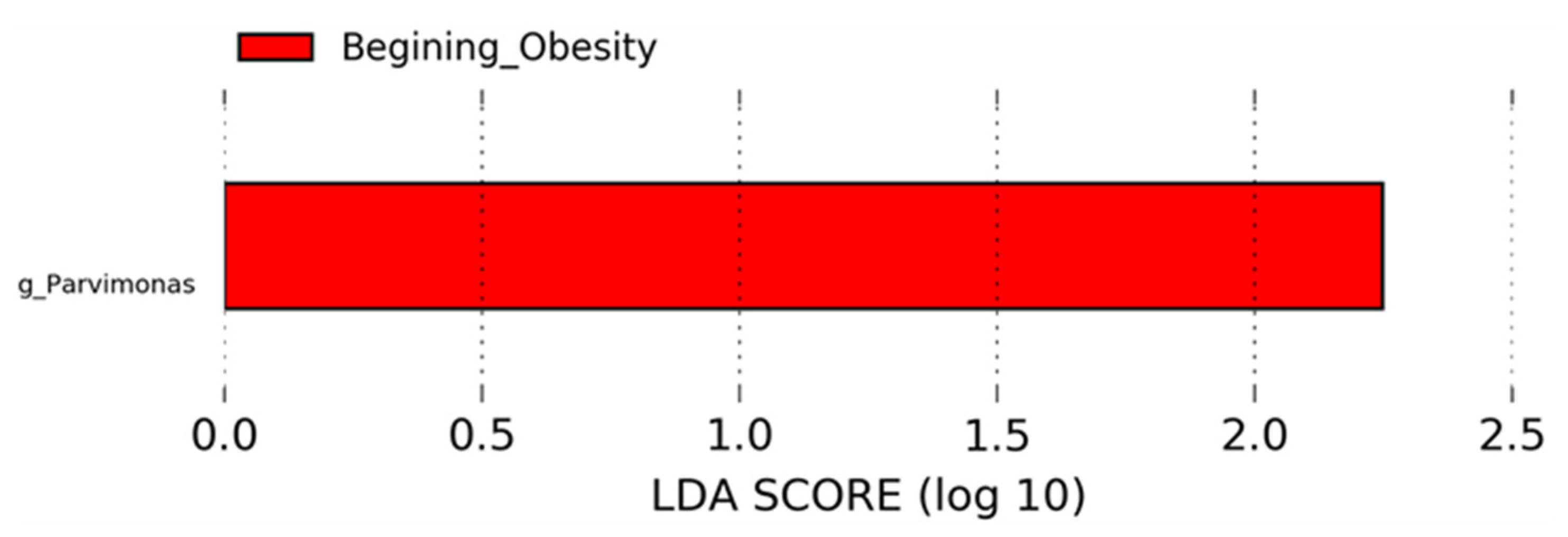
3.4. Nopal and Caloric Restriction Diet Intervention Lead to an Increase of Specific Bacteria in Faecal Samples
