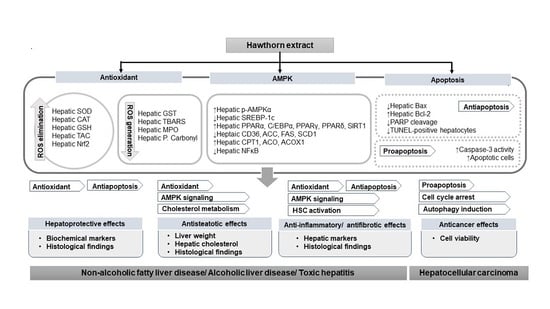
Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases
Abstract
1. Introduction
2. Phytochemistry of Hawthorn
3. Pharmacological Properties of Hawthorn
3.1. Hepatoprotective Effect
3.2. Antisteatotic Effect
3.3. Anti-Inflammatory and Antifibrotic Effects
3.4. Anticancer Effects
4. Safety of Hawthorn
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef] [PubMed]
- Bernal, W.; Wendon, J. Acute Liver Failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Huang, J.L.; George, J.; Huang, J.; Leung, C.; Eslam, M.; Chan, H.L.; Ng, S.C. The changing epidemiology of liver diseases in the Asia–Pacific region. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Liangpunsakul, S.; Haber, P.; McCaughan, G.W. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 2016, 150, 1786–1797. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hébrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: From physiology to therapeutic perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef]
- Malhotra, P.; Gill, R.K.; Saksena, S.; Alrefai, W.A. Disturbances in cholesterol homeostasis and non-alcoholic fatty liver diseases. Front. Med. 2020, 7, 467. [Google Scholar] [CrossRef]
- Fingas, C.D.; Gerken, G.; Canbay, A. Apoptosis in selected liver diseases. Turk. J. Gastroenterol. 2009, 20, 171–179. [Google Scholar]
- Song, E.J.; Kim, N.Y.; Heo, M.Y. Protective effect of korean medicinal plants on ethanol-induced cytotoxicity in HepG2 Cells. Nat. Prod. Sci. 2013, 19, 329–336. [Google Scholar]
- Liu, Z.L.; Xie, L.Z.; Zhu, J.; Li, G.Q.; Grant, S.J.; Liu, J.P. Herbal medicines for fatty liver diseases. Cochrane Database Syst. Rev. 2013, 24, CD009059. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Bras. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Caliskan, O. The Mediterranean Diet, 1st ed.; Elsevier: San Diego, CA, USA, 2015; pp. 621–628. [Google Scholar]
- Wu, J.; Peng, W.; Qin, R.; Zhou, H. Crataegus pinnatifida: Chemical constituents, pharmacology, and potential applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef] [PubMed]
- Korea Ministry of Food and Drug Safety. The Korean Pharmacopoeia, 12th ed.; The KFDA Notification: Cheongju, Korea, 2020; p. 53.
- National Pharmacopoeia Committee of China Ministry of Health. Pharmacopoeia of the People’s Republic of China, 11th ed.; The NMPA Notification: Beijing, China, 2020; pp. 33–34.
- Council of Europe. European Pharmacopoeia, 4th ed.; European Directorate for the Quality of Medicines: Stragbourg, France, 2002; pp. 1292–1295. [Google Scholar]
- The United States Pharmacopeial Convention. The United States Pharmacopeia, 38th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2015; pp. 6102–6106. [Google Scholar]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef]
- Wen, L.; Guo, R.; You, L.; Abbasi, A.M.; Li, T.; Fu, X.; Liu, R.H. Major triterpenoids in Chinese hawthorn “Crataegus pinnatifida” and their effects on cell proliferation and apoptosis induction in MDA-MB-231 cancer cells. Food Chem. Toxicol. 2017, 100, 149–160. [Google Scholar] [CrossRef]
- Caligiani, A.; Malavasi, G.; Palla, G.; Marseglia, A.; Tognolini, M.; Bruni, R. A simple GC–MS method for the screening of betulinic, corosolic, maslinic, oleanolic and ursolic acid contents in commercial botanicals used as food supplement ingredients. Food Chem. 2013, 136, 735–741. [Google Scholar] [CrossRef]
- Alirezalu, A.; Salehi, P.; Ahmadi, N.; Sonboli, A.; Aceto, S.; Hatami Maleki, H.; Ayyari, M. Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int. J. Food Prop. 2018, 21, 452–470. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Lü, D.; Zhou, C.; Yang, B. Quantitative analysis of phenolic compounds in Chinese hawthorn (Crataegus spp.) fruits by high performance liquid chromatography–electrospray ionisation mass spectrometry. Food Chem. 2011, 127, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Bell, A. Lipid metabolism in liver and selected tissues and in the whole body of ruminant animals. Prog. Lipid Res. 1979, 18, 117–164. [Google Scholar] [CrossRef]
- Tahmasebi, M.; Sadeghi, H.; Nazem, H.; Kokhdan, E.P.; Omidifar, N. Hepatoprotective effects of Berberis vulgaris leaf extract on carbon tetrachloride-induced hepatotoxicity in rats. J. Educ. Health Promot. 2018, 7, 147. [Google Scholar]
- Czaja, M.J. Cell signaling in oxidative stress-induced liver injury. Semin. Liver Dis. 2007, 27, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.; Gores, G. Apoptosis: A mechanism of acute and chronic liver injury. Gut 2005, 54, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, G.; Jeivad, F.; Goharbari, M.; Gheshlaghi, G.H.; Sabzevari, O. Ethanol extract of Crataegus oxyacantha L. ameliorate dietary non-alcoholic fatty liver disease in rat. Drug Res. 2018, 68, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Al Humayed, S. Protective and therapeutic effects of Crataegus aronia in non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 2017, 123, 23–30. [Google Scholar] [CrossRef]
- Rezaei-Golmisheh, A.; Malekinejad, H.; Asri-Rezaei, S.; Farshid, A.A.; Akbari, P. Hawthorn ethanolic extracts with triterpenoids and flavonoids exert hepatoprotective effects and suppress the hypercholesterolemia-induced oxidative stress in rats. Iran. J. Basic Med. Sci. 2015, 18, 691–699. [Google Scholar]
- Wang, S.Y.; Xu, Q.Y.; Zhang, W.J.; Liu, X.; Ying, X.X.; Kang, T.G. Study on effects of hawthorn leaves extract on fatty liver in rats. China J. Tradit. Chin. Med. Pharm. 2011, 26, 2955–2959. [Google Scholar]
- Lee, J.-J.; Lee, H.-J.; Oh, S.-W. Antiobesity Effects of Sansa (Crataegi fructus) on 3T3-L1 Cells and on High-Fat–High-Cholesterol Diet-Induced Obese Rats. J. Med. Food. 2017, 20, 19–29. [Google Scholar] [CrossRef]
