Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease
Abstract
1. Introduction
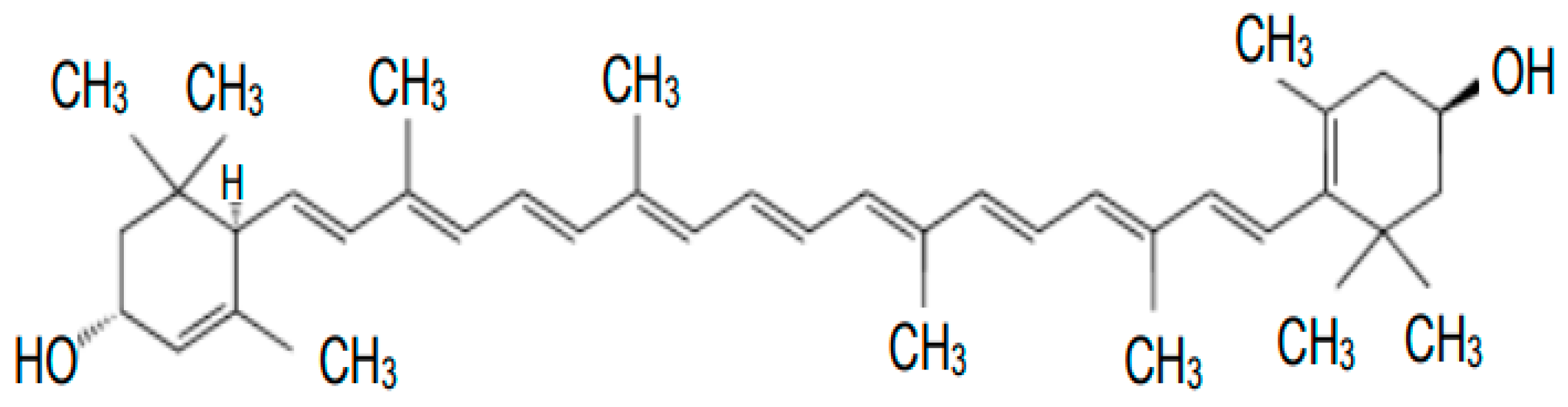
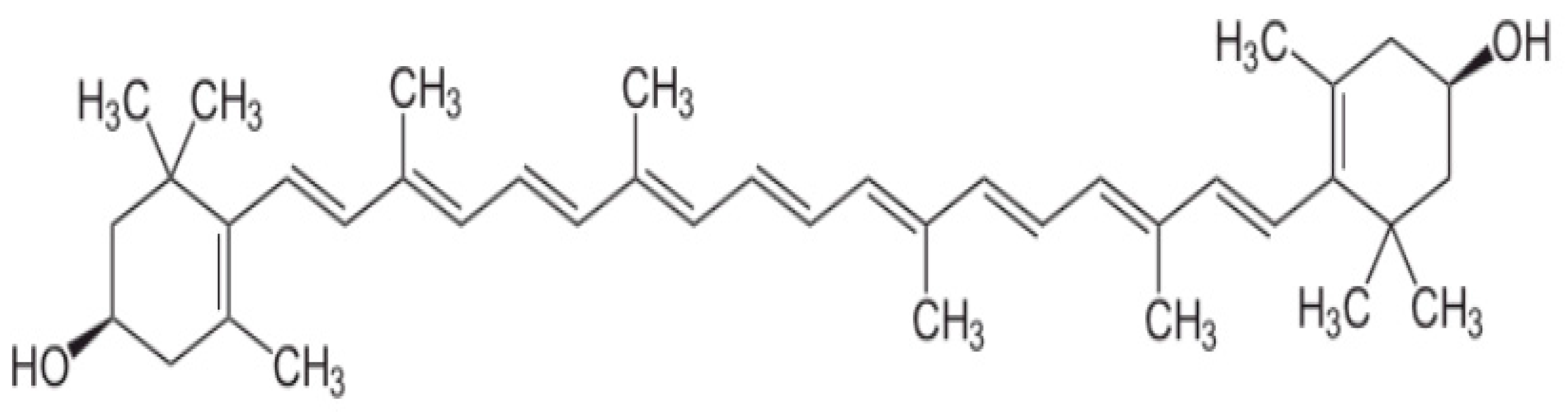
2. Metabolism and Functions of Carotenoids
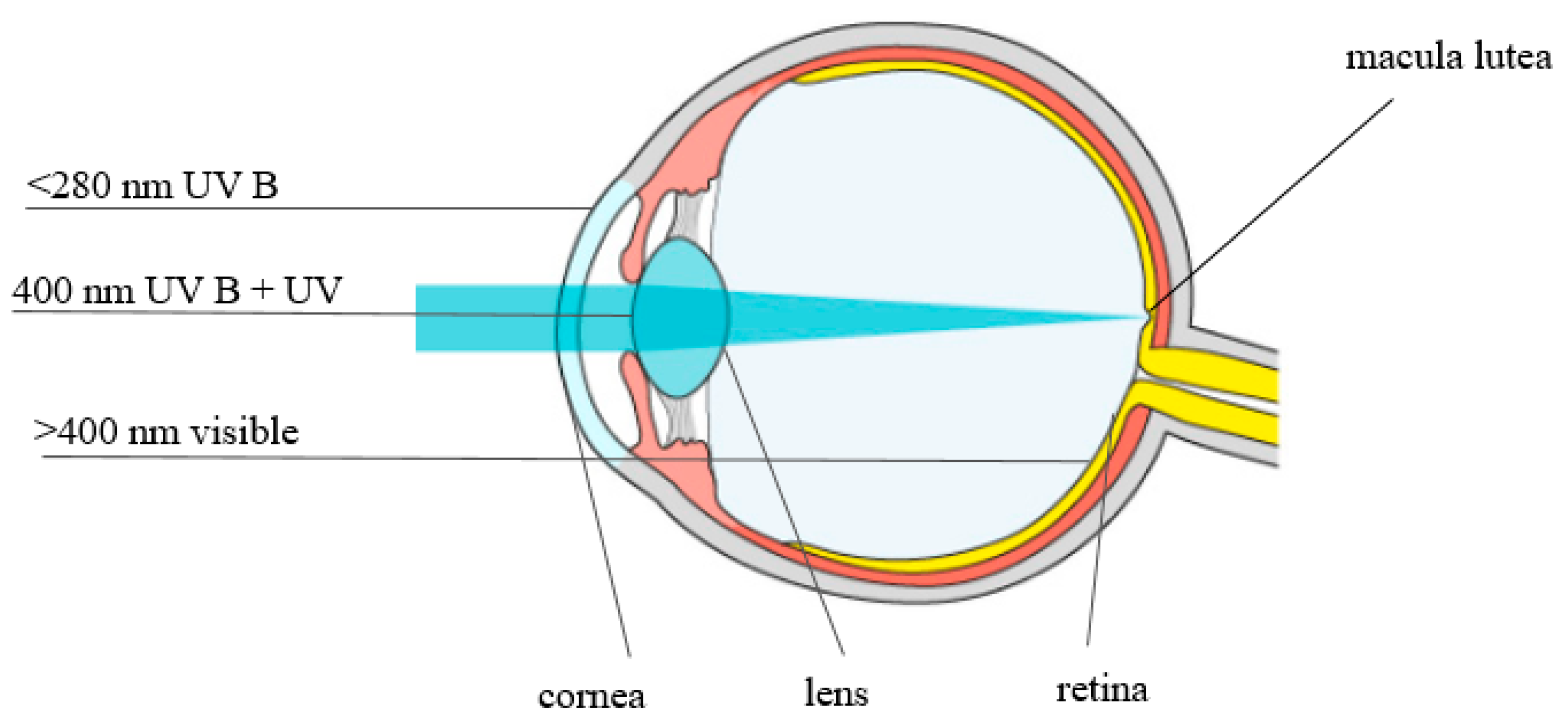
3. The Role of Lutein and Zeaxanthin in Eye Vision
4. Lutein, Zeaxanthin, and Macular Degeneration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirdyani, H.; Sheth, M. Lutein—The less explored carotenoid. World J. Pharm. Res. 2017, 6, 528–553. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, E. Lutein—Diet source and potential disease prevention. Postępy Fitoter. 2010, 2, 97–100. [Google Scholar]
- EFSA: Statement on the safety of synthetic zeaxanthin as an ingredient in food supplements. EFSA J. 2012, 10, 2981.
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Castenmiller, J.J.M.; West, C.E. Bioavailability of carotenoids. Pure Appl. Chem. 1997, 89, 2145–2150. [Google Scholar] [CrossRef]
- Li, B.X.; Vachali, P.; Bernstein, P.S. Human ocular carotenoid-binding proteins. Photochem. Photobiol. Sci. 2010, 9, 1418–1425. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Vishwanathan, R.; Schalch, W.; Johnson, E.J. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr. Neurosci. 2016, 19, 95–101. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and Zeaxanthin and Their Potential Roles in Disease Prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar] [CrossRef] [PubMed]
- Jaswir, I.; Monsur, H.A. Anti-inflammatory compounds of macroalgae origin: A review. J. Med. Plants Res. 2011, 5, 7146–7154. [Google Scholar]
- Santoyo, S.; Jaime, L.; Plaza, M.; Herrero, M.; Rodriguez-Meizoso, I.; Ibañez, E.; Reglero, G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2012, 24, 731–741. [Google Scholar] [CrossRef]
- Lee, E.H.; Faulhaber, D.; Hanson, K.M.; Ding, W.; Peters, S.; Kodali, S.; Granstein, R.D. Dietary lutein reduces ultraviolet radiation-induced inflammation and immunosuppression. J. Investig. Dermatol. 2004, 122, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Shahzad, T.; Malik, A.; Shariati, M.A.; Laishevtcev, A.; Plygun, S.; Heydari, M.; et al. Xanthophyll: Health benefits and therapeutic insights. Life Sci. 2020, 240, 117104. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Effects of carotenoids and retinoids on gap junctional communication. BioFactors 2001, 15, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kima, J.H.; Naa, J.H.; Kima, C.K.; Kima, J.Y.; Haa, K.S.; Leeb, H.; Chungc, H.T.; Kwond, H.J.; Kwone, Y.G.; Kim, Y.M. The non-provitamin A carotenoid, lutein, inhibits NF-κB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-κB-inducing kinase pathways: Role of H2O2 in NF-κB activation. Free Radic. Biol. Med. 2008, 45, 885–896. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, S.; Son, H.Y.; Park, S.B.; Kim, M.S.; Choi, E.J.; Singh, T.S.; Ha, J.H.; Lee, M.G.; Kim, J.E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharmacol. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.-Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef]
- Widomska, J.; SanGiovanni, J.P.; Subczynski, W.K. Why Is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients 2020, 12, 1333. [Google Scholar] [CrossRef]
- Shyam, R.; Gorusupudi, A.; Nelson, K.; Horvath, M.P.; Bernstein, P.S. RPE65 Has an Additional Function as the Lutein to Meso-Zeaxanthin Isomerase in the Vertebrate Eye. Proc. Natl. Acad. Sci. USA 2017, 114, 10882–10887. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; de Moura, F.F.; Zhao, D.Y.; Aebischer, C.P.; Bernstein, P.S. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3383–3392. [Google Scholar]
- Khachik, F.; de Moura, F.F.; Chew, E.Y.; Douglass, L.W.; Ferris, F.L.; Kim, J.; Thompson, D.J.S. The effect of lutein and zeaxanthin supplementation on metabolites of these carotenoids in the serum of persons aged 60 or older. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5234–5242. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Neuringer, M.; Russell, R.M.; Schalch, W.; Snodderly, D.M. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investig. Ophthalmol. Vis. Sci. 2005, 46, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, E.J.; Li, B.; Bernstein, P.S.; Vishwanathan, R.; Johnson, M.A.; Poon, L.; Johnson, E.J. Relationship between Concentrations of Lutein and StARD3 among Pediatric and Geriatric Human Brain Tissue. PLoS ONE 2016, 11, e0159877. [Google Scholar]
- Bhosale, P.; Li, B.; Sharifzadeh, M.; Gellermann, W.; Frederick, J.M.; Tsuchida, K.; Bernstein, P.S. Purification and Partial Characterization of a Lutein-Binding Protein from Human Retina. Biochemistry 2009, 48, 4798–4807. [Google Scholar] [CrossRef]
