Associations of Metabolic and Obstetric Risk Parameters with Timing of Lactogenesis II
Abstract
1. Introduction
2. Materials and Methods
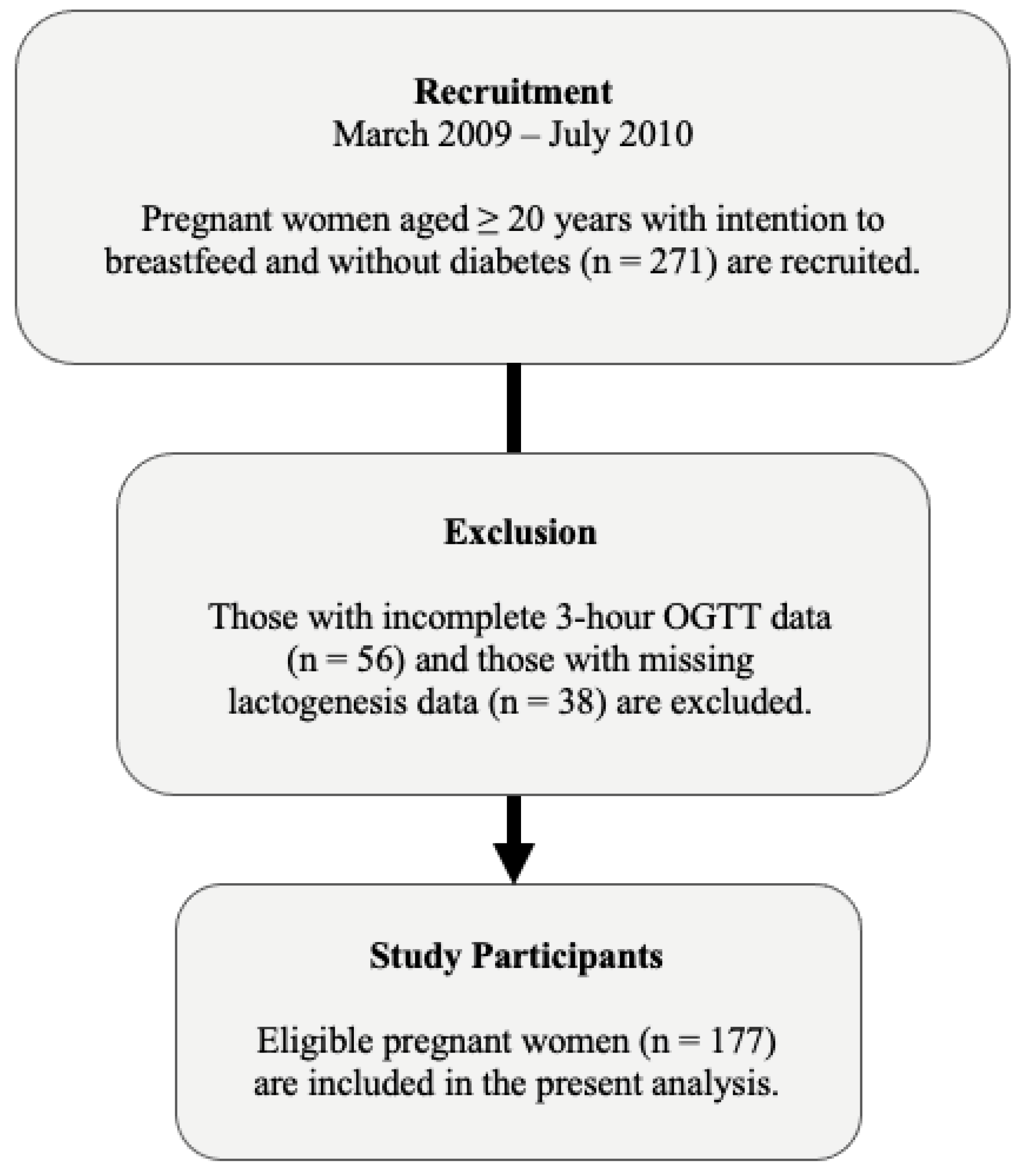
2.1. Study Design and Population
2.2. Data Collection
2.3. Biochemical Procedures and Analyses
2.4. Timing of Lactogenesis II
2.5. Exposure Variables
2.6. Statistical Analyses
3. Results
3.1. Study Population Characteristics
3.2. Association of Metabolic and Obstetric Variables with DLII
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Infant and Young Child Feeding 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 20 December 2021).