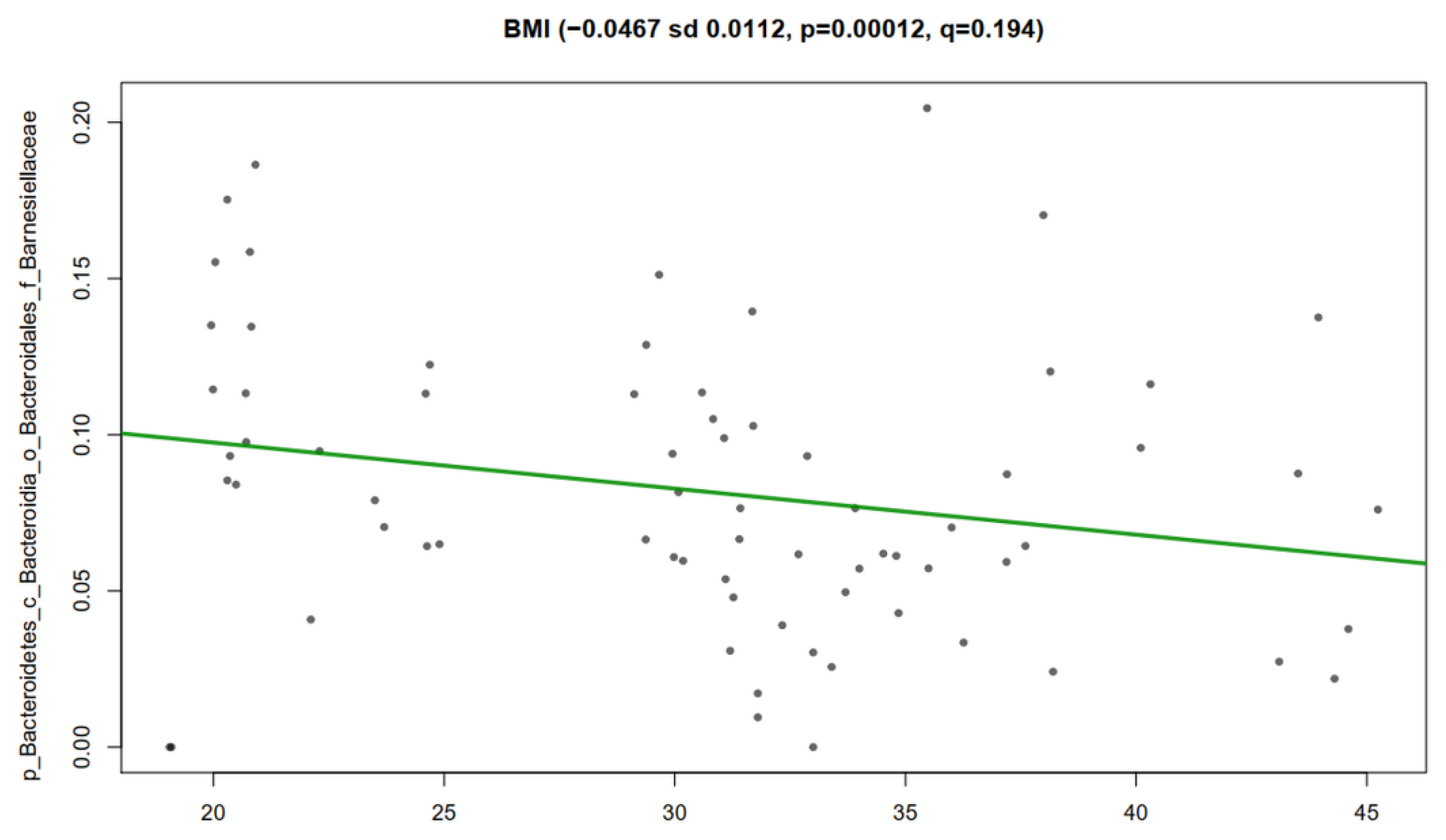
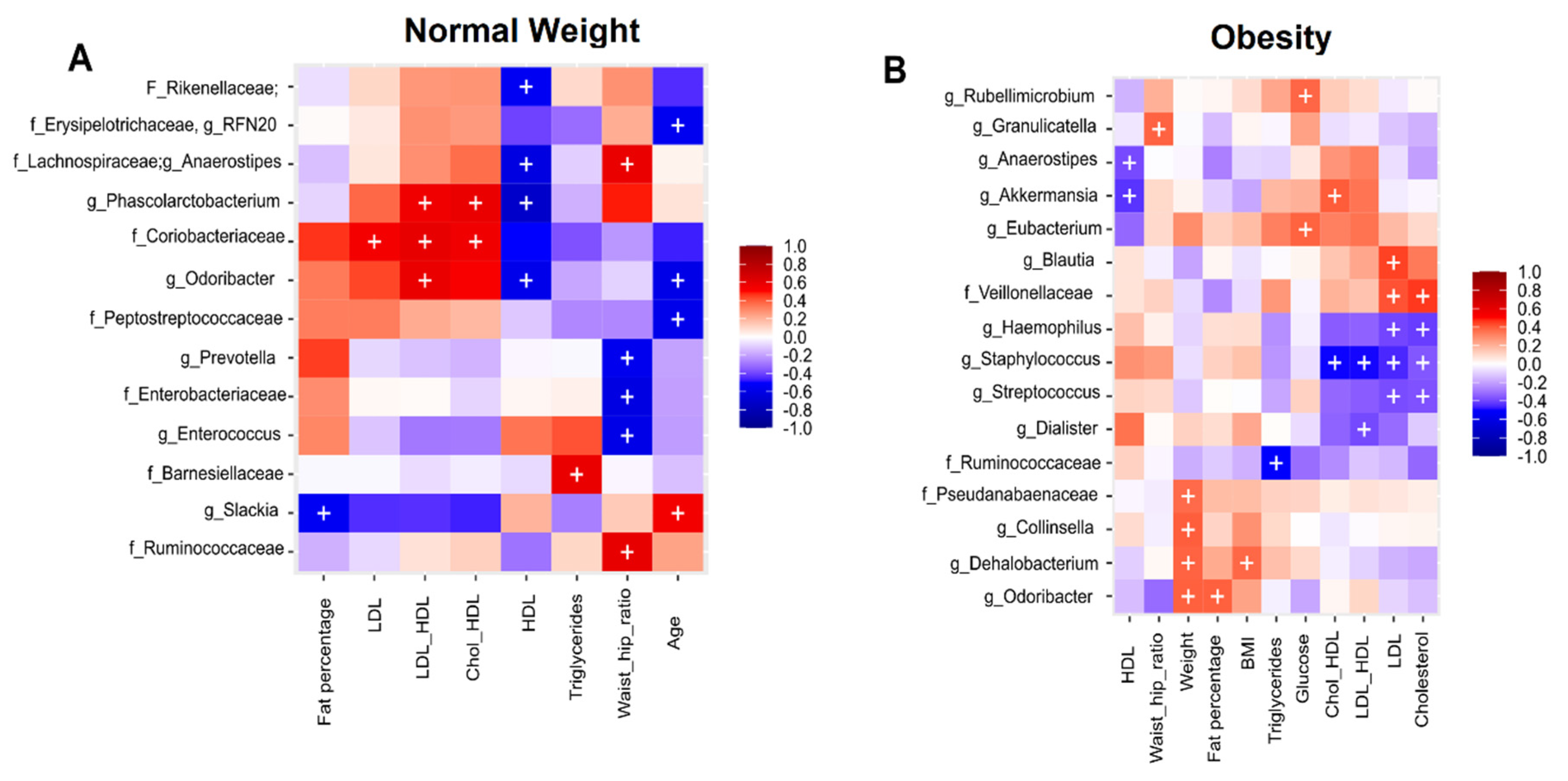
3.5. Association Analysis of Clinical Metadata with Faecal Bacteria during Dietary Intervention with Nopal
3.6. Correlation Analysis of Clinical Metadata with Faecal Bacteria during Dietary Intervention with Nopal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 February 2022).