- Hammond, B.R.; Fletcher, L.M.; Elliott, J.G. Glare Disability, Photostress Recovery, and Chromatic Contrast: Relation to Macular Pigment and Serum Lutein and Zeaxanthin. Investig. Ophthalmol. Vis. Sci. 2013, 54, 476–481. [Google Scholar] [CrossRef]
- Lindbergh, C.A.; Lv, J.; Zhao, Y.; Mewborn, C.M.; Puente, A.N.; Terry, D.P.; Renzi-Hammond, L.M.; Hammond, B.R.; Liu, T.; Miller, L.S. The effects of lutein and zeaxanthin on resting state functional connectivity in older Caucasian adults: A randomized controlled trial. Brain Imaging Behav. 2020, 14, 668–681. [Google Scholar] [CrossRef]
- Contín, M.; Benedetto, M.; Quinteros-Quintana, M.; Guido, M.E. Light pollution: The possible consequences of excessive illumination on retina. Eye 2016, 30, 255–263. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and mesozeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Blakely, S.; Herbert, A.; Collins, M.; Jenkins, M.; Mitchell, G.; Grundel, E.; O’Neill, K.R.; Khachik, F. Lutein Interacts with Ascorbic Acid More Frequently than with α-Tocopherol to Alter Biomarkers of Oxidative Stress in Female Zucker Obese Rats. J. Nutr. 2003, 133, 2838–2844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khachik, F.; Bernstein, P.S.; Garland, D.L. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1802–1811. [Google Scholar]
- Stringham, J.M.; O’Brien, K.J.; Stringham, N.T. Contrast Sensitivity and Lateral Inhibition Are Enhanced With Macular Carotenoid Supplementation. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2291–2295. [Google Scholar] [CrossRef] [PubMed]
- Machida, N.; Kosehira, M.; Kitaichi, N. Clinical Effects of Dietary Supplementation of Lutein with High Bio-Accessibility on Macular Pigment Optical Density and Contrast Sensitivity: A Randomized Double-Blind Placebo-Controlled Parallel-Group Comparison Trial. Nutrients 2020, 12, 2966. [Google Scholar] [CrossRef]
- Howells, O.; Eperjesi, F.; Bartlett, H. Improving the repeatability of heterochromatic flicker photometry for measurement of macular pigment optical density. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Stringham, J.M.; Hammond, B.R. Macular Pigment and Visual Performance under Glare Conditions. Optom. Vis. Sci. 2008, 85, 82–88. [Google Scholar] [CrossRef]
- Stringham, J.M.; Garcia, P.V.; Smith, P.A.; McLin, L.N.; Foutch, B.K. Macular Pigment and Visual Performance in Glare: Benefