Impact of Ready-Meal Consumption during Pregnancy on Birth Outcomes: The Japan Environment and Children’s Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Variables
2.3. Outcomes
2.4. Exposures and Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, L.P.; Ng, S.W.; Popkin, B.M. Trends in US home food preparation and consumption: Analysis of national nutrition surveys and time use studies from 1965–1966 to 2007–2008. Nutr. J. 2013, 12, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachat, C.; Khanh, L.N.B.; Khan, N.C.; Dung, N.Q.; van Anh, N.D.; Roberfroid, D.; Kolsteren, P. Eating out of home in Vietnamese adolescents: Socioeconomic factors and dietary associations. Am. J. Clin. Nutr. 2009, 90, 1648–1655. [Google Scholar] [CrossRef]
- Alkerwi, A.; Crichton, G.E.; Hebert, J.R. Consumption of ready-made meals and increased risk of obesity: Findings from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study. Br. J. Nutr. 2015, 113, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Soriano, G.; de Barreto, P.S.; Rolland, Y.; Plessz, M.; Goisser, S.; Guyonnet, S.; Fougere, B.; Vellas, B.; Andrieu, S.; Sourdet, S. Ready-meal consumption in older people: Association with obesity and dietary intake. Aging Clin. Exp. Res. 2019, 31, 855–861. [Google Scholar] [CrossRef]
- Lee, S.; Min, J.Y.; Kim, H.J.; Min, K.B. Association Between the Frequency of Eating Non-home-prepared Meals and Women Infertility in the United States. J. Prev. Med. Public Health 2020, 53, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Miyake, Y.; Tanaka, K.; Sasaki, S.; Hirota, Y. Maternal total caffeine intake, mainly from Japanese and Chinese tea, during pregnancy was associated with risk of preterm birth: The Osaka Maternal and Child Health Study. Nutr. Res. 2015, 35, 309–316. [Google Scholar] [CrossRef]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Sonta, S.; Makino, T.; Suzumori, K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 2005, 20, 2325–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Sonta, S.; Makino, T.; Suzumori, K. PCBs, hexachlorobenzene and DDE are not associated with recurrent miscarriage. Am. J. Reprod. Immunol. 2003, 50, 485–489. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Nakayama, S.F.; Kishi, R.; Mori, C.; Yamagata, Z.; Ohya, Y.; Kawamoto, T.; Kamijima, M. Japan Environment and Children’s Study: Backgrounds, activities, and future directions in global perspectives. Environ. Health Prev. Med. 2017, 22, 61. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai-Shimada, M.; Nakayama, S.F.; Isobe, T.; Michikawa, T.; Yamazaki, S.; Nitta, H.; Takeuchi, A.; Kobayashi, Y.; Tamura, K.; Suda, E.; et al. Questionnaire results on exposure characteristics of pregnant women participating in the Japan Environment and Children Study (JECS). Environ. Health Prev. Med. 2018, 23, 45. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, M.A.; André, L.C.; Cardeal, Z.L. Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int. J. Environ. Res. Public Health 2013, 11, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewska, K.; Stragierowicz, J.; Gromadzinska, J. Bisphenol A—Application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health 2015, 28, 209–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.S.; Kwack, S.J.; Kim, K.B.; Kim, H.S.; Lee, B.M. Potential risk of bisphenol A migration from polycarbonate containers after heating, boiling, and microwaving. J. Toxicol. Environ. Health A 2009, 72, 1285–1291. [Google Scholar] [CrossRef]
- Kawamura, Y.; Etoh, M.; Hirakawa, Y.; Abe, Y.; Mutsuga, M. Bisphenol A in domestic and imported canned foods in Japan. Food Addit. Contam. Part A 2014, 31, 330–340. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Vom Saal, F.S.; Welshons, W.V. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ. Res. 2006, 100, 50–76. [Google Scholar] [CrossRef]
- Lathi, R.B.; Liebert, C.A.; Brookfield, K.F.; Taylor, J.A.; vom Saal, F.S.; Fujimoto, V.Y.; Baker, V.L. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 2014, 102, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Allard, P.; Colaiácovo, M.P. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA 2010, 107, 20405–20410. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.A.; Rovira, J.; Sharma, R.P.; Nadal, M.; Schuhmacher, M.; Kumar, V. Prenatal exposure estimation of BPA and DEHP using integrated external and internal dosimetry: A case study. Environ. Res. 2017, 158, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Radke, E.G.; Glenn, B.S.; Braun, J.M.; Cooper, G.S. Phthalate exposure and female reproductive and developmental outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2019, 130, 104580. [Google Scholar] [CrossRef] [PubMed]
- Dagher, J.B.; Hahn-Townsend, C.K.; Kaimal, A.; Mansi, M.A.; Henriquez, J.E.; Tran, D.G.; Laurent, C.R.; Bacak, C.J.; Buechter, H.E.; Cambric, C.; et al. Independent and combined effects of Bisphenol A and Diethylhexyl Phthalate on gestational outcomes and offspring development in Sprague-Dawley rats. Chemosphere 2021, 263, 128307. [Google Scholar] [CrossRef] [PubMed]
- Gelbke, H.P.; Banton, M.; Block, C.; Dawkins, G.; Eisert, R.; Leibold, E.; Pemberton, M.; Puijk, I.M.; Sakoda, A.; Yasukawa, A. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem. Toxicol. 2019, 124, 151–167. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.W.; Wu, Y.; Neelakantan, N.; Chong, M.F.; Pan, A.; van Dam, R.M. Maternal caffeine intake during pregnancy and risk of pregnancy loss: A categorical and dose-response meta-analysis of prospective studies. Public Health Nutr. 2016, 19, 1233–1244. [Google Scholar] [CrossRef] [Green Version]
- Janssen, H.G.; Davies, I.G.; Richardson, L.D.; Stevenson, L. Determinants of takeaway and fast food consumption: A narrative review. Nutr. Res. Rev. 2018, 31, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Rivera, X.C.S.; Azapagic, A. Life cycle environmental impacts of ready-made meals considering different cuisines and recipes. Sci. Total Environ. 2019, 660, 1168–1181. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, N.; Vivekadhish, S. Millennium development goals (MDGS) to sustainable development goals (SDGS): Addressing unfinished agenda and strengthening sustainable development and partnership. Indian J. Community Med. 2016, 41, 1. [Google Scholar] [CrossRef]
