The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Review of the Literature
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lewkowitz, A.K.; Gupta, A.; Simon, L.; Sabol, B.A.; Stoll, C.; Cooke, E.; Rampersad, R.A.; Tuuli, M.G. Intravenous compared with oral iron for the treatment of iron-deficiency anemia in pregnancy: A systematic review and meta-analysis. J. Perinatol. 2019, 39, 519–532. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Anemia in Pregnancy: ACOG Practice Bulletin, Number 233. Obstet. Gynecol. 2021, 138, e55–e64. [Google Scholar] [CrossRef] [PubMed]
- James, A.H. Iron Deficiency Anemia in Pregnancy. Obstet. Gynecol. 2021, 138, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Turawa, E.; Awotiwon, O.; Dhansay, M.A.; Cois, A.; Labadarios, D.; Bradshaw, D.; Wyk, V.P.-V. Prevalence of Anaemia, Iron Deficiency, and Iron Deficiency Anaemia in Women of Reproductive Age and Children under 5 Years of Age in South Africa (1997–2021): A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12799. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Abernathy, J.; Juul, S.; Short, V.; Derman, R. Prevalence of iron deficiency in first trimester, non-anemic pregnant women. J. Matern. Fetal Neonatal Med. 2021, 34, 1002–1005. [Google Scholar] [CrossRef]
- Coad, J.; Conlon, C. Iron deficiency in women. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Loy, S.L.; Lim, L.M.; Chan, S.-Y.; Tan, P.T.; Chee, Y.L.; Quah, P.L.; Chan, J.K.Y.; Tan, K.H.; Yap, F.; Godfrey, K.M.; et al. Iron status and risk factors of iron deficiency among pregnant women in Singapore: A cross-sectional study. BMC Public Health 2019, 19, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, T.; Lambourne, J.; Thorp-Jones, D.; Thomas, D. Implementation of early management of iron deficiency in pregnancy during the SARS-CoV-2 pandemic. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 258, 60–62. [Google Scholar] [CrossRef]
- Vandermeulen, H.; Strauss, R.; Lin, Y.; McLeod, A.; Barrett, J.; Sholzberg, M.; Callum, J. The contribution of iron deficiency to the risk of peripartum transfusion: A retrospective case control study. BMC Pregnancy Childbirth 2020, 20, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Fernandez, J.; Ochoa, J.J.; Latunde-Dada, G.O.; Diaz-Castro, J. Iron Deficiency and Iron Homeostasis in Low Birth Weight Preterm Infants: A Systematic Review. Nutrients 2019, 11, 1090. [Google Scholar] [CrossRef] [Green Version]
- Abu-Ouf, N.M.; Jan, M.M. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med. J. 2015, 36, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Garzon, S.; Cacciato, P.M.; Certelli, C.; Salvaggio, C.; Magliarditi, M.; Rizzo, G. Iron Deficiency Anemia in Pregnancy: Novel Approaches for an Old Problem. Oman Med. J. 2020, 35, e166. [Google Scholar] [CrossRef]
- Karakoc, G.; Orgul, G.; Sahin, D.; Yucel, A. Is every other day iron supplementation effective for the treatment of the iron deficiency anemia in pregnancy? J. Matern. Neonatal Med. 2021, 35, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Adenan, A.M.; Ali, A.; Ismail, N.A.S. Living through the COVID-19 Pandemic: Impact and Lessons on Dietary Behavior and Physical Well-Being. Int. J. Environ. Res. Public Health 2022, 19, 642. [Google Scholar] [CrossRef] [PubMed]
- Suwalska, J.; Napierała, M.; Bogdański, P.; Łojko, D.; Wszołek, K.; Suchowiak, S.; Suwalska, A. Perinatal Mental Health during COVID-19 Pandemic: An Integrative Review and Implications for Clinical Practice. J. Clin. Med. 2021, 10, 2406. [Google Scholar] [CrossRef]