- Housein, Z.M.I.; Othman, G.Q.; Mustafa, T.A. The liver protective role of hawthorn (Crataegus sp.) in hypertriglycerdimic induced rats. Polytech. J. 2017, 7, 111–118. [Google Scholar]
- Li, Z.; Xu, J.; Zheng, P.; Xing, L.; Shen, H.; Yang, L.; Zhang, L.; Ji, G. Hawthorn leaf flavonoids alleviate nonalcoholic fatty liver disease by enhancing the adiponectin/AMPK pathway. Int. J. Clin. Exp. 2015, 8, 17295–17307. [Google Scholar]
- Gao, Z.Q.; Xie, M.J.; Chen, L.B. Effects of fruits and leaves of hawthorn on lipid metabolism and oxidative stress in fatty liver rats. J. Tradit. Chin. Med. 2016, 34, 50–53. [Google Scholar]
- Wang, D.; Cai, Y.; Pan, S.; Zhang, L.; Chen, Y.; Chen, F.; Jin, M.; Yan, M.; Li, X.; Chen, Z. Effect of total flavone of haw Leaves on nuclear factor erythroid-2 related factor and other related factors in nonalcoholic steatohepatitis rats. Chin. J. Integr. Med. 2018, 24, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Al Humayed, S.; Eid, R.A.; Shatoor, A.S.; Haidara, M.A.; Zaki, M.S.A.; Al-Ani, B. Differential Therapeutic Effects of Crataegus aronia and Simvastatin on the Hepatocyte Ultrastructure in Hepatic Steatosis. J. Morphol. 2017, 35, 578–583. [Google Scholar] [CrossRef]
- Han, X.; Li, W.; Huang, D.; Yang, X. Polyphenols from hawthorn peels and fleshes differently mitigate dyslipidemia, inflammation and oxidative stress in association with modulation of liver injury in high fructose diet-fed mice. Chem.-Biol. Interact. 2016, 257, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Seo, B. Preventive effects of water extracts from Crataegi fructus on hyperlipiderma and liver damage induced by alcohol. Korea J. Herbol. 2005, 20, 35–43. [Google Scholar]
- Kim, J.-S. Hypoglycemic Effect and Hepatic Detoxification Activity of Extracts from Crataegus fructus and Morus alba L. in Alcohol-treated Rats. Biomed. Sci. Lett. 2007, 13, 17–23. [Google Scholar]
- Kim, N.Y.; Song, E.J.; Heo, M.Y. Protective Effect of Crataegus pinnatifida and Cinnamomum cassia on Ethanol-induced Cytotoxicity and DNA Damage in HepG2 Cells. Nat. Prod. Sci. 2014, 20, 237–242. [Google Scholar]
- Martínez-Rodríguez, J.L.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; AdriÃ, J. Antioxidant, hypolipidemic and preventive effect of Hawthorn (Crataegus oxyacantha) on alcoholic liver damage in rats. J. Pharmacogn. Phytother. 2016, 8, 193–202. [Google Scholar]
- Kao, E.-S.; Wang, C.-J.; Lin, W.-L.; Yin, Y.-F.; Wang, C.-P.; Tseng, T.-H. Anti-inflammatory potential of flavonoid contents from dried fruit of Crataegus pinnatifida in vitro and in vivo. J. Agric. Food Chem. 2005, 53, 430–436. [Google Scholar] [CrossRef]
- Hamza, A.A.; Lashin, F.M.; Gamel, M.; Hassanin, S.O.; Abdalla, Y.; Amin, A. Hawthorn herbal preparation from Crataegus oxyacantha attenuates in vivo carbon tetrachloride-induced hepatic fibrosis via modulating oxidative stress and inflammation. Antioxidants 2020, 9, 1173. [Google Scholar] [CrossRef]
- Salam, O.M.A.; Sleem, A.A.; Shafee, N. Effect of Crataegus extract on carbon tetrachloride-induced hepatic damage. Comp. Clin. Pathol. 2012, 21, 1719–1726. [Google Scholar] [CrossRef]
- Shin, J.-H.; Jo, M.-J.; Park, S.-M.; Park, S.-J.; Kim, S.-C. Hepatoprotective activity of Crataegii fructus water extract against cadmium-induced toxicity in rats. J. Physiol. Pathol. 2010, 24, 249–257. [Google Scholar]
- Keskin, N.; Mammadov, R.; Ili, P. The effects of Crataegus aronia var. dentata Browicz extract on biochemical indices and apoptosis in partially hepatectomized liver in rats. Bosn. J. Basic Med. Sci. 2012, 12, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and prevention of hepatic steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Musso, G.; Gambino, R.; Cassader, M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 2009, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P.; James, O.F. Steatohepatitis: A tale of two “hits”? Gastroenterology 1998, 144, 842–845. [Google Scholar] [CrossRef]
- Alfaradhi, M.Z.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Musial, B.; Fowden, A.; Ozanne, S.E. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R26–R34. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Vendemiale, G. Free radical biology for medicine: Learning from nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2013, 65, 952–968. [Google Scholar] [CrossRef]
- Li, T.; Zhu, R.; Dong, Y.; Liu, Y.; Li, S.; Chen, G. Effects of pectin pentaoligosaccharide from Hawthorn (Crataegus pinnatifida Bunge. var. Major) on the activity and mRNA levels of enzymes involved in fatty acid oxidation in the liver of mice fed a high-fat diet. J. Agric. Food Chem. 2013, 61, 7599–7605. [Google Scholar] [CrossRef]
- Zhu, R.; Li, T.; Dong, Y.; Liu, Y.; Li, S.; Chen, G.; Zhao, Z.; Jia, Y. Pectin pentasaccharide from hawthorn (Crataegus pinnatifida Bunge. Var. major) ameliorates disorders of cholesterol metabolism in high-fat diet fed mice. Food Res. Int. 2013, 54, 262–268. [Google Scholar] [CrossRef]
- Zhu, R.-G.; Sun, Y.-D.; Hou, Y.-T.; Fan, J.-G.; Chen, G.; Li, T.-P. Pectin penta-oligogalacturonide reduces cholesterol accumulation by promoting bile acid biosynthesis and excretion in high-cholesterol-fed mice. Chem.-Biol. Interact. 2017, 272, 153–159. [Google Scholar] [CrossRef]
- Zhu, R.; Hou, Y.; Sun, Y.; Li, T.; Fan, J.; Chen, G.; Wei, J. Pectin penta-oligogalacturonide suppresses intestinal bile acids absorption and downregulates the FXR-FGF15 axis in high-cholesterol fed mice. Lipids 2017, 52, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.-Y.; Wong, C.N.-Y.; Yau, M.Y.-C.; Yu, P.H.-F.; Au, A.L.S.; Poon, C.C.-W.; Seto, S.-W.; Lam, T.-Y.; Kwan, Y.-W.; Chan, S.-W. Consumption of dried fruit of Crataegus pinnatifida (hawthorn) suppresses high-cholesterol diet-induced hypercholesterolemia in rats. J. Funct. Foods. 2010, 2, 179–186. [Google Scholar] [CrossRef]