- Marino, M.; Puppo, F.; Del Bo, C.; Vinelli, V.; Riso, P.; Porrini, M.; Martini, D. A Systematic Review of Worldwide Consumption of Ultra-Processed Foods: Findings and Criticisms. Nutrients 2021, 13, 2778. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Lisy, K.; Riitano, D.; Jordan, Z.; Aromataris, E. Caring for families experiencing stillbirth: Evidence-based guidance for maternity care providers. Women Birth 2015, 28, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]

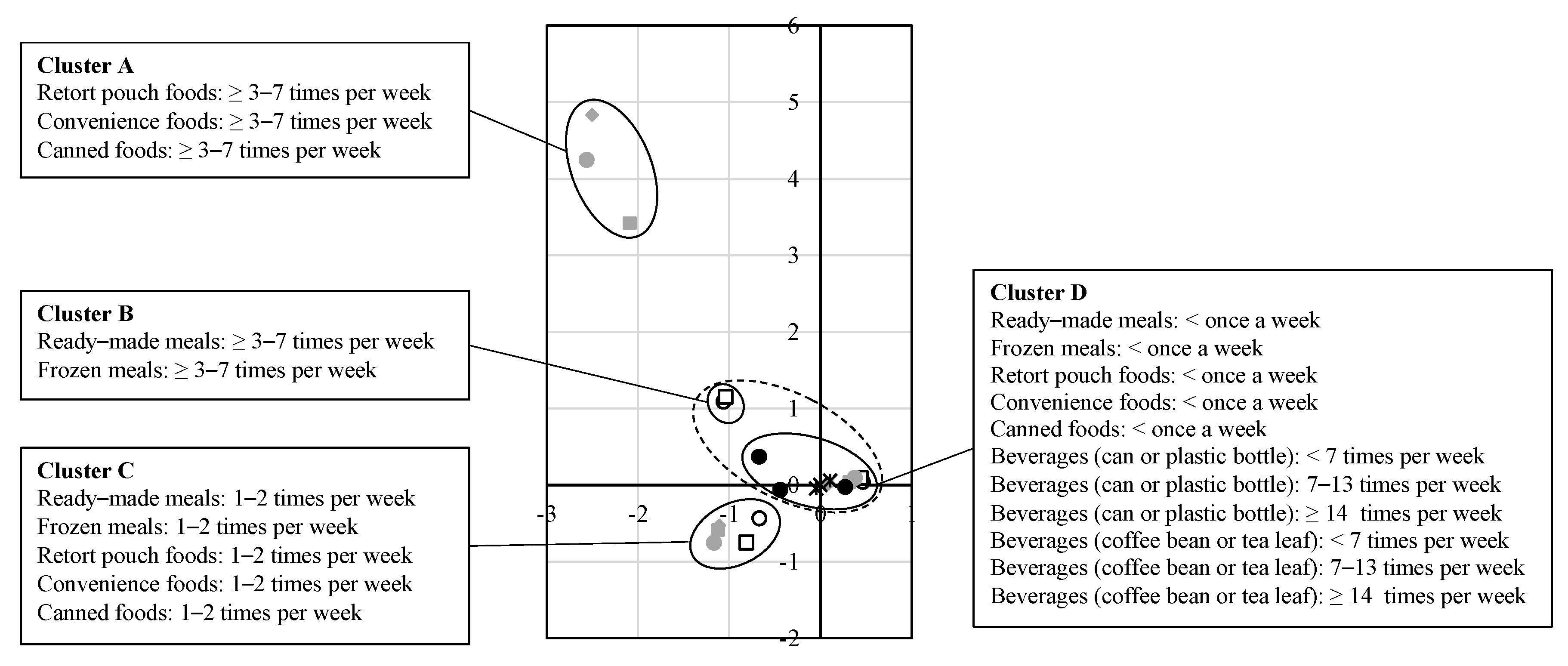
| Variables | n | (%) | |
|---|---|---|---|
| Stillbirth (≥12 weeks gestation) | 842 | (0.9) | |
| Pre-term birth (<37 weeks gestation) | 4547 | (4.8) | |
| Small for gestational age infant | 6599 | (7.0) | |
| Low birth weight (<2500 g) | 7601 | (8.1) | |
| Maternal age at registration | |||
| <20 | 1131 | (1.2) | |
| 20—29 | 37,882 | (40.3) | |
| 30–39 | 51,554 | (54.8) | |
| ≥40 | 3263 | (3.5) | |
| Missing | 232 | (0.2) | |
| Smoking histories during second/third trimester | |||
| Non-smokers | 51,049 | (54.3) | |
| Ex-smokers who quit before pregnancy | 21,183 | (22.5) | |
| Ex-smokers who quit during early pregnancy | 12,186 | (13.0) | |
| Current smokers | 4049 | (4.3) | |
| Missing | 5595 | (5.9) | |
| Maternal educational status | |||
| Junior high school or high school | 32,362 | (34.4) | |
| Higher professional school or professional school | 37,256 | (39.6) | |
| Junior college or college | 17,789 | (18.9) | |
| Postgraduate college | 1285 | (1.4) | |
| Missing | 5370 | (5.7) | |
| Annual income (JPY × 10,000) | |||
| <200 | 4746 | (5.0) | |
| 200–<400 | 28,775 | (30.6) | |
| 400–<600 | 27,330 | (29.1) | |
| 600–<800 | 13,080 | (13.9) | |
| 800–<1000 | 5362 | (5.7) | |
| ≥1000 | 3489 | (3.7) | |
| Missing | 11,280 | (12.0) | |
| Alcohol intake during second/third trimesters | |||
| Never | 29,632 | (31.5) | |
| Abstinence before pregnancy | 15,196 | (16.2) | |
| Abstinence from this pregnancy | 41,171 | (43.8) | |
| Continuance drinking | 2477 | (2.6) | |
| Missing | 5586 | (5.9) | |
| In vitro fertilization and embryo transfer | |||
| No | 90,591 | (96.3) | |
| Yes | 2887 | (3.1) | |
| Missing | 584 | (0.6) | |
| Maternal BMI | |||
| <18.5 | 15,080 | (16.0) | |
| 18.5–<25.0 | 68,255 | (72.6) | |
| ≥ 25.0 | 10,050 | (10.7) | |
| Missing | 677 | (0.7) | |
| Histories of pregnancy loss | |||
| Never | 71,555 | (76.1) | |
| Once | 16,297 | (17.3) | |
| Twice | 3604 | (3.8) | |
| More than 3 times | 1055 | (1.1) | |
| Missing | 1551 | (1.6) | |
| Histories of live birth | |||
| No | 36,792 | (39.1) | |
| Yes | 54,424 | (57.9) | |
| Missing | 2846 | (3.0) | |
| Hypertensive disorders of pregnancy | |||
| No | 87,754 | (93.3) | |
| Yes | 2759 | (2.9) | |
| Missing | 3549 | (3.8) | |
| Gestational diabetes | |||
| No | 89,570 | (95.2) | |
| Yes | 943 | (1.0) | |
| Missing | 90,513 | (96.2) | |
| Frequency of ready-made meals | |||
| <once a week | 55,354 | (58.8) | |