- Citu, C.; Neamtu, R.; Sorop, V.-B.; Horhat, D.I.; Gorun, F.; Tudorache, E.; Gorun, O.M.; Boarta, A.; Tuta-Sas, I.; Citu, I.M. Assessing SARS-CoV-2 Vertical Transmission and Neonatal Complications. J. Clin. Med. 2021, 10, 5253. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Bialy, A.I.; Kołomańska-Bogucka, D.; Tim, S.; Opławski, M. Pregnancy and Childbirth in the COVID-19 Era—The Course of Disease and Maternal–Fetal Transmission. J. Clin. Med. 2020, 9, 3749. [Google Scholar] [CrossRef]
- Citu, C.; Gorun, F.; Motoc, A.; Sas, I.; Gorun, O.M.; Burlea, B.; Tuta-Sas, I.; Tomescu, L.; Neamtu, R.; Malita, D.; et al. The Predictive Role of NLR, d-NLR, MLR, and SIRI in COVID-19 Mortality. Diagnostics 2022, 12, 122. [Google Scholar] [CrossRef]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
- Timircan, M.; Bratosin, F.; Vidican, I.; Suciu, O.; Tirnea, L.; Avram, V.; Marincu, I. Exploring Pregnancy Outcomes Associated with SARS-CoV-2 Infection. Medicina 2021, 57, 796. [Google Scholar] [CrossRef]
- Citu, C.; Gorun, F.; Motoc, A.; Sas, I.; Gorun, O.M.; Burlea, B.; Serban, D.M.; Neamtu, R.; Citu, I.M. Hysteroscopy as a Primary Tool in Exploration and Treatment of Infertility: Single Center Experience in Western Romania. Diagnostics 2021, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Timircan, M.; Bratosin, F.; Vidican, I.; Suciu, O.; Turaiche, M.; Bota, A.V.; Mitrescu, S.; Marincu, I. Coping Strategies and Health-Related Quality of Life in Pregnant Women with SARS-CoV-2 Infection. Medicina 2021, 57, 1113. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Myers, B.; Robinson, S.; Allard, S.; Strong, J.; Oppenheimer, C.; on behalf of the British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2012, 156, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynecol. Obstet. 2015, 131, S213–S253. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, G.; de Andreis, F.B.; Aronico, N.; Lenti, M.V.; Barteselli, C.; Merli, S.; Pellegrino, I.; Coppola, L.; Cremonte, E.M.; Croce, G.; et al. Anemia in patients with Covid-19: Pathogenesis and clinical significance. Clin. Exp. Med. 2021, 21, 239–246. [Google Scholar] [CrossRef]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Quadretti, L.; Fogato, L.; Zuliani, G.; Roncon, L. Prognostic Role of Anemia in COVID-19 Patients: A Meta-Analysis. Infect. Dis. Rep. 2021, 13, 930–937. [Google Scholar] [CrossRef]
- Al-Jarallah, M.; Rajan, R.; Al Saber, A.; Pan, J.; Al-Sultan, A.T.; Abdelnaby, H.; Alroomi, M.; Dashti, R.; Aboelhassan, W.; Almutairi, F.; et al. In-hospital mortality in SARS-CoV-2 stratified by hemoglobin levels: A retrospective study. eJHaem 2021, 2, 335–339. [Google Scholar] [CrossRef]
- Bonnesen, B.; Jensen, J.-U.S.; Jeschke, K.N.; Mathioudakis, A.G.; Corlateanu, A.; Hansen, E.F.; Weinreich, U.M.; Hilberg, O.; Sivapalan, P. Management of COVID-19-Associated Acute Respiratory Failure with Alternatives to Invasive Mechanical Ventilation: High-Flow Oxygen, Continuous Positive Airway Pressure, and Noninvasive Ventilation. Diagnostics 2021, 11, 2259. [Google Scholar] [CrossRef]
- Covali, R.; Socolov, D.; Socolov, R.; Pavaleanu, I.; Carauleanu, A.; Akad, M.; Boiculese, V.L.; Adam, A.M. Complete Blood Count Peculiarities in Pregnant SARS-CoV-2-Infected Patients at Term: A Cohort Study. Diagnostics 2021, 12, 80. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm. Rep. 1998, 47, 1–29. [Google Scholar]
- World Health Organization. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. Available online: http://repository.poltekkes-kaltim.ac.id/1183/1/22.%20Dairy%20iron%20and%20folic%20acid%20supplementation%20in%20pregnant%20women.pdf (accessed on 6 January 2022).
- Stoffel, N.U.; von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B. Oral iron supplementation in iron-deficient women: How much and how often? Mol. Asp. Med. 2020, 75, 100865. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2021, 32, e2261. [Google Scholar] [CrossRef]
- Muñoz, M.; Peña-Rosas, J.P.; Robinson, S.; Milman, N.; Holzgreve, W.; Breymann, C.; Goffinet, F.; Nizard, J.; Christory, F.; Samama, C.-M.; et al. Patient blood management in obstetrics: Management of anaemia and haematinic deficiencies in pregnancy and in the post-partum period: NATA consensus statement. Transfus. Med. 2017, 28, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Kemper, A.R.; Fan, T.; Grossman, D.C.; Phipps, M.G. Gaps in evidence regarding iron deficiency anemia in pregnant women and young children: Summary of US Preventive Services Task Force recommendations. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1555S–1558S. [Google Scholar] [CrossRef]
- Marcewicz, L.H.; Anderson, B.L.; Byams, V.R.; Grant, A.M.; Schulkin, J. Screening and Treatment for Iron Deficiency Anemia in Women: Results of a Survey of Obstetrician-Gynecologists. Matern. Child Health J. 2017, 21, 1627–1633. [Google Scholar] [CrossRef] [Green Version]
- Scholz, R.; Young, D.; Scavone, B.; Hofer, J.; Siddiqui, M. Anemia in Pregnancy and Risk of Blood Transfusion [11C]. Obstet. Gynecol. 2018, 131, 33S. [Google Scholar] [CrossRef]

| Variables * | COVID-19 Positive (n = 95) | COVID-19 Negative (n = 351) | p |
|---|---|---|---|
| Maternal characteristics | |||
| Age | 0.269 | ||
| <25 | 8 (8.4%) | 44 (12.5%) | |
| 25–34 | 65 (68.4%) | 247 (70.4%) | |
| >34 | 22 (23.2%) | 60 (17.1%) | |
| Gravidity | 0.826 | ||
| 1 | 49 (51.6%) | 171 (48.7%) | |
| 2 | 29 (30.5%) | 108 (30.8%) | |
| ≥3 | 17 (17.9%) | 72 (20.5%) | |
| Parity | 0.296 | ||
| 1 | 56 (58.9%) | 189 (53.8%) | |
| 2 | 32 (33.7%) | 116 (33.0%) | |
| ≥3 | 7 (7.4%) | 46 (13.1%) | |