- American Academy of Pediatrics (AAP). AAP Recommendations on Breastfeeding 2021. Available online: https://publications.aap.org/redbook/book/347/chapter-abstract/5749497/AAP-Recommendations-on-Breastfeeding?redirectedFrom=fulltext (accessed on 20 December 2021).
- Pound, C.M.; Unger, S.L.; Canadian Paediatric Society, Nutrition and Gastroenterology Committee, Hospital Paediatrics Section. The baby-friendly initiative: Protecting, promoting and supporting breastfeeding. Paediatr. Child Health 2012, 17, 317–321. [Google Scholar]
- Ley, S.H.; O’Connor, D.L.; Retnakaran, R.; Hamilton, J.K.; Sermer, M.; Zinman, B.; Hanley, A.J. Impact of maternal metabolic abnormalities in pregnancy on human milk and subsequent infant metabolic development: Methodology and design. BMC Public Health 2010, 10, 590. [Google Scholar] [CrossRef]
- Panuganti, P.L.; Bazzano, L.A.; Ley, S.H. Hormones in Human Milk: A Summary of the Quantity, Determinants, and Health Outcomes of Milk Hormones. In Human Milk: Sampling and Measurement of Energy-Yielding Nutrients and Other Macromolecules; Academic Press: Cambridge, MA USA, 2021; pp. 235–274. [Google Scholar]
- Ley, S.H.; Chavarro, J.E.; Li, M.; Bao, W.; Hinkle, S.N.; Wander, P.L.; Rich-Edwards, J.; Olsen, S.; Vaag, A.; Damm, P. Lactation duration and long-term risk for incident type 2 diabetes in women with a history of gestational diabetes mellitus. Diabetes Care 2020, 43, 793–798. [Google Scholar] [CrossRef]
- Demirci, J.; Schmella, M.; Glasser, M.; Bodnar, L.; Himes, K.P. Delayed lactogenesis II and potential utility of antenatal milk expression in women developing late-onset preeclampsia: A case series. BMC Pregnancy Childbirth 2018, 18, 68. [Google Scholar] [CrossRef]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess. 2007, 153, 1–186. [Google Scholar]
- Ley, S.H. Role of lactation in cardiometabolic health consequences. J. Women’s Health 2019, 28, 3–4. [Google Scholar] [CrossRef]
- Pillay, J.; Davis, T.J. Physiology, Lactation. StatPearls 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499981/ (accessed on 20 December 2021).
- Pang, W.W.; Hartmann, P.E. Initiation of human lactation: Secretory differentiation and secretory activation. J. Mammary Gland. Biol. Neoplasia. 2007, 12, 211–221. [Google Scholar] [CrossRef]
- Lawrence, R.A. Physiology of Lactation. In Breastfeeding: A guide for the Medical Profession, 9th ed.; Elsevier: Philadelphia, PA USA, 2022; pp. 58–92. [Google Scholar]
- Neville, M.C.; Morton, J.; Umemura, S. Lactogenesis: The transition from pregnancy to lactation. Pediatr. Clin. N. Am. 2001, 48, 35–52. [Google Scholar] [CrossRef]
- Brownell, E.; Howard, C.R.; Lawrence, R.A.; Dozier, A.M. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J. Pediatr. 2012, 161, 608–614. [Google Scholar] [CrossRef]
- Chapman, D.J.; Perez-Escamilla, R. Identification of risk factors for delayed onset of lactation. J. Am. Diet. Assoc. 1999, 99, 450–454. [Google Scholar] [CrossRef]
- Dewey, K.G.; Nommsen-Rivers, L.A.; Heinig, M.J.; Cohen, R.J. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 2003, 112, 607–619. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A.; Chantry, C.J.; Peerson, J.M.; Cohen, R.J.; Dewey, K.G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr. 2010, 92, 574–584. [Google Scholar] [CrossRef]
- Yu, X.; Li, J.; Lin, X.; Luan, D. Association between delayed lactogenesis II and early milk volume among mothers of preterm infants. Asian Nurs. Res. 2019, 13, 93–98. [Google Scholar] [CrossRef]