| 1–2 times per week | 25,342 | (26.9) | |
| ≥3–7 times per week | 8030 | (8.5) | |
| Missing | 5336 | (5.7) | |
| Frequency of frozen meals | |||
| <once a week | 59,163 | (62.9) | |
| 1–2 times per week | 20,747 | (22.1) | |
| ≥3–7 times per week | 8709 | (9.3) | |
| Missing | 5443 | (5.8) | |
| Frequency of retort pouch foods | |||
| <once a week | 68,609 | (72.9) | |
| 1–2 times per week | 18,200 | (19.3) | |
| ≥3–7 times per week | 1712 | (1.8) | |
| Missing | 5541 | (5.9) | |
| Frequency of convenience foods in plastics container | |||
| <once a week | 70,390 | (74.8) | |
| 1–2 times per week | 16,196 | (17.2) | |
| ≥ 3–7 times per week | 1934 | (2.1) | |
| Missing | 5542 | (5.9) | |
| Frequency of canned foods | |||
| <once a week | 81,808 | (87.0) | |
| 1–2 times per week | 6070 | (6.5) | |
| ≥3–7 times per week | 307 | (0.3) | |
| Missing | 5877 | (6.2) | |
| Frequency of beverages (can or plastic bottle) | |||
| <7 times per week | 56,446 | (60.0) | |
| 7–13 times per week | 22,676 | (24.1) | |
| ≥14 times per week | 7284 | (7.7) | |
| Missing | 7656 | (8.1) | |
| Frequency of beverages (coffee bean or tea leaf) | |||
| <7 times per week | 49,422 | (52.5) | |
| 7–13 times per week | 21,666 | (23.0) | |
| ≥14 times per week | 15,179 | (16.1) | |
| Missing | 7795 | (8.3) | |
| Maternal working hour (h), mean (SD) | 4.0 | (4.0) | |
| Maternal energy intake (kcal/day), mean (SD) | 1715.2 | (647.4) | |
| Stillbirth (≥12 Weeks Gestation) a | Pre-Term Birth (<37 Weeks Gestation) b | Small for Gestational Age Infant | Low Birth Weight (<2500 g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 842 | n = 4547 | n = 6599 | n = 7601 | ||||||
| q-value | Adjusted ORs (95% CI) | q-value | Adjusted ORs (95% CI) | q-value | Adjusted ORs (95% CI) | q-value | Adjusted ORs (95% CI) | ||
| Ready-made meals | <once a week | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| (cooked with microwave heating in general) | 1–2 times per week | 0.002 | 2.054 (1.442–2.926) | 0.030 | 1.100 (1.024–1.181) | 0.050 | 0.929 (0.874–0.987) | 0.840 | 0.990 (0.936–1.048) |
| ≥3–7 times per week | 0.007 | 2.632 (1.507–4.597) | 0.950 | 0.993 (0.877–1.125) | 0.375 | 0.940 (0.853–1.036) | 0.542 | 0.961 (0.875–1.056) | |
| Frozen meals | <once a week | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| (cooked with microwave heating in general) | 1–2 times per week | 0.000 | 2.225 (1.679–2.949) | 0.231 | 1.068 (0.985–1.158) | 0.542 | 1.026 (0.962–1.095) | 0.449 | 1.034 (0.971–1.102) |
| ≥3–7 times per week | 0.005 | 2.170 (1.418–3.322) | 0.099 | 1.126 (1.005–1.261) | 0.781 | 1.020 (0.93–1.119) | 0.961 | 1.003 (0.918–1.097) | |
| Retort pouch foods | <once a week | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| (heated with boiling water in general) | 1–2 times per week | 0.542 | 1.123 (0.856–1.475) | 0.498 | 1.043 (0.957–1.135) | 0.283 | 1.052 (0.982–1.128) | 0.012 | 1.105 (1.035–1.18) |
| ≥3–7 times per week | 0.077 | 0.312 (0.109–0.891) | 0.161 | 0.786 (0.605–1.021) | 0.542 | 1.081 (0.89–1.313) | 0.242 | 1.155 (0.963–1.385) | |