| Complications | |||
| Anemia | 40 (42.1%) | 103 (29.3%) | 0.018 |
| Gestational hypertension | 4 (4.2%) | 12 (3.4%) | 0.712 |
| Gestational diabetes mellitus | 5 (5.3%) | 20 (5.7%) | 0.870 |
| PROM | 10 (10.5%) | 22 (6.3%) | 0.153 |
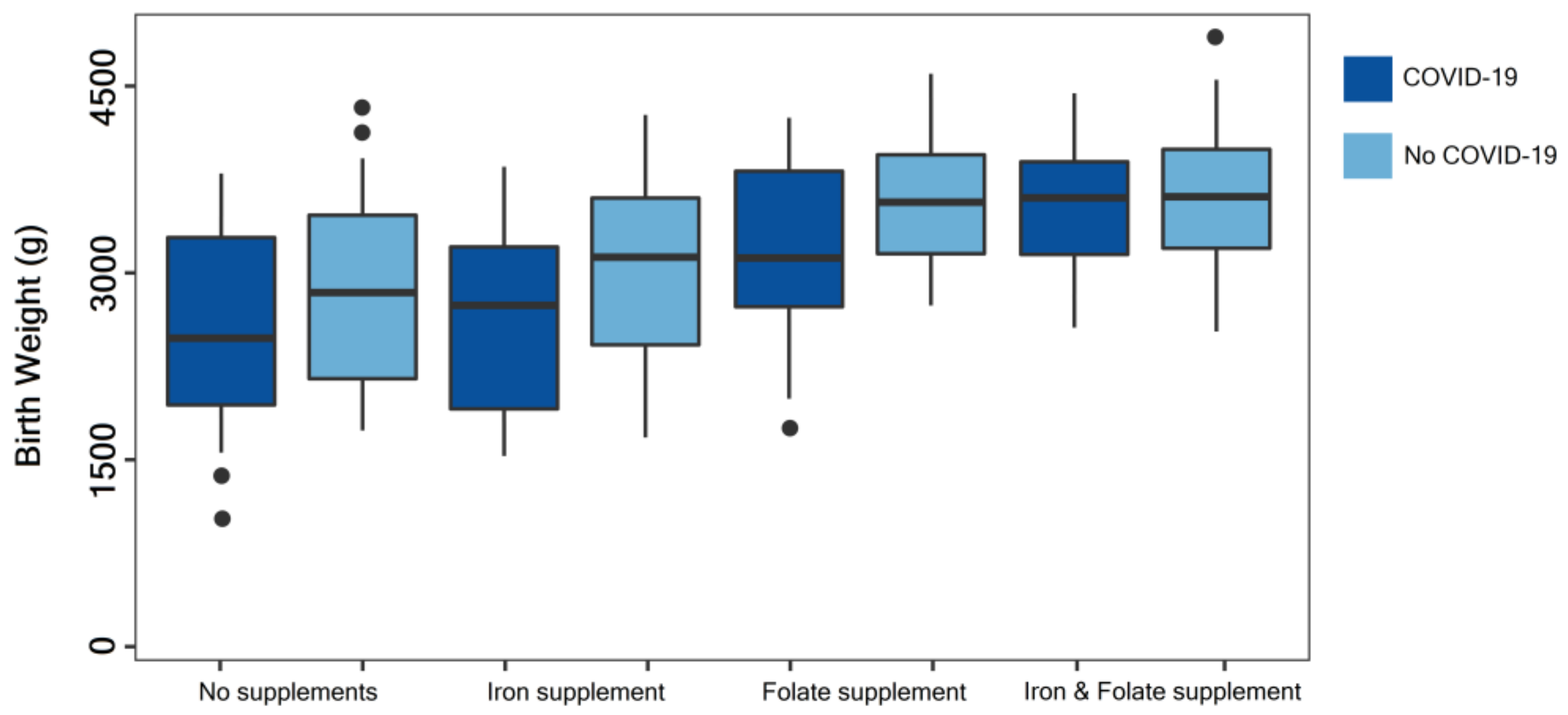
| Nutritional supplementation | 0.009 | ||
| No Iron/Folic Acid | 18 (18.9%) | 92 (26.2%) | |
| Iron | 17 (17.9%) | 98 (27.9%) | |
| Folic Acid | 10 (10.6%) | 42 (12.0%) | |
| Iron and Folic Acid | 50 (52.6%) | 119 (33.9%) | |
| Neonatal characteristics | |||
| Complications | |||
| Anemia | 30 (31.6%) | 76 (21.7%) | 0.043 |
| Puerperal infection | 7 (7.4%) | 18 (5.3%) | 0.399 |
| Premature birth | 14 (14.7%) | 28 (7.9%) | 0.045 |
| Malformations | 2 (2.1%) | 6 (1.7%) | 0.796 |
| Birth weight | 0.027 | ||
| <1500 g | 4 (4.2%) | 5 (1.4%) | |
| 1500–2500 g | 15 (15.8%) | 31 (8.8%) | |
| >2500 g | 76 (80.0%) | 315 (89.7%) | |
| APGAR score | 0.029 | ||
| ≥9 | 75 (78.8%) | 308 (87.7%) | |
| 7–8 | 10 (10.6%) | 29 (8.3%) | |
| ≤6 | 10 (10.6%) | 14 (4.0%) |
| Laboratory Data * | Normal Range | COVID-19 Positive (n = 40) | COVID-19 Negative (n = 103) | p |
|---|---|---|---|---|
| RBC (millions/mm3) | 4.0–5.0 | 3.4 ± 1.6 | 3.8 ± 1.2 | 0.012 |
| Platelets (thousands/mm3) | 150–450 | 168 ± 31 | 171 ± 36 | 0.643 |
| WBC (thousands/mm3) | 4.0–10.0 | 12.6 ± 4.8 | 7.5 ± 2.2 | <0.001 |
| Hemoglobin (g/dL) | 11.5–14.0 | 10.1 ± 2.9 | 11.0 ± 2.1 | 0.041 |
| Hematocrit (g/dL) | 35–44 | 33.4 ± 5.3 | 34.7 ± 4.6 | 0.148 |
| Mean Corpuscular Volume (fL) | 80–96 | 88.7 ± 9.5 | 87.2 ± 9.0 | 0.379 |
| Ferritin (ng/mL) | 30–150 | 21.4 ± 4.2 | 23.3 ± 4.6 | 0.024 |
| Sideremia (µg/dL) | 50–170 | 42.8 ± 6.2 | 45.3 ± 5.9 | 0.026 |
| Transferrin (saturation %) | 15–45 | 12.3 ± 2.5 | 13.8 ± 2.0 | <0.001 |
| Fe (μmol/L) | 10–30 | 7.6 ± 2.1 | 8.8 ± 2.3 | 0.004 |
| Reticulocyte count (%) | 0.5–2.5 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.008 |