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut Microbiota: A New Path to Treat Obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. A Review on Gut Microbiota: A Central Factor in the Pathophysiology of Obesity. Lipids Health Dis. 2021, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- Kaur, M. Pharmacological Actions of Opuntia ficus indica: A Review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus Stem (Opuntia ficus-indica Mill): Anatomy, Physiology and Chemical Composition with Emphasis on Its Biofunctional Properties. J. Sci. Food Agric. 2017, 97, 5065–5073. [Google Scholar] [CrossRef]
- El-Mostafa, K.; el Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; el Kebbaj, M.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [Green Version]
- Trombetta, D.; Puglia, C.; Perri, D.; Licata, A.; Pergolizzi, S.; Lauriano, E.R.; de Pasquale, A.; Saija, A.; Bonina, F.P. Effect of Polysaccharides from Opuntia ficus-indica (L.) Cladodes on the Healing of Dermal Wounds in the Rat. Phytomedicine 2006, 13, 352–358. [Google Scholar] [CrossRef]
- Sánchez-Tapia, M.; Aguilar-López, M.; Pérez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, A.R.; Torres, N. Nopal (Opuntia ficus indica) Protects from Metabolic Endotoxemia by Modifying Gut Microbiota in Obese Rats Fed High Fat/Sucrose Diet. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the Diversity and Short-Chain Fatty Acids Production by the Bacterial Community in Overweight and Obese Mexican Children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef]
- García-Mena, J.; Murugesan, S.; Pérez-Muñoz, A.A.; García-Espitia, M.; Maya, O.; Jacinto-Montiel, M.; Monsalvo-Ponce, G.; Piña-Escobedo, A.; Domínguez-Malfavón, L.; Gómez-Ramírez, M.; et al. Airborne Bacterial Diversity from the Low Atmosphere of Greater Mexico City. Microb. Ecol. 2016, 72, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Lahti, L.; Shetty, S. Tools for microbiome analysis in R. Version. 2017. Available online: http://microbiome.github.com/microbiome (accessed on 25 December 2021).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the Gut Microbial Community between Obese and Lean Peoples Using 16S Gene Sequencing in a Japanese Population. J. Clin. Biochem. Nutr 2016, 59, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, F.C.; Carle, R. Cactus Stems (Opuntia Spp.): A Review on Their Chemistry, Technology, and Uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, X.; Luo, T.; Wang, D.; Sun, Y.; Dai, J. Effects of Short-Term Dietary Fiber Intervention on Gut Microbiota in Young Healthy People. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3507–3516. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; de Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, J.; Yamamoto, A.; Palermo-Conde, L.A.; Higashi, K.; Sonomoto, K.; Tan, J.; Lee, Y.-K. Impact of Westernized Diet on Gut Microbiota in Children on Leyte Island. Front. Microbiol. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Stegen, J.C.; Maldonado-Gómez, M.X.; Eren, A.M.; Siba, P.M.; Greenhill, A.R.; Walter, J. The Gut Microbiota of Rural Papua New Guineans: Composition, Diversity Patterns, and Ecological Processes. Cell Rep. 2015, 11, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Precup, G.; Vodnar, D.-C. Gut Prevotella as a Possible Biomarker of Diet and Its Eubiotic versus Dysbiotic Roles: A Comprehensive Literature Review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef]