- Kwon, S.H.; Kim, J.B. Effects of Crataegii fructus on the diet-induced hyperlipidemia in rats. J. Orient. Physiol. Pathol. 2010, 24, 67–73. [Google Scholar]
- Kwok, C.-Y.; Li, C.; Cheng, H.-L.; Ng, Y.-F.; Chan, T.-Y.; Kwan, Y.-W.; Leung, G.P.-H.; Lee, S.M.-Y.; Mok, D.K.-W.; Yu, P.H.-F. Cholesterol lowering and vascular protective effects of ethanolic extract of dried fruit of Crataegus pinnatifida, hawthorn (Shan Zha), in diet-induced hypercholesterolaemic rat model. J. Funct. Foods. 2013, 5, 1326–1335. [Google Scholar] [CrossRef]
- Zhang, Z.; Ho, W.K.; Huang, Y.; Chen, Z.-Y. Hypocholesterolemic activity of hawthorn fruit is mediated by regulation of cholesterol-7α-hydroxylase and acyl CoA: Cholesterol acyltransferase. Food Res. Int. 2002, 35, 885–891. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, M.-S. Changes of Plasma and Hepatic Lipids, Hydroxy-Methyl-Glutaryl CoA Reductase Activity and Acyl-CoA: Cholesterol Acyltransferase Activity by Supplementation of Hot Water Extracts from Rosa rugosa, Crataegus pinnatifida and Polygonum cuspidatum in High-Cholesterol Fed Rats. Prev. Nutr. Food Sci. 1998, 3, 344–350. [Google Scholar]
- Kim, Y.-S.; Byun, S.-H.; Kim, S.-C.; Kuk, M.; Cho, E.-H. Effects on cure and prevention of an obesity (Ⅳ). Korea J. Herbol. 2000, 15, 37. [Google Scholar]
- Go, K.J.; Song, Y.S. Influence of fructus Crataegi water extract on the obese mouse models. J. Tradit. Korean Med. 1998, 8, 1–8. [Google Scholar]
- Gao, Y.; Xiao, Y. Effect of hawthorn and hawthorn flavonoids extract on rats with hyperlipidemia. Chin. J. Food Hyg. 2002, 14, 14–16. [Google Scholar]
- Ban, S.-S.; Yoon, H.-D.; Shin, O.-C.; Shin, Y.-J.; Park, C.-S.; Park, J.-H.; Seo, B.-I. The effects of Artemisiae capillaris, Ponciri fructus and Cartaegi fructus in obese rats induced by high fat diet. Korea J. Herbol. 2006, 21, 55–67. [Google Scholar]
- Gal, S.-W.; Choi, Y.-J.; Cho, S.-J. Anti-obesity effect of Crataegus fructus extract from Chinese cultivation. J. Life Sci. 2011, 21, 1586–1591. [Google Scholar] [CrossRef][Green Version]
- Hu, H.; Dong, Q.; Wang, Q.; Pan, L.; Zhang, T.; Luo, X. Studies on protective effects of zhongtian hawthorn extract on hepatic steatosis. J. Tianjin Univ. Sci. Technol. 2018, 33, 14–19. [Google Scholar]
- Hu, H.-J.; Luo, X.-G.; Dong, Q.-Q.; Mu, A.; Shi, G.-L.; Wang, Q.-T.; Chen, X.-Y.; Zhou, H.; Zhang, T.-C.; Pan, L.-W. Ethanol extract of Zhongtian hawthorn lowers serum cholesterol in mice by inhibiting transcription of 3-hydroxy-3-methylglutaryl-CoA reductase via nuclear factor-kappa B signal pathway. Exp. Biol. Med. 2016, 241, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.; Abuharfeil, N.; Shabsoug, B. The effect of Crataegus aronica aqueous extract in rabbits fed with high cholesterol diet. Eur. J. Sci. Res. 2008, 22, 352–360. [Google Scholar]
- Rajendran, S.; Deepalakshmi, P.; Parasakthy, K.; Devaraj, H.; Devaraj, S.N. Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis 1996, 123, 235–241. [Google Scholar] [CrossRef]
- He, Z.; Kwek, E.; Hao, W.; Zhu, H.; Liu, J.; Ma, K.Y.; Chen, Z.-Y. Hawthorn fruit extract reduced trimethylamine-N-oxide (TMAO)-exacerbated atherogenesis in mice via anti-inflammation and anti-oxidation. Nutr. Metab. 2021, 18, 1–15. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kong, R.; Han, H.-S.; Kang, D.-h.; Lee, S.-J.; Lee, C.-C.; Wang, S.; Kwon, D.-Y.; Kang, O.-H. Non-alcoholic fatty liver protective effects, and studies on the mechanism of action of Crataegi fructus. Korea J. Herbol. 2018, 33, 61–70. [Google Scholar]
- Yoo, J.-H.; Liu, Y.; Kim, H.-S. Hawthorn Fruit Extract Elevates Expression of Nrf2/HO-1 and Improves Lipid Profiles in Ovariectomized Rats. Nutrients 2016, 8, 283. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Dong, Y.; Li, S.; Zhu, R. Anti-fat deposition and antioxidant effects of haw pectic oligosaccharide in the liver of high-fat-fed mice. CYTA-J. Food 2014, 12, 27–31. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Dong, Y.; Zhu, R.; Liu, Y. Antioxidant activity of penta-oligogalacturonide, isolated from haw pectin, suppresses triglyceride synthesis in mice fed with a high-fat diet. Food Chem. 2014, 145, 335–341. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Huang, Z.; Xie, W.; Tong, C.; Bao, R.; Sun, X.; Li, W.; Li, S. Pectin oligosaccharide from hawthorn fruit ameliorates hepatic inflammation via NF-κB inactivation in high-fat diet fed mice. J. Funct. Foods 2019, 57, 345–350. [Google Scholar] [CrossRef]
- Greuter, T.; Shah, V.H. Hepatic sinusoids in liver injury, inflammation, and fibrosis: New pathophysiological insights. J. Gastroenterol. 2016, 51, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014, 5, e996. [Google Scholar] [CrossRef]
- Somade, O.T.; Ajayi, B.O.; Olunaike, O.E.; Jimoh, L.A. Hepatic oxidative stress, up-regulation of pro-inflammatory cytokines, apoptotic and oncogenic markers following 2-methoxyethanol administrations in rats. Biochem. Biophys. Rep. 2020, 24, 100806. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Fu, M.; Wang, X.; Chang, X. Regulatory effects of hawthorn polyphenols on hyperglycemic, inflammatory, insulin resistance responses, and alleviation of aortic injury in type 2 diabetic rats. Food Res. Int. 2021, 142, 110239. [Google Scholar] [CrossRef]
- Zheng, K.; He, Z.; Kitazato, K.; Wang, Y. Selective Autophagy Regulates Cell Cycle in Cancer Therapy. Theranostics 2019, 9, 104–125. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Flavonoids as Anticancer Agents: Structure-Activity Relationship Study. Curr. Med. Chem. Anticancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef]
- Peng, F.H.; Ma, X.; Hu, X.Y. Effect of hawthorn extract on apoptosis and related factors of HepG2 cells. Zhongguo Shiyan Fangjixue Zazhi 2016, 22, 169–172. [Google Scholar]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, antioxidant and anti-proliferative properties and zinc content of five south Portugal herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar] [CrossRef]
- Qiao, A.; Wang, Y.; Xiang, L.; Zhang, Z.; He, X. Novel triterpenoids isolated from hawthorn berries functioned as antioxidant and antiproliferative activities. J. Funct. Foods 2015, 13, 308–313. [Google Scholar] [CrossRef]
- Guo, R.; Lin, B.; Shang, X.Y.; Zhou, L.; Yao, G.D.; Huang, X.X.; Song, S.J. Phenylpropanoids from the fruit of Crataegus pinnatifida exhibit cytotoxicity on hepatic carcinoma cells through apoptosis induction. Fitoterapia 2018, 127, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, A.; Lumlerdkij, N.; Heinrich, M. Caucasian endemic medicinal and nutraceutical plants: In-vitro antioxidant and cytotoxic activities and bioactive compounds. J. Pharm. Pharmacol. 2019, 71, 1152–1161. [Google Scholar] [CrossRef]
- Rodrigues, S.; Calhelha, R.C.; Barreira, J.C.M.; Dueñas, M.; Carvalho, A.M.; Abreu, R.M.V.; Santos-Buelga, C.; Ferreira, I.C.F.R. Crataegus monogyna buds and fruits phenolic extracts: Growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC–DAD–ESI/MS. Food Res. Int. 2012, 49, 516–523. [Google Scholar] [CrossRef]
- Huang, X.X.; Zhou, C.C.; Li, L.Z.; Li, F.F.; Lou, L.L.; Li, D.M.; Ikejima, T.; Peng, Y.; Song, S.J. The cytotoxicity of 8-O-4′ neolignans from the seeds of Crataegus pinnatifida. Bioorganic Med. Chem. Lett. 2013, 23, 5599–5604. [Google Scholar] [CrossRef]
- Huang, X.-X.; Zhou, C.-C.; Li, L.-Z.; Peng, Y.; Lou, L.-L.; Liu, S.; Li, D.-M.; Ikejima, T.; Song, S.-J. Cytotoxic and antioxidant dihydrobenzofuran neolignans from the seeds of Crataegus pinnatifida. Fitoterapia 2013, 91, 217–223. [Google Scholar] [CrossRef]
- Guo, R.; Shang, X.Y.; Lv, T.M.; Yao, G.D.; Lin, B.; Wang, X.B.; Huang, X.X.; Song, S.J. Phenylpropanoid derivatives from the fruit of Crataegus pinnatifida Bunge and their distinctive effects on human hepatoma cells. Phytochemistry 2019, 164, 252–261. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Yu, L.; Gao, G. Comparisons of pharmacological effect and LD50 among four kinds of hawthorn fruit. China J. Chin. Mater. Med. 1994, 19, 454–455, 510. [Google Scholar]
- Jouad, H.; Lemhadri, A.; Maghrani, M.; Burcelin, R.; Eddouks, M. Hawthorn evokes a potent anti-hyperglycemic capacity in streptozotocin-induced diabetic rats. J. Herb. Pharmacother. 2003, 3, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Abu-Gharbieh, E.; Shehab, N.G. Therapeutic potentials of Crataegus azarolus var. eu-azarolus Maire leaves and its isolated compounds. BMC Complement. Altern. Med. 2017, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Shatoor, A.S. Acute and sub-acute toxicity of Crataegus aronia syn. azarolus (L.) whole plant aqueous extract in wistar rats. Am. J. Pharmacol. Toxicol. 2011, 6, 37–45. [Google Scholar] [CrossRef]
- Daniele, C.; Mazzanti, G.; Pittler, M.H.; Ernst, E. Adverse-Event Profile of Crataegus spp. Drug Saf. 2006, 29, 523–535. [Google Scholar] [CrossRef]
- Holubarsch, C.J.; Colucci, W.S.; Eha, J. Benefit-risk assessment of Crataegus extract WS 1442: An evidence-based review. Am. J. Cardiovasc. Drugs 2018, 18, 25–36. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Chawla, Y.K. Herbal medicines for liver diseases. Dig. Dis. Sci. 2005, 50, 1807–1812. [Google Scholar] [CrossRef]
- Girish, C.; Pradhan, S.C. Drug development for liver diseases: Focus on picroliv, ellagic acid and curcumin. Fundam. Clin. Pharmacol. 2008, 22, 623–632. [Google Scholar] [CrossRef]
- Kumari, R.; Sahu, M.K.; Tripathy, A.; Uthansingh, K.; Behera, M. Hepatocellular carcinoma treatment: Hurdles, advances and prospects. Hepatic Oncol. 2018, 5, HEP08. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, R.; Liang, Z.; Fan, A.; Kang, D. Hyperoside attenuates non-alcoholic fatty liver disease through targeting Nr4A1 in macrophages. Int. Immunopharmacol. 2021, 94, 107438. [Google Scholar] [CrossRef]
- Inamdar, S.; Joshi, A.; Malik, S.; Boppana, R.; Ghaskadbi, S. Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice. Biochem. Biophys. Res. Commun. 2019, 519, 106–112. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Yuan, X.; He, L.; Li, X.; Huang, S.; Hou, S.; Liang, J. Vitexin ameliorates chronic stress plub high fat diet-induced nonalcoholic fatty liver disease by inhibiting inflammation. Eur. J. Pharmacol. 2020, 882, 173264. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Duan, S.; Guan, T.; Yuan, X.; Lin, J.; Hou, S.; Lai, X.; Huang, S.; Du, X.; Chen, S. Vitexin protects against ethanol-induced liver injury through Sirt1/p53 signaling pathway. Eur. J. Pharmacol. 2020, 873, 173007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Feng, W.-J.; Liu, G.-C.; Ma, Q.-Q.; Li, H.-L.; Gao, X.-Y.; Liu, H.-Z.; Piao, G.-C.; Yuan, H.-D. Corosolic acid attenuates hepatic lipid accumulation and inflammatory response via AMPK/SREBPs and NF-κ B/MAPK signaling pathways. Am. J. Chin. Med. 2020, 48, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cui, Z.; Gao, X.; Liu, H.; Wang, L.; Gong, J.; Wang, A.; Zhang, J.; Ma, Q.; Huang, Y. Corosolic acid ameliorates non-alcoholic steatohepatitis induced by high-fat diet and carbon tetrachloride by regulating TGF-β1/Smad2, NF-κB, and AMPK signaling pathways. Phytother. Res. 2021, 35, 5214–5226. [Google Scholar] [CrossRef]
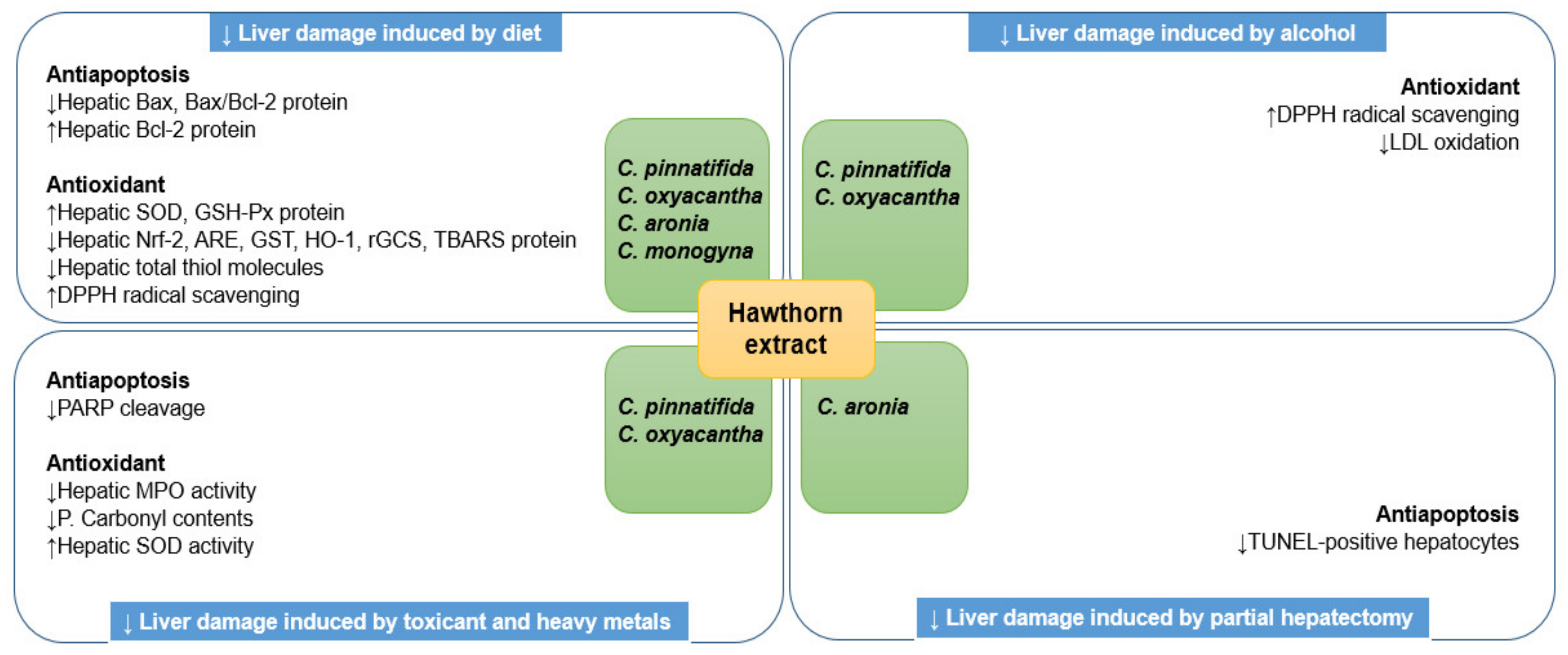
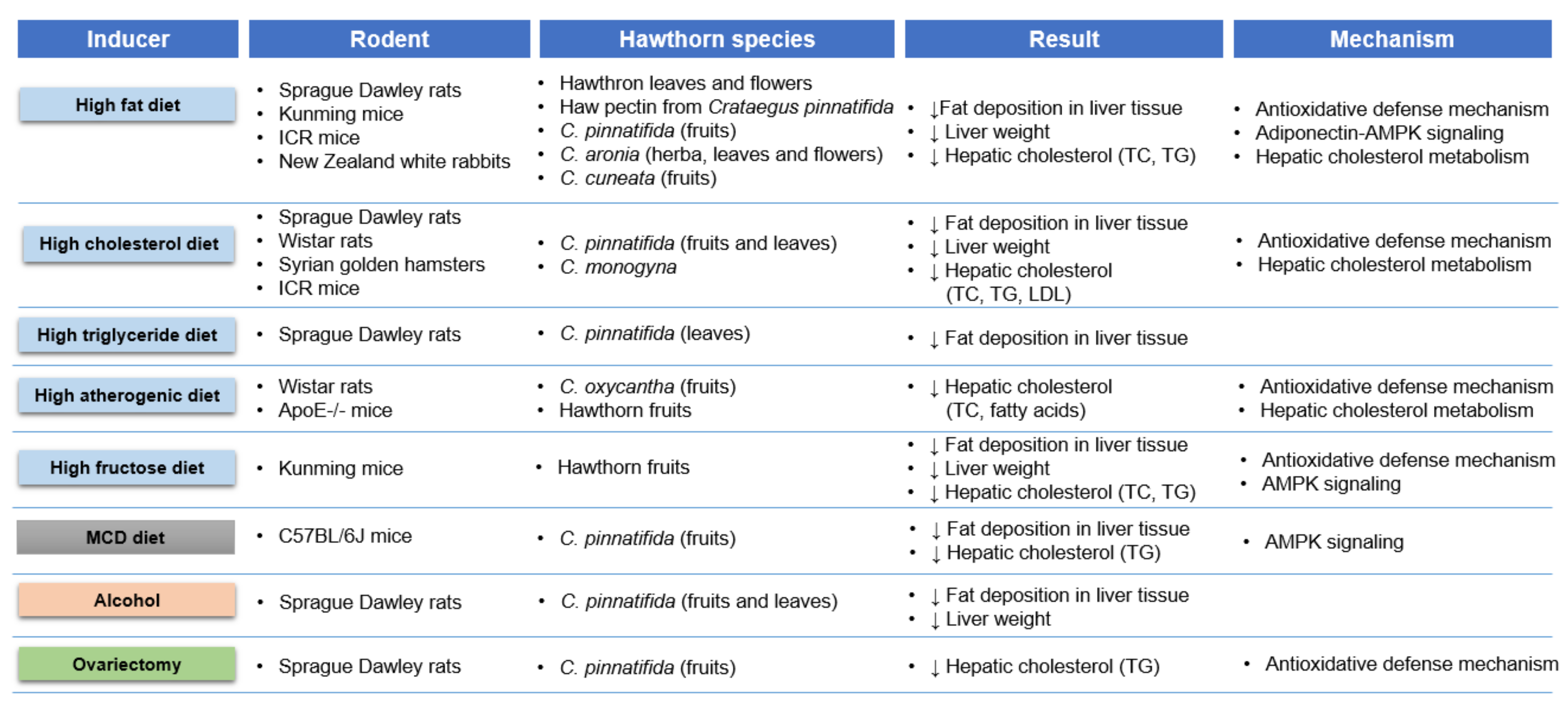
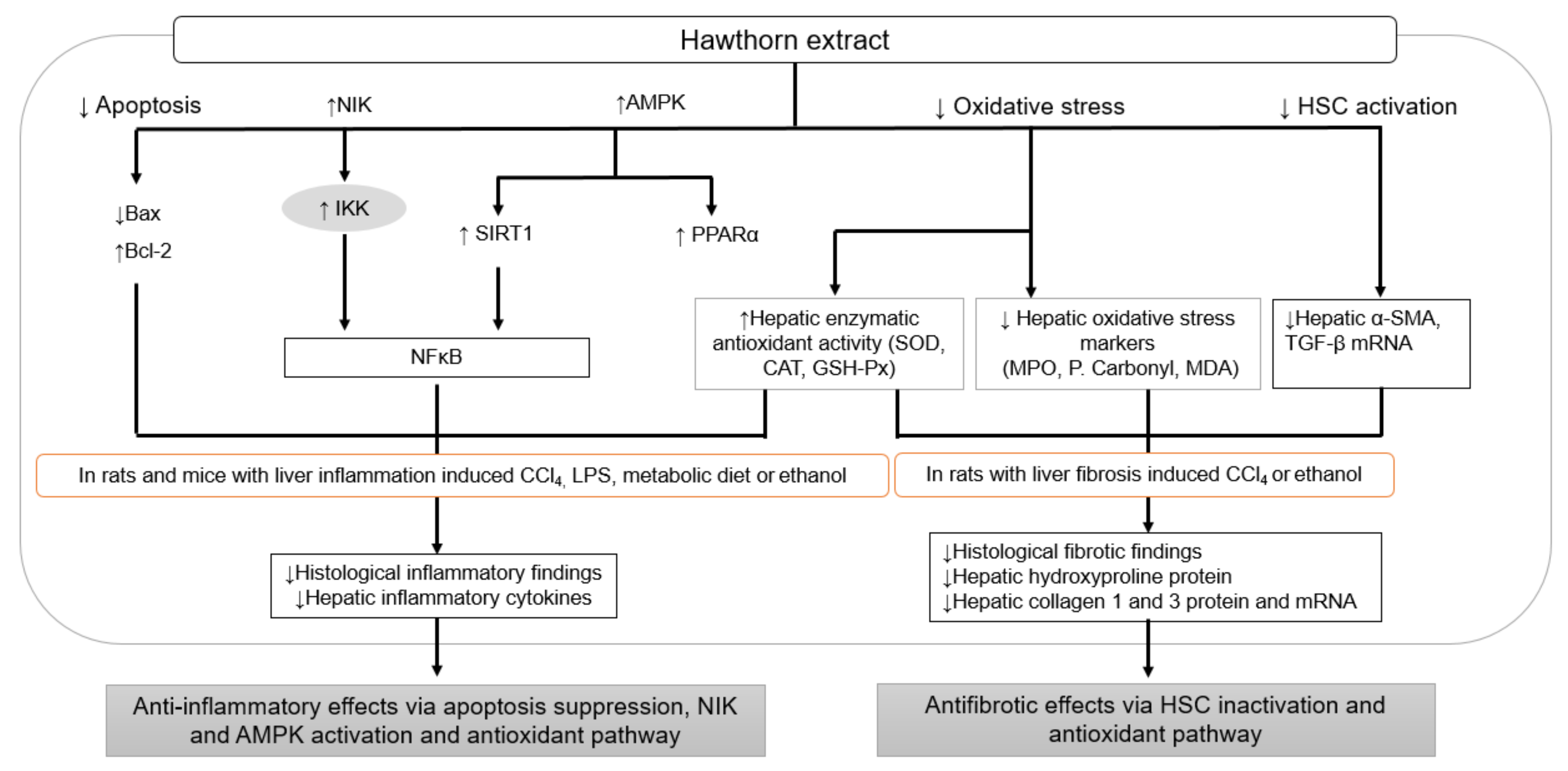

| Sources | Models | Doses | Results and Mechanisms | Reference |
|---|---|---|---|---|