| Serum folate (nmol/L) | 10–45 | 15.7 ± 3.1 | 16.2 ± 3.0 | 0.376 |
| Total iron-binding capacity (µg/dL) | 41–73 | 68.0 ± 9.1 | 65.4 ± 8.6 | 0.112 |
| Haptoglobin (g/L) | 0.3–2.0 | 3.3 ± 0.4 | 0.5 ± 0.1 | <0.001 |
| Variables * | Total (n = 95) | Anemia (n = 40) | No Anemia (n = 55) | p |
|---|---|---|---|---|
| Maternal outcomes | ||||
| Severe COVID-19 | 5 (5.3%) | 3 (7.5%) | 2 (3.6%) | 0.405 |
| Puerperal infection | 7 (26.3%) | 6 (15.0%) | 1 (1.8%) | 0.015 |
| Postpartum hemorrhage | 18 (18.9%) | 10 (25.0%) | 8 (14.5%) | 0.199 |
| Antepartum hemorrhage | 18 (18.9%) | 9 (22.5%) | 9 (16.4%) | 0.451 |
| Transfusion necessity | 5 (5.3%) | 4 (10.0%) | 1 (1.8%) | 0.077 |
| Abnormal placentation | 9 (9.5%) | 4 (10.0%) | 5 (9.1%) | 0.881 |
| PROM ** | 10 (10.5%) | 7 (17.5%) | 3 (5.4%) | 0.058 |
| Gestational hypertension | 4 (4.2%) | 3 (7.5%) | 1 (1.8%) | 0.173 |
| Gestational diabetes mellitus | 5 (5.3%) | 4 (10.0%) | 1 (1.8%) | 0.077 |
| Pre-eclampsia | 4 (4.2%) | 2 (5.0%) | 2 (3.6%) | 0.743 |
| Emergency c-section | 27 (28.4%) | 17 (42.6%) | 10 (18.2%) | 0.009 |
| Neonatal outcomes | ||||
| Small for gestational age | 22 (23.2%) | 14 (35.0%) | 8 (14.5%) | 0.019 |
| Low birth weight | 19 (20.0%) | 11 (27.5%) | 8 (14.5%) | 0.119 |
| Prematurity | 14 (14.7%) | 9 (22.5%) | 5 (9.1%) | 0.068 |
| Sepsis | 5 (5.3%) | 3 (7.5%) | 2 (3.6%) | 0.405 |
| Low APGAR score (<7) | 10 (10.5%) | 6 (15.0%) | 4 (7.3%) | 0.225 |
| Variables * | COVID-19 Positive | COVID-19 Negative | ||
|---|---|---|---|---|
| r | p | r | p | |
| Nutritional supplementation | ||||
| No Iron/Folic Acid | 0.229 | 0.148 | 0.204 | 0.319 |
| Iron | −0.310 | 0.033 | −0.248 | 0.127 |
| Folic Acid | −0.243 | 0.106 | −0.255 | 0.202 |
| Iron and Folic Acid | −0.646 | 0.005 | −0.410 | 0.024 |
| Maternal outcomes | ||||
| Severe COVID−19 | 0.332 | 0.044 | − | − |
| Puerperal infection | 0.508 | 0.002 | 0.426 | 0.017 |