- Dubois, G.; Girard, C.; Lapointe, F.-J.; Shapiro, B.J. The Inuit Gut Microbiome Is Dynamic over Time and Shaped by Traditional Foods. Microbiome 2017, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of Diet and Individual Variation on Intestinal Microbiota Composition and Fermentation Products in Obese Men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.-P.; et al. Insight into the Prebiotic Concept: Lessons from an Exploratory, Double Blind Intervention Study with Inulin-Type Fructans in Obese Women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [Green Version]
- López-Romero, P.; Pichardo-Ontiveros, E.; Avila-Nava, A.; Vázquez-Manjarrez, N.; Tovar, A.R.; Pedraza-Chaverri, J.; Torres, N. The Effect of Nopal (Opuntia ficus indica) on Postprandial Blood Glucose, Incretins, and Antioxidant Activity in Mexican Patients with Type 2 Diabetes after Consumption of Two Different Composition Breakfasts. J. Acad. Nutr. Diet. 2014, 114, 1811–1818. [Google Scholar] [CrossRef]
- Wolfram, R.M.; Kritz, H.; Efthimiou, Y.; Stomatopoulos, J.; Sinzinger, H. Effect of Prickly Pear (Opuntia robusta) on Glucose- and Lipid-Metabolism in Non-Diabetics with Hyperlipidemia—A Pilot Study. Wien. Klin. Wochenschr. 2002, 114, 840–846. [Google Scholar] [PubMed]
- Vrieze, A.; Holleman, F.; Zoetendal, E.G.; de Vos, W.M.; Hoekstra, J.B.L.; Nieuwdorp, M. The Environment within: How Gut Microbiota May Influence Metabolism and Body Composition. Diabetologia 2010, 53, 606–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delzenne, N.M.; Neyrinck, A.M.; Bäckhed, F.; Cani, P.D. Targeting Gut Microbiota in Obesity: Effects of Prebiotics and Probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Udayappan, S.; Manneras-Holm, L.; Chaplin-Scott, A.; Belzer, C.; Herrema, H.; Dallinga-Thie, G.M.; Duncan, S.H.; Stroes, E.S.G.; Groen, A.K.; Flint, H.J.; et al. Oral Treatment with Eubacterium Hallii Improves Insulin Sensitivity in Db/Db Mice. NPJ Biofilms Microbiomes 2016, 2, 16009. [Google Scholar] [CrossRef] [Green Version]
- Cho, G.-S.; Ritzmann, F.; Eckstein, M.; Huch, M.; Briviba, K.; Behsnilian, D.; Neve, H.; Franz, C.M.A.P. Quantification of Slackia and Eggerthella Spp. in Human Feces and Adhesion of Representatives Strains to Caco-2 Cells. Front. Microbiol. 2016, 7, 658. [Google Scholar] [CrossRef] [Green Version]
- Del Chierico, F.; Abbatini, F.; Russo, A.; Quagliariello, A.; Reddel, S.; Capoccia, D.; Caccamo, R.; Ginanni Corradini, S.; Nobili, V.; de Peppo, F.; et al. Gut Microbiota Markers in Obese Adolescent and Adult Patients: Age-Dependent Differential Patterns. Front. Microbiol. 2018, 9, 1210. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef] [Green Version]
- Maffei, V.J.; Kim, S.; Blanchard, E.; Luo, M.; Jazwinski, S.M.; Taylor, C.M.; Welsh, D.A. Biological Aging and the Human Gut Microbiota. J. Gerontol. Ser. A 2017, 72, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Wang, D.; Ren, H.; Cai, K.; Chen, P.; Fang, C.; Shi, Z.; Zhang, P.; Wang, J.; Yang, H.; et al. Effect of Caloric Restriction on BMI, Gut Microbiota, and Blood Amino Acid Levels in Non-Obese Adults. Nutrients 2020, 12, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Normal Weight Group | Obesity Group | |||||