- Matias, S.L.; Dewey, K.G.; Quesenberry, C.P., Jr.; Gunderson, E.P. Maternal prepregnancy obesity and insulin treatment during pregnancy are independently associated with delayed lactogenesis in women with recent gestational diabetes mellitus. Am. J. Clin. Nutr. 2014, 99, 115–121. [Google Scholar] [CrossRef]
- Rocha, B.d.O.; Machado, M.P.; Bastos, L.L.; Barbosa Silva, L.; Santos, A.P.; Santos, L.C.; Ferrarez Bouzada, M.C. Risk factors for delayed onset of lactogenesis II among primiparous mothers from a brazilian baby-friendly hospital. J. Hum. Lact. 2020, 36, 146–156. [Google Scholar] [CrossRef]
- Scott, J.A.; Binns, C.W.; Oddy, W.H. Predictors of delayed onset of lactation. Matern. Child Nutr. 2007, 3, 186–193. [Google Scholar] [CrossRef]
- Nommsen-Rivers, L.A.; Chantry, C.J.; Dewey, K.G. Early breastfeeding outcomes in gestational diabetic primiparas delivering term infants. FASEB J. 2010, 24, 91–94. [Google Scholar] [CrossRef]
- Arthur, P.G.; Smith, M.; Hartmann, P.E. Milk lactose, citrate, and glucose as markers of lactogenesis in normal and diabetic women. J. Pediatr. Gastroenterol. Nutr. 1989, 9, 488–496. [Google Scholar] [CrossRef]
- Neubauer, S.H.; Ferris, A.M.; Chase, C.G.; Fanelli, J.; Thompson, C.A.; Lammi-Keefe, C.J.; Clark, R.M.; Jensen, R.G.; Bendel, R.B.; Green, K.W. Delayed lactogenesis in women with insulin-dependent diabetes mellitus. Am. J. Clin. Nutr. 1993, 58, 54–60. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Kjolhede, C.L. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 2004, 113, e465–e471. [Google Scholar] [CrossRef]
- Ley, S.H.; Hanley, A.J.; Sermer, M.; Zinman, B.; O’Connor, D.L. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am. J. Clin. Nutr. 2012, 95, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hanley, A.J.; Retnakaran, R.; Sermer, M.; Zinman, B.; O’Connor, D.L. Effect of macronutrient intake during the second trimester on glucose metabolism later in pregnancy. Am. J. Clin. Nutr. 2011, 94, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hanley, A.J.; Sermer, M.; Zinman, B.; O’connor, D.L. Lower dietary vitamin E intake during the second trimester is associated with insulin resistance and hyperglycemia later in pregnancy. Eur. J. Clin. Nutr. 2013, 67, 1154–1156. [Google Scholar] [CrossRef][Green Version]
- LeMay-Nedjelski, L.; Butcher, J.; Ley, S.H.; Asbury, M.R.; Hanley, A.J.; Kiss, A.; Unger, S.; Copeland, J.K.; Wang, P.W.; Zinman, B. Examining the relationship between maternal body size, gestational glucose tolerance status, mode of delivery and ethnicity on human milk microbiota at three months post-partum. BMC Microbiol. 2020, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- LeMay-Nedjelski, L.; Yonemitsu, C.; Asbury, M.R.; Butcher, J.; Ley, S.H.; Hanley, A.J.; Kiss, A.; Unger, S.; Copeland, J.K.; Wang, P.W. Oligosaccharides and microbiota in human milk are interrelated at 3 months postpartum in a cohort of women with a high prevalence of gestational impaired glucose tolerance. J. Nutr. 2021, 151, 3431–3441. [Google Scholar] [CrossRef]
- LeMay-Nedjelski, L.; Asbury, M.R.; Butcher, J.; Ley, S.H.; Hanley, A.J.; Kiss, A.; Unger, S.; Copeland, J.K.; Wang, P.W.; Stintzi, A. Maternal diet and infant feeding practices are associated with variation in the human milk microbiota at 3 months postpartum in a cohort of women with high rates of gestational glucose intolerance. J. Nutr. 2021, 151, 320–329. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: New York, NY, USA, 2005; Available online: https://books.google.com/books?hl=en&lr=&id=lBlu7UKI3aQC&oi=fnd&pg=PR11&ots=RXOyXW6tlz&sig=VDqvPKxKRHhWz9aysm3Pl8jS6zk#v=onepage&q&f=true (accessed on 20 December 2021).