| Convenience foods in plastic container | <once a week | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| (heated with boiling water in general) | 1–2 times per week | 0.296 | 1.265 (0.908–1.762) | 0.388 | 1.052 (0.966–1.144) | 0.542 | 1.031 (0.96–1.108) | 0.375 | 1.043 (0.975–1.115) |
| ≥3–7 times per week | 0.050 | 0.391 (0.18–0.849) | 0.449 | 0.886 (0.707–1.11) | 0.542 | 0.927 (0.769–1.117) | 0.619 | 0.943 (0.793–1.121) | |
| Canned foods | <once a week | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| (without heating in general) | 1–2 times per week | 0.911 | 1.047 (0.666–1.646) | 0.050 | 1.157 (1.027–1.305) | 0.936 | 1.008(0.908–1.119) | 0.231 | 1.082 (0.983–1.191) |
| ≥3–7 times per week | NA | NA NA | 0.388 | 0.694 (0.378–1.277) | 0.911 | 0.956 (0.61–1.499) | 0.888 | 0.946 (0.623–1.435) | |
| Beverage (can or plastic bottle) | <7 times per week | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| 7–13 times per week | 0.000 | 3.484 (2.611–4.649) | 0.012 | 1.125 (1.042–1.214) | 0.652 | 0.981 (0.922–1.044) | 0.027 | 1.084 (1.022–1.149) | |
| ≥14 times per week | 0.000 | 2.930 (1.837–4.673) | 0.000 | 1.294 (1.160–1.444) | 0.161 | 1.091 (0.993.–1.199) | 0.006 | 1.160 (1.062–1.268) | |
| Beverage (coffee bean or tea leaf) | <7 times per week | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| 7–13 times per week | 0.000 | 3.752 (2.923–4.816) | 0.053 | 1.094 (1.014–1.180) | 0.007 | 1.105 (1.040–1.175) | 0.027 | 1.082 (1.022–1.147) | |
| ≥14 times per week | 0.021 | 1.754 (1.192–2.581) | 0.542 | 0.965 (0.884–1.054) | 0.001 | 1.150 (1.073–1.232) | 0.375 | 1.043 (0.975–1.115) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamada, H.; Ebara, T.; Matsuki, T.; Kato, S.; Sato, H.; Ito, Y.; Saitoh, S.; Kamijima, M.; Sugiura-Ogasawara, M.; on behalf of the Japan Environment and Children’s Study Group. Impact of Ready-Meal Consumption during Pregnancy on Birth Outcomes: The Japan Environment and Children’s Study. Nutrients 2022, 14, 895. https://doi.org/10.3390/nu14040895
Tamada H, Ebara T, Matsuki T, Kato S, Sato H, Ito Y, Saitoh S, Kamijima M, Sugiura-Ogasawara M, on behalf of the Japan Environment and Children’s Study Group. Impact of Ready-Meal Consumption during Pregnancy on Birth Outcomes: The Japan Environment and Children’s Study. Nutrients. 2022; 14(4):895. https://doi.org/10.3390/nu14040895
Chicago/Turabian StyleTamada, Hazuki, Takeshi Ebara, Taro Matsuki, Sayaka Kato, Hirotaka Sato, Yuki Ito, Shinji Saitoh, Michihiro Kamijima, Mayumi Sugiura-Ogasawara, and on behalf of the Japan Environment and Children’s Study Group. 2022. "Impact of Ready-Meal Consumption during Pregnancy on Birth Outcomes: The Japan Environment and Children’s Study" Nutrients 14, no. 4: 895. https://doi.org/10.3390/nu14040895
APA StyleTamada, H., Ebara, T., Matsuki, T., Kato, S., Sato, H., Ito, Y., Saitoh, S., Kamijima, M., Sugiura-Ogasawara, M., & on behalf of the Japan Environment and Children’s Study Group. (2022). Impact of Ready-Meal Consumption during Pregnancy on Birth Outcomes: The Japan Environment and Children’s Study. Nutrients, 14(4), 895. https://doi.org/10.3390/nu14040895