| Extract of C. pinnatifida (leaves) | In vivo, male Sprague Dawley rats fed with high-fat diets | 100 mg/kg | ↓Liver damage induced by high-fat diet ↓Serum ALP, LDH | [34] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-fat diets | 10 mL/kg | ↓Liver damage induced by high-fat diet ↓Serum ALP, GGT ↓Liver pyknotic nuclei | [35] |
| Extract of hawthorn (leaves) | In vivo, male Sprague Dawley rats fed with high-fat diets | 160 mg/kg | ↓Liver damage induced by high-fat diet ↓Ballooning degeneration necrosis in liver tissue Antioxidant ↓Nrf2-positive stained hepatocytes ↑Hepatic Nrf2 mRNA ↓Hepatic GST, HO-1, rGCS mRNA and protein | [36] |
| Ethanolic extract of C. oxycantha | In vivo, male Wistar rats fed with high-fat diets | 20 mg/kg | ↓Liver damage induced by high-fat diet ↓Serum AST, ALT, GGT, ALP, total bilirubin, direct bilirubin, LDH, and MDA | [28] |
| 70% ethanol extract of C. aronia (leaves and flowers) | In vivo, male Wistar rats fed with high-fat diets | 200 mg/kg | ↓Liver damage induced by high-fat diet ↓Highly vacuolated hepatocytes ↓Damaged endoplasmic reticuli ↓Distorted intercellular spaces ↓Irregular nuclear membranes Antioxidant ↑Hepatic GSH ↓Hepatic TBARS | [37] |
| Water extract of C. aronia | In vivo, male Wistar rats fed with high-fat diets | 200 mg/kg | ↓Liver damage induced by high-fat diet ↓Serum AST, ALT, and GGT | [29] |
| 70% ethanol extract of C. monogyna | In vivo, male Wistar rats fed with high-cholesterol diet | 100 mg/kg | ↓Liver damage induced by high cholesterol diet ↓Serum AST, ALT, and GGT ↓Hepatic MDA protein ↓Highly vacuolated hepatocytes and nuclear chromatin condensation in liver tissue Antioxidant ↓Hepatic total thiol molecules ↑DPPH radical scavenging | [30] |
| 70% ethanol extract of C. pinnatifida31 (leaves) | In vivo, Sprague Dawley rats fed with high-cholesterol diet | 5, 7.5, 10 mL/kg | ↓Liver damage induced by high cholesterol diet ↓Serum AST, ALT, and GGT ↓Cell necrosis, sinusoidal distension in liver tissue | [31] |
| 80% ethanol extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed high-fat and high-cholesterol diets | 5, 10% | ↓Liver damage induced by high fat and cholesterol diet ↓Serum AST, ALT, ALP, and LDH | [32] |
| Powder from dried C. pinnatifida (leaves) | In vivo, Sprague Dawley rats fed with high-triglyceride diet | 2% | ↓Liver damage induced by high triglyceride diet ↓Serum ALT, ALP ↓Hepatocyte enlargement | [33] |
| Polyphenols from 80% ethanol extract of hawthorn peels and fleshes | In vivo, male Kunming mice fed with high-fructose diet | 400 mg/kg | ↓Liver damage induced by high-fructose diet ↓Serum AST, ALT, and ALP ↓Hepatic MDA protein ↓Hepatocyte necrosis, cytoplasmic vacuolation, cellular degeneration, and the loss of cellular boundaries in liver tissue Antiapoptosis ↓Hepatic Bax, Bax/Bcl-2 protein ↑Hepatic Bcl-2 protein (only in hawthorn peels) Antioxidant ↑Hepatic SOD, GSH-Px protein ↓Hepatic Nrf-2, ARE protein | [38] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed 25% alcohol for 55 days | 1 cc/100 g | ↓Liver damage induced by alcohol ↓Serum AST, ALT, ALP, and LDH | [39] |
| 95% ethanol extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed 50% alcohol for 6 weeks | 100 mg/kg | ↓Liver damage induced by alcohol ↓Hepatic ADH activity ↑Hepatic ALDH activity | [40] |
| 80% methanol extract of C. pinnatifida | In vitro, human hepatoma HepG2 cells induced by 1.3% ethanol | 0.4% | ↓Liver damage induced by alcohol ↑Cell viability 152.5% Antioxidant ↑DPPH radical scavenging ↓LDL oxidation | [10] |
| 70% ethanol extract of dried C. pinnatifida (branches) | In vitro, human hepatoma HepG2 cells induced by 1.3% ethanol | 0.4, 1% | ↓Liver damage induced by alcohol ↑Cell viability 136.3% ↓Liver cell DNA damage ↓CYP2E1 enzyme expression ↓Catalytic activity of CYP2E1 | [41] |
| Methanol extract of C. oxycantha (leaves) | In vivo, male Wistar rats administered with 3 g/kg/day of 35% ethanol | 50 mg/kg | ↓Liver damage induced by alcohol ↓Serum AST, ALT, GGT, ACP, and bilirubin ↑Liver glycogen ↓Hepatic MDA ↓Cell congestion, necrosis, and sinusoidal distension in liver tissue | [42] |
| 70% ethanol extract of the leaves of Crataegus pinnatifida from a local market in China | In vivo, Sprague Dawley rats fed 56% alcohol for 8 weeks | 5 mL/kg | ↓Liver damage induced by alcohol ↓Serum AST, ALT, and GGT ↓Cell necrosis, sinusoidal distension in liver tissue | [31] |
| Flavonoids from C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats injected with LPS | 50, 100, 200 mg/kg | ↓Liver damage induced by LPS ↓Serum AST, ALT ↓Extensive hepatocyte necrosis in liver tissue | [43] |
| Hawthorn capsule extracted from C. oxyacantha (leaves and flowers) | In vivo, male Wistar albino rats received an oral administration of CCl4 | 350 mg/kg | ↓Liver damage induced by toxic substances ↓Serum AST, ALT, GGT, and bilirubin ↑Serum albumin ↓Hepatic MDA, Antioxidant ↓Hepatic MPO activity ↓Hepatic P. Carbonyl activity ↑Hepatic SOD activity | [44] |
| Extract of hawthorn | In vivo, Sprague Dawley rats of both sexes received an oral administration of CCl4 | 40 mg/kg | ↓Liver damage induced by toxic substances ↓Serum AST, ALT, and ALP ↓Vacuolar degeneration in hepatocytes ↑Protein and mucopolysaccharide contents in hepatocytes | [45] |
| Water extract of C. pinnatifida (fruits) | In vitro, Rat hepatocytes H4IIE induced by cadmium | 0.1, 0.3 mg/mL | ↓Liver damage induced by heavy metals ↑Cell viability Antiapoptosis ↓PARP cleavage | [46] |
| In vivo, male Sprague Dawley rats intravenously injected with cadmium 4 mg/kg | 50, 100 mg/kg | ↓Liver damage induced by heavy metals ↓Serum AST, ALT, and LDH Hepatic degenerative regions and cells Hepatic centrolobular necrosis with peripheral hemorrhages/congestions | ||
| 70% ethyl alcohol extract of C. aronia (flowers) | In vivo, male albino rats with 50% partial hepatectomy | 0.5, 1% | ↓Liver damage induced by partial hepatectomy ↓Serum AST, ALT Antiapoptosis ↓TUNEL-positive hepatocytes | [47] |
| Sources | Models | Doses | Results and Mechanisms | Reference |
|---|---|---|---|---|
| Extract of hawthorn (leaves) | In vivo, male Sprague Dawley rats fed with high-fat diets | 160 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Fat deposition in liver tissue Antioxidant ↑Nrf2-positive stained hepatocytes ↑Hepatic Nrf2 mRNA ↓Hepatic GST, HO-1, rGCS mRNA, and protein | [36] |
| Extract of C. pinnatifida (leaves) | In vivo, male Sprague Dawley rats fed with high-fat diets | 100 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Liver weight ↓Fat deposition in liver tissue ↓Hepatic TC, TG Adiponectin/AMPK signaling ↑Serum adiponectin ↑Hepatic adiponectin receptor 2 mRNA and protein ↑Hepatic p-AMPKα protein ↓Hepatic SREBP-1c mRNA and protein ↑Hepatic PPARα mRNA and protein ↓Heptatic CD36, FAS, and SCD1 mRNA ↑Hepatic CPT1, ACO, and ACOX1 mRNA | [34] |
| Haw pectin pentaoligosaccharide from C. pinnatifida (fruits) | In vivo, male Kunming mice fed with high-fat diets | 150 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Fat deposition in liver tissue AMPK signaling ↑Hepatic CPT1, ACO mRNA ↑Hepatic PPARα mRNA and protein | [53] |
| Haw pectin from C. pinnatifida (fruits) | In vivo, male Kunming mice fed with high-fat diets | 50, 150, 300 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Hepatic TC Hepatic cholesterol and bile acid metabolism ↓Hepatic HMG-CoA reductase, ACAT mRNA, and protein ↑Hepatic cholesterol 7α-hydroxylase mRNA and protein | [54] |
| Haw pectin from C. pinnatifida (fruits) | In vivo, male Kunming mice fed with high-fat diets | 300 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Liver weight ↓Hepatic TC Hepatic cholesterol and bile acid metabolism ↓Hepatic bile acids ↑Gallbladder bile acids ↑Hepatic cholesterol 7α-hydroxylase mRNA and protein ↑Hepatic ABCA1, SR-BI, LXRα, BSEP mRNA, and protein | [55] |
| Haw pectin from C. pinnatifida (fruits) | In vivo, male Kunming mice fed with high-fat diets | 300 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Hepatic TC Hepatic cholesterol and bile acid metabolism ↓Hepatic FGFR4 mRNA and protein ↑Hepatic cholesterol 7α-hydroxylase mRNA and protein ↑Fecal bile acids ↓Hepatic bile acids | [56] |
| Haw pectin from C. pinnatifida (fruits) | In vivo, male Kunming mice fed with high-fat diets | 50, 150, 300 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Liver weight ↓Fat deposition in liver tissue Antioxidant ↑Hepatic SOD activity | [74] |
| Haw pectin from C. pinnatifida (fruits) | In vivo, male Kunming mice fed with high-fat diets | 50, 150, 300 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Hepatic TG Antioxidant ↑Hepatic SOD, CAT, and GSH-Px activity ↑Hepatic TAC, GSH levels | [75] |
| Haw pectin from C. pinnatifida (fruits) | In vivo, male Kunming mice fed with high-fat diets | 150 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Liver weight ↓Fat deposition in liver tissue ↓Hepatic TG, total lipids AMPK/SIRT1/NFκB signaling ↑Hepatic AMPKα, SIRT1 mRNA ↓Hepatic NFκB mRNA and protein | [77] |
| 70% ethanol extract of C. aronia (leaves and flowers) | In vivo, male Wistar rats fed with high-fat diets | 200 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Fat deposition in liver tissue Antioxidant ↑Hepatic GSH ↓Hepatic TBARS | [37] |
| 80% ethanol extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed high-cholesterol diet | 2% | ↓Hepatic steatosis induced by high-cholesterol diet ↓Liver weight ↓Fat deposition in liver tissue Antioxidant ↑Hepatic SOD, CAT activity | [57] |
| 70% ethanol extract of C. monogyna | In vivo, male Wistar rats fed with high-cholesterol diet | 100 mg/kg | ↓Hepatic steatosis induced by high-cholesterol diet ↓Liver/body weight ↓Hepatic TC, TG, and LDL Antioxidant ↓Hepatic total thiol molecules ↑DPPH radical scavenging | [30] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-fat diets | 397.3 mg/kg | ↓Hepatic steatosis induced by high-cholesterol diet ↓Liver weight | [58] |
| 80% ethanol extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-fat and high-cholesterol diets | 5, 10% | ↓Hepatic steatosis induced by high-fat and cholesterol diet ↓Fat deposition in liver tissue ↓Hepatic TC, TG | [32] |
| 80% ethanol extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-cholesterol diet | 100 mg/kg | ↓Hepatic steatosis induced by high-cholesterol diet ↓Hepatic lipid contents Hepatic cholesterol metabolism ↑Hepatic cholesterol 7α-hydroxylase mRNA | [59] |
| 80% ethanol extract of C. pinnatifida (fruits) | In vivo, male Syrian golden hamsters fed with high-cholesterol diet | 0.5% | ↓Hepatic steatosis induced by high-cholesterol diet ↓Hepatic FFA contents Hepatic cholesterol metabolism ↑Hepatic cholesterol 7α-hydroxylase protein | [60] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-cholesterol diet | 2% | ↓Hepatic steatosis induced by high-cholesterol diet ↓Hepatic TC, TG Hepatic cholesterol metabolism ↓Hepatic ACAT activity | [61] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-cholesterol diet | 124 mg/kg | ↓Hepatic steatosis induced by high-cholesterol diet ↓Hepatic lipid contents | [62] |
| Water extract of C. pinnatifida (fruits) | In vivo, female ICR mice fed with high-cholesterol diet | 50, 100 mg/kg | ↓Hepatic steatosis induced by high-cholesterol diet ↓Liver weight ↓Hepatic TC, TG | [63] |
| 70% ethanol extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-fat diets | 500, 1000 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Hepatic TC, TG | [64] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-fat diets | 10 mL/kg | ↓Hepatic steatosis induced by high-fat diet ↓Liver weight ↓Fat deposition in liver tissue ↓Hepatic TC, TG | [65] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-fat diets | 10 mL/kg | ↓Hepatic steatosis induced by high-fat diet ↓Fat deposition in liver tissue | [35] |
| Methanol extract of C. pinnatifida (fruits) | In vivo, female ICR mice fed with high-fat diets | 100 μg | ↓Hepatic steatosis induced by high-fat diet ↓Liver weight | [66] |
| 95% ethanol extract of C. cuneata (fruits) | In vivo, male Kunming mice fed with high-fat diet | 90, 130 mg/kg | ↓Hepatic steatosis induced by high-cholesterol diet ↓Fat deposition in liver tissue | [67] |
| Ethanol extract of C. cuneata (fruits) | In vivo, male mice fed with high-fat diet | 130 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Hepatic TC Hepatic cholesterol metabolism ↓Hepatic HMG-CoA reductase mRNA ↓Hepatic HMG-CoA reductase promoter activity ↓Hepatic NFκB p65 mRNA | [68] |
| Water extract of C. aronia (herba) | In vivo, male Wistar rats fed with high-fat diet | 200 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Liver weight ↓Fat deposition in liver tissue | [29] |
| 70% ethanol extract of C. pinnatifida (leaves) | In vivo, Sprague Dawley rats fed with high-cholesterol diet | 5, 7.5, 10 mL/kg | ↓Hepatic steatosis induced by high-cholesterol diet ↓Liver weight ↓Fat deposition in liver tissue | [31] |
| Ethanol extract of C. oxycantha | In vivo, male Wistar rats fed high-fat diet | 20 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Fat deposition in liver tissue | [28] |
| Water extract of C. aronia (fruits) | In vivo, New Zealand white rabbits fed high-fat diet | 10 mg/kg | ↓Hepatic steatosis induced by high-fat diet ↓Hepatic TC, TG, FFA, and phospholipids Hepatic cholesterol metabolism ↓Hepatic HMG-CoA reductase, ACAT activity ↑Hepatic cholesterol 7-hydroxylase activity | [69] |
| Polyphenols from 80% ethanol extracs of hawthorn peels and fleshes | In vivo, male Kunming mice fed with high-fructose diet | 400 mg/kg | ↓Hepatic steatosis induced by high-fructose diet ↓Liver weight ↓Fat deposition in liver tissue ↓Hepatic TC, TG Antioxidant ↑Hepatic SOD, GSH-Px protein ↓Hepatic Nrf-2, ARE protein AMPK signaling ↑Hepatic PPARα protein ↓Hepatic FAS protein | [38] |
| Ethanol extract of C. oxycantha (fruits) | In vivo, male Wistar rats fed atherogenic diet | 0.5 mL/100 g | ↓Hepatic steatosis induced by atherogenic diet ↓Hepatic TC Hepatic cholesterol catabolism to bile acids ↑Hepatic LDL receptors ↓Hepatic cholesterol biosynthesis ↑Hepatic bile acids ↑Fecal bile acidss | [70] |
| 80% ethanol extract of hawthorn (fruits) | In vivo, ApoE−/− mice fed atherogenic diet | 2% | ↓Hepatic steatosis induced by atherogenic diet ↓Hepatic TC ↓Hepatic fatty acids Antioxidant ↑Hepatic TAC protein ↑Hepatic SOD mRNA and protein ↑Hepatic GSH-Px mRNA ↑Hepatic CAT mRNA and protein | [71] |
| 70% ethanol extract of C. pinnatifida (fruits) | In vivo, male C57BL/6J mice fed MCD diet | 300 mg/kg | ↓Hepatic steatosis induced by MCD diet ↓Fat deposition in liver tissue ↓Hepatic TG AMPK signaling ↑Hepatic p-AMPKα protein ↓Hepatic SREBP-1c, C/EBPα, and PPARγ protein ↓Hepatic ACC, FAS protein | [72] |
| 70% ethanol extract of C. pinnatifida (fruits) | In vivo, female ovariectomized Sprague Dawley rats | 100, 200 mg/kg | ↓Hepatic steatosis after ovariectomy ↓Hepatic TG Antioxidant ↑Hepatic Nrf2 mRNA and protein ↑Hepatic HO-1 mRNA and protein ↑Hepatic GSH-Px mRNA and protein ↑Hepatic CAT mRNA and protein | [73] |
| Water extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed 25% alcohol for 55 days | 1 cc/100 g | ↓Hepatic steatosis induced by alcohol ↓Liver weight | [39] |
| 70% ethanol extract of C. pinnatifida (leaves) | In vivo, Sprague Dawley rats fed 56% alcohol for 8 weeks | 5 mL/kg | ↓Hepatic steatosis induced by alcohol ↓Liver weight ↓Fat deposition in liver tissue | [31] |
| Sources | Models | Doses | Results and Mechanisms | Reference |
|---|---|---|---|---|
| Hawthorn capsule extracted from C. oxyacantha (leaves and flowers) | In vivo, male Wistar albino rats orally administered with CCl4 | 350 mg/kg | ↓Hepatic inflammation induced by CCl4 ↓Hepatic IL-1β, TNF-α mRNA ↓Hepatic COX-2 mRNA Antioxidant ↓Hepatic MPO activity ↓Hepatic P. carbonyl contents ↑Hepatic SOD activity NFκB signaling ↓Hepatic NFκB mRNA | [44] |
| Flavonoids from C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats injected with LPS | 50, 100, 200 mg/kg | ↓Hepatic inflammation induced by LPS ↓Neutrophil leukocyte infiltration in liver tissue ↓Hepatic expression of iNOS and COX-2 | [43] |
| 80% ethanol extract of C. pinnatifida (fruits) | In vivo, male Sprague Dawley rats fed with high-cholesterol diets | 2% | ↓Hepatic inflammation induced by high-cholesterol diet ↓Hepatic expression of NOS Antioxidant ↑Hepatic SOD, CAT activity | [57] |
| 70% ethanol extract of C. monogyna | In vivo, male Wistar rats fed with high-cholesterol diets | 100 mg/kg | ↓Hepatic inflammation induced by high-cholesterol diet ↓Inflammatory cells infiltration in liver tissue Antioxidant ↓Hepatic total thiol molecules ↑DPPH radical scavenging | [30] |
| 70% ethanol extract of C. pinnatifida (leaves) | In vivo, Sprague Dawley rats fed with high-cholesterol diets | 5, 7.5, 10 mL/kg | ↓Hepatic inflammation induced by high cholesterol diet ↓Inflammatory cells infiltration in liver tissue | [31] |
| Powder from dried C. pinnatifida (leaves) | In vivo, Sprague Dawley rats fed with high-triglyceride diets | 2% | ↓Hepatic inflammation induced by high triglyceride diet ↓Mononuclear inflammatory cells around the hepatic blood vessels | [33] |