|---|---|---|---|---|---|---|
| Variable | Beginning n = 11 | End n =11 | p | Beginning n = 25 | End n = 25 | p |
| Age | 22.1 ± 2.6 21 (18–27) | 22.1 ± 2.6 21 (18–27) | ND | 40.6 ± 10.7 42 (22–59) | 40.6 ± 10.7 42 (22–59) | ND |
| Weight | 54.3 ± 6.7 53 (45–65) | 54.1 ± 6.7 53 (45–65) | 0.79 b | 84.9 ± 13.6 78 (65–111) | 83.1 ± 13.9 76 (64–110) | <0.001 a |
| BMI | 21.5 ± 1.9 20 (19–24) | 21.4 ± 1.9 20 (19–24) | 0.793 b | 35.1 ± 4.5 33 (30–44) | 34.1 ± 4.7 32 (29–45) | <0.001 a |
| Hip | 70.9 ± 6.1 72 (62–85) | 70.4 ± 6.1 71 (62–24) | 0.974 b | 99.7 ± 8.8 99 (82–118) | 99.1 ± 8.7 99 (82–118) | <0.001 b |
| Waist | 91.7 ± 8.6 91 (72–102) | 91.6 ± 8.4 91 (72–102) | 0.742 b | 114 ± 10 113 (100–139) | 114 ± 10 113 (100–138) | 0.380 b |
| Hip/Waist | 0.77 ± 0.05 0.7 (0.6–0.8) | 0.77 ± 0.06 0.7 (0.6–0.7) | ND | 0.87 ± 0.04 0.87 (0.8–0.9) | 0.86 ± 0.04 0.87 (0.8–0.9) | 0.008 a |
| % Fat | 26.4 ± 5.0 24 (20–36) | 26.1 ± 4.9 25 (20–36) | 0.887 a | 46.7 ± 5.3 47 (36–55) | 46.1 ± 6.7 46 (28–59) | 0.273 b |
| Glucose | 83.8 ± 4.5 85 (76–92) | 82.5 ± 4.8 82 (73–90) | 0.535 a | 112 ± 41 95 (82–248) | 98 ± 26 97 (81–150) | 0.030 b |
| Tryglicerides | 111 ± 24 170 (133–193) | 106 ± 25 169 (115–188) | 0.618 a | 172 ± 97 163 (50–493) | 138 ± 42 133 (75–203) | 0.069 b |
| Cholesterol | 167 ± 19 14 (9–25) | 159 ± 24 14 (9–25) | 0.390 a | 190 ± 34 192 (113–273) | 178 ± 23 181 (113–216) | 0.032 a |
| HDL-Chol | 60.5 ± 10.2 62 (40–78) | 61.8 ± 10.3 63 (43–79) | 0.775 a | 41.3 ± 9.9 40 (25–70) | 39.2 ± 9.0 37 (25–59) | 0.036 a |
| LDL-Chol | 84.9 ± 21.7 85 (44–117) | 76.4 ± 26.6 88 (39–118) | 0.420 a | 115 ± 30 120 (55–186) | 111 ± 20 114 (59–137) | 0.237 a |
| LDL/HDL | 1.47 ± 0.59 1.3 (0.5–2.9) | 1.24 ± 0.49 1.3 (0.4–2.4) | 0.338 a | 2.96 ± 1.13 2.7 (1.1–5.2) | 2.99 ± 0.86 2.8 (1.0–4.5) | 0.270 b |
| Chol/HDL | 2.85 ± 0.63 2.7 (1.8–4.4) | 2.64 ± 0.56 2.6 (1.7–3.4) | 0.415 a | 4.90 ± 1.55 4.6 (2.3–8.1) | 4.76 ± 1.14 4.7 (2.2–6.9) | 0.590 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corona-Cervantes, K.; Parra-Carriedo, A.; Hernández-Quiroz, F.; Martínez-Castro, N.; Vélez-Ixta, J.M.; Guajardo-López, D.; García-Mena, J.; Hernández-Guerrero, C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients 2022, 14, 1008. https://doi.org/10.3390/nu14051008
Corona-Cervantes K, Parra-Carriedo A, Hernández-Quiroz F, Martínez-Castro N, Vélez-Ixta JM, Guajardo-López D, García-Mena J, Hernández-Guerrero C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients. 2022; 14(5):1008. https://doi.org/10.3390/nu14051008
Chicago/Turabian StyleCorona-Cervantes, Karina, Alicia Parra-Carriedo, Fernando Hernández-Quiroz, Noemí Martínez-Castro, Juan Manuel Vélez-Ixta, Diana Guajardo-López, Jaime García-Mena, and César Hernández-Guerrero. 2022. "Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment" Nutrients 14, no. 5: 1008. https://doi.org/10.3390/nu14051008
APA StyleCorona-Cervantes, K., Parra-Carriedo, A., Hernández-Quiroz, F., Martínez-Castro, N., Vélez-Ixta, J. M., Guajardo-López, D., García-Mena, J., & Hernández-Guerrero, C. (2022). Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients, 14(5), 1008. https://doi.org/10.3390/nu14051008