- Canadian Diabetes Association. 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. 2008. Available online: http://www.diabetesclinic.ca/en/pdf/CDA_cpg-2008.pdf (accessed on 20 December 2021).
- Retnakaran, R.; Qi, Y.; Sermer, M.; Connelly, P.W.; Hanley, A.J.; Zinman, B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care 2008, 31, 2026–2031. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef]
- Matsuda, M.; De Fronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Retnakaran, R.; Qi, Y.; Goran, M.I.; Hamilton, J.K. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabetic Med. 2009, 26, 1198–1203. [Google Scholar] [CrossRef]
- Chapman, D.J.; Pérez-Escamilla, R. Maternal perception of the onset of lactation is a valid, public health indicator of lactogenesis stage II. J. Nutr. 2000, 130, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Cregan, M. Lactogenesis and the effects of insulin-dependent diabetes mellitus and prematurity. J. Nutr. 2001, 131, 3016S–3020S. [Google Scholar] [CrossRef] [PubMed]
- Perinatal Services BC Health Promotion Guideline. Breastfeeding Healthy Term Infants. 2015. Available online: http://www.perinatalservicesbc.ca/search?k=breastfeedinghealthyterminfantguideline.pdf (accessed on 20 December 2021).
- Baby-Friendly Hospital Initiative. Ten Steps to Successful Breastfeeding, from UNICEF and the World Health Organization. 2018. Available online: https://www.unicef.org/documents/baby-friendly-hospital-initiative (accessed on 20 December 2021).
- Lind, J.N.; Perrine, C.G.; Li, R. Relationship between use of labor pain medications and delayed onset of lactation. J. Hum. Lact. 2014, 30, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Kutlucan, L.; Seker, İ.S.; Demiraran, Y.; Ersoy, Ö.; Karagöz, İ.; Sezen, G.; Köse, S.A. Effects of different anesthesia protocols on lactation in the postpartum period. J. Turk. Ger. Gynecol. Assoc. 2014, 15, 233. [Google Scholar] [CrossRef]
- Kocaöz, F.Ş.; Destegül, D.; Kocaöz, S. Comparison of the breastfeeding outcomes and self-efficacy in the early postpartum period of women who had given birth by cesarean under general or spinal anesthesia. J. Matern. Fetal Neonatal Med. 2021, 34, 1545–1549. [Google Scholar] [CrossRef]
| Risk Factor | DLII—Yes n (%) or Mean ± SD | DLII—No n (%) or Mean ± SD |
|---|---|---|
| Age (y) | 34.7 ± 4.5 | 35.0 ± 4.2 |
| Race | ||
| White | 53 (54.1) | 50 (63.3) |
| Non-white | 45 (45.9) | 29 (36.7) |
| Pregravid BMI (kg/m2) | 24.8 ± 4.6 | 25.0 ± 5.5 |
| Family history of T2DM | ||
| Yes | 53 (54.1) | 42 (53.2) |
| No | 45 (45.9) | 37 (46.8) |
| Gestational diabetes | ||
| Yes | 24 (24.5) | 16 (20.3) |
| No | 74 (75.5) | 63 (79.7) |
| Serum concentrations at OGTT | ||
| Fasting glucose (mmol/L) | 4.7 ± 0.6 | 4.7 ± 0.9 |
| HOMA-IR | 2.3 ± 1.4 | 2.3 ± 1.6 |
| ISogtt 1 | 10.9 (8.3, 15.8) | 13.0 (8.3, 18.2) |
| Adiponectin (μg/mL) | 7.1 ± 2.4 | 7.4 ± 2.2 |
| Gestational age (wk) | 39.3 ± 1.2 | 39.0 ± 1.2 |
| Primiparous | ||
| Yes | 60 (61.2) | 33 (41.8) |
| No | 38 (38.8) | 46 (58.2) |
| Mode of delivery | ||
| Scheduled cesarean | 10 (10.2) | 17 (21.8) |
| Unscheduled cesarean | 31 (31.6) | 12 (15.4) |
| Spontaneous | 57 (58.2) | 49 (62.8) |
| Infant birth weight (kg) | 3.4 ± 0.5 | 3.4 ± 0.5 |
| Time to first breast contact (h) | 3.8 ± 7.0 | 2.3 ± 4.5 |
| Time to onset of lactogenesis II (h) | 98.3 ± 30.1 | 57.2 ± 11.7 |
| Obstetric Variable | OR (95% CI) 1 | OR (95% CI) 2 |
|---|---|---|
| Mode of delivery | 0.52 (0.21–1.26) | 0.62 (0.24–1.57) |
| Scheduled cesarean | 2.24 (1.02–4.92) | 1.93 (0.86–4.35) |
| Unscheduled cesarean Spontaneous | 1.00 [Reference] | 1.00 [Reference] |
| Infant birth weight | ||
| <3.2 kg | 0.98 (0.46–2.07) | 1.03 (0.47–2.23) |
| 3.2 kg–3.6 kg | 1.00 [Reference] | 1.00 [Reference] |
| >3.6 kg | 1.21 (0.58–2.54) | 1.20 (0.56–2.56) |
| Time to first breast contact | ||
| ≤1 h | 1.00 [Reference] | 1.00 [Reference] |
| 1.1–2 h | 1.35 (0.62–2.96) | 1.30 (0.57–2.97) |
| ≥2 h | 2.30 (1.09–4.86) | 2.43 (1.09–5.45) |
| Metabolic Variable | OR (95% CI) 1 | OR (95% CI) 2 |
|---|---|---|
| Family history of T2DM | ||
| Yes | 1.02 (0.55–1.87) | 1.03 (0.55–1.92) |
| No | 1.00 [Reference] | 1.00 [Reference] |
| Gestational diabetes | ||
| Yes | 1.21 (0.57–2.57) | 1.26 (0.58–2.73) |
| No | 1.00 [Reference] | 1.00 [Reference] |
| Fasting glucose | 0.92 (0.60–1.41) | 0.97 (0.62–1.52) |
| HOMA-IR | 0.99 (0.80–1.22) | 1.05 (0.82–1.35) |
| ISogtt | 0.99 (0.96–1.02) | 0.99 (0.95–1.02) |
| Adiponectin | 0.97 (0.84–1.11) | 0.96 (0.83–1.11) |
| Variable | OR (95% CI) 1 |
|---|---|
| Age (y) | 0.99 (0.91–1.08) |
| Race | |
| White | 1.00 [Reference] |
| Non-White | 1.29 (0.66–2.53) |
| Pregravid BMI | 0.98 (0.92–1.05) |
| Primiparity | |
| Yes | 1.96 (0.92–4.18) |
| No | 1.00 [Reference] |
| Mode of delivery | |
| Scheduled cesarean | 0.48 (0.17–1.33) |
| Unscheduled cesarean | 1.42 (0.60–3.40) |
| Spontaneous | 1.00 [Reference] |
| Time to first breast contact | |
| ≤1 h | 1.00 [Reference] |
| 1.1–2 h | 1.34 (0.57–3.17) |
| ≥2 h | 2.71 (1.12–6.53) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullen, A.J.; O’Connor, D.L.; Hanley, A.J.; Piedimonte, G.; Wallace, M.; Ley, S.H. Associations of Metabolic and Obstetric Risk Parameters with Timing of Lactogenesis II. Nutrients 2022, 14, 876. https://doi.org/10.3390/nu14040876
Mullen AJ, O’Connor DL, Hanley AJ, Piedimonte G, Wallace M, Ley SH. Associations of Metabolic and Obstetric Risk Parameters with Timing of Lactogenesis II. Nutrients. 2022; 14(4):876. https://doi.org/10.3390/nu14040876
Chicago/Turabian StyleMullen, Amber J., Deborah L. O’Connor, Anthony J. Hanley, Giovanni Piedimonte, Maeve Wallace, and Sylvia H. Ley. 2022. "Associations of Metabolic and Obstetric Risk Parameters with Timing of Lactogenesis II" Nutrients 14, no. 4: 876. https://doi.org/10.3390/nu14040876
APA StyleMullen, A. J., O’Connor, D. L., Hanley, A. J., Piedimonte, G., Wallace, M., & Ley, S. H. (2022). Associations of Metabolic and Obstetric Risk Parameters with Timing of Lactogenesis II. Nutrients, 14(4), 876. https://doi.org/10.3390/nu14040876