| Postpartum hemorrhage | 0.204 | 0.313 | 0.148 | 0.352 |
| Antepartum hemorrhage | 0.197 | 0.390 | 0.124 | 0.479 |
| Transfusion necessity | 0.691 | 0.001 | 0.662 | 0.001 |
| Abnormal placentation | 0.301 | 0.446 | 0.347 | 0.320 |
| PROM ** | 0.374 | 0.098 | 0.355 | 0.127 |
| Gestational hypertension | 0.126 | 0.670 | 0.134 | 0.558 |
| Gestational diabetes mellitus | 0.108 | 0.642 | 0.128 | 0.643 |
| Pre-eclampsia | 0.214 | 0.573 | 0.246 | 0.540 |
| Emergency c−section | 0.497 | 0.038 | 0.366 | 0.192 |
| Neonatal outcomes | ||||
| Small for gestational age | 0.418 | 0.045 | 0.338 | 0.173 |
| Birth weight | −0.525 | 0.001 | −0.386 | 0.010 |
| Prematurity | 0.429 | 0.040 | 0.347 | 0.095 |
| Sepsis | 0.321 | 0.127 | 0.304 | 0.144 |
| APGAR score | −0.431 | 0.008 | −0.315 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uta, M.; Neamtu, R.; Bernad, E.; Mocanu, A.G.; Gluhovschi, A.; Popescu, A.; Dahma, G.; Dumitru, C.; Stelea, L.; Citu, C.; et al. The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection. Nutrients 2022, 14, 836. https://doi.org/10.3390/nu14040836
Uta M, Neamtu R, Bernad E, Mocanu AG, Gluhovschi A, Popescu A, Dahma G, Dumitru C, Stelea L, Citu C, et al. The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection. Nutrients. 2022; 14(4):836. https://doi.org/10.3390/nu14040836
Chicago/Turabian StyleUta, Mihaela, Radu Neamtu, Elena Bernad, Adelina Geanina Mocanu, Adrian Gluhovschi, Alin Popescu, George Dahma, Catalin Dumitru, Lavinia Stelea, Cosmin Citu, and et al. 2022. "The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection" Nutrients 14, no. 4: 836. https://doi.org/10.3390/nu14040836
APA StyleUta, M., Neamtu, R., Bernad, E., Mocanu, A. G., Gluhovschi, A., Popescu, A., Dahma, G., Dumitru, C., Stelea, L., Citu, C., Bratosin, F., & Craina, M. (2022). The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection. Nutrients, 14(4), 836. https://doi.org/10.3390/nu14040836