| Haw pectin from the water extract of C. pinnatifida | In vivo, male Kunming mice fed with high-fat diets | 150 mg/kg | ↓Hepatic inflammation induced by high-fat diet ↓Hepatic TNF-α, IL-6 contents ↑Hepatic IL-10 contents NIK/IKK/NFκB signaling ↓Hepatic RIP1, NIK, IKKα, TNFα, TNFR1, and TRAF2 mRNA ↓Hepatic NFκB mRNA and protein AMPK/SIRT1/NFκB signaling ↑Hepatic AMPKα, SIRT1 mRNA ↓Hepatic NFκB mRNA and protein | [77] |
| Polyphenols from the ethanol extract of C. pinnatifida (fruits) | In vivo, male Wistar rats fed with high-fat diets and streptozotocin | 300 mg/kg | ↓Hepatic inflammation induced by high-fat diet and streptozotocin ↓Inflammatory cells infiltration in liver tissue ↓Hepatic TNF-α, MCP-1, and IL-6 protein AMPK/SIRT1/NFκB signaling ↑Hepatic AMPKα, PPARδ, and SIRT1 protein ↓NFκB p65 protein | [82] |
| Water extract of hawthorn fruits and leaves | In vivo, male Sprague Dawley rats fed with high-fat diets | 1 mg/100 g | ↓Hepatic inflammation induced by high-fat diet ↓Inflammatory cells infiltration in liver tissue | [35] |
| 80% ethanol extract of hawthorn (fruits) | In vivo, ApoE−/− mice fed with atherogenic diets | 2% | ↓Hepatic inflammation induced by atherogenic diet ↓Hepatic MCP-1, TNF-α, IL-1β, IL-6, and IL-10 mRNA and protein Antioxidant ↑Hepatic TAC protein ↑Hepatic SOD mRNA and protein ↑Hepatic GSH-Px mRNA ↑Hepatic CAT mRNA and protein | [71] |
| Polyphenols from 80% ethanol extract of hawthorn peels and fleshes | In vivo, male Kunming mice fed with high-fructose diets | 400 mg/kg | ↓Hepatic inflammation induced by high-fructose diet ↓Hepatic expression of IL-1, IL-6, and TNF-α Antiapoptosis ↓Hepatic Bax, Bax/Bcl-2 protein ↑Hepatic Bcl-2 protein (only in hawthorn peels) Antioxidant ↓Hepatic MDA protein ↑Hepatic SOD, GSH-Px protein ↓Hepatic Nrf-2, ARE protein AMPK signaling ↑Hepatic PPARα protein | [38] |
| 70% ethanol extract of C. pinnatifida (leaves) | In vivo, Sprague Dawley rats fed with 56% alcohol for 8 weeks | 5 mL/kg | ↓Hepatic inflammation induced by alcohol ↓Inflammatory cells infiltration in liver tissue | [31] |
| Extract of hawthorn | In vivo, Sprague Dawley rats orally administered with CCl4 | 40 mg/kg | ↓Liver fibrosis induced by toxic substances ↓Marked fibrosis around the main blood vessels in liver tissue | [45] |
| Hawthorn capsule extracted from C. oxyacantha (leaves and flowers) | In vivo, male Wistar albino rats orally administered with CCl4 | 350 mg/kg | ↓Liver fibrosis induced by toxic substances ↓Hepatic fibrotic septa ↓Severity score of Masson ↓Masson-positive area ↓Hepatic hydroxyproline protein ↓Hepatic collagen 1 and 3 protein and mRNA HSC inactivation ↓Hepatic α-SMA-positive cells ↓Hepatic α-SMA mRNA ↓Hepatic TGF-β mRNA Antioxidant ↓Liver MPO activity ↓Liver P. carbonyl contents ↑Liver SOD activity | [44] |
| Methanol extract of C. oxycantha (leaves) | In vivo, male Wistar rats administered with 3 g/kg/day of 35% ethanol | 50 mg/kg | ↓Liver fibrosis induced by alcohol ↓Moderate fibrosis near lobule central veins in liver tissue Antioxidant ↓Hepatic MDA | [42] |
| Sources | Models | Doses | Results and Mechanisms | Reference |
|---|---|---|---|---|
| Ethanol extract of C. pinnatifida (fruits) | In vitro, human hepatoma HepG2 cells | 0.8 mg/mL | ↓Cell viability Apoptosis induction ↑Caspase-3 mRNA ↓Bcl-2 mRNA and protein ↑Bax mRNA and protein | [86] |
| 80% ethanol extract of C. monogyna (buds and fruits) | In vitro, human hepatoma HepG2 cells | 0.5, 1 mg/mL | ↓Cell viability | [87] |
| Triterpenoids from 80% acetone extract of C. pinnatifida (fruits) | In vitro, human hepatoma HepG2 cells | ↓Cell viability (IC50 < 5 μM) | [88] | |
| Phenylpropanoids from 70% ethanol extract of C. pinnatifida (fruits) | In vitro, human hepatoma HepG2 and Hep3B cells | 100 μM. | ↓Cell viability (IC50: 17.5–27.36 μM in HepG2 and 38.96–43.58 μM in Hep3B) Apoptosis induction | [89] |
| Phenylpropanoids from 70% ethanol extract of C. pinnatifida (fruits) | In vitro, human hepatoma HepG2 and Hep3B cells | 50 μM | ↓Cell viability Apoptosis induction ↑Apoptotic cells Autophagy induction Monodansylcadaverine positive cells Cell cycle arrest G2/M arrest by (−)-crataegusanoid A G0/G1 arrest by (−)-crataegusanoid B | [94] |
| Lignans from 70% ethanol extract of C. pinnatifida (seeds) | In vitro, human hepatoma HepG2 cells | ↓Cell viability (IC50: 38.54–39.97 μM) | [92] | |
| 80% methanol extract of C. monogyna (buds and fruits) | In vitro, human hepatoma HepG2 cells | ↓Cell viability | [91] | |
| 80% acetone extract of C. pinnatifida obtained from Shandong Institue of (fruits) | In vitro, human hepatoma HepG2 cells | 5–30 μg/ml | ↓Cell viability (IC50: 11.58 μg/mL) ↓Cell proliferation IC50 of ursolic acid 12.58 μM IC50 of corosolic acid 9.44 μM IC50 of maslinic acid 23.42 μM IC50 of oleanolic acid 54.02 μM | [20] |
| Dihydrobenzofuran neolignan from 70% ethanol extract of C. pinnatifida (seeds) | In vitro, human hepatoma HepG2 cells | ↓Cell viability (IC50 = 30.96 μM) | [93] | |
| 80% ethanol extract of of C. armena (shoots, flowers, and fruits) | In vitro, human hepatoma HepG2 cells | ↓Cell viability (IC50 = 8.66 μg/mL) | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Jang, E.; Lee, J.-H. Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases. Nutrients 2022, 14, 867. https://doi.org/10.3390/nu14040867
Kim E, Jang E, Lee J-H. Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases. Nutrients. 2022; 14(4):867. https://doi.org/10.3390/nu14040867
Chicago/Turabian StyleKim, Eujin, Eungyeong Jang, and Jang-Hoon Lee. 2022. "Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases" Nutrients 14, no. 4: 867. https://doi.org/10.3390/nu14040867
APA StyleKim, E., Jang, E., & Lee, J.-H. (2022). Potential Roles and Key Mechanisms of Hawthorn Extract against Various Liver Diseases. Nutrients, 14(4), 867. https://doi.org/10.3390/nu14040867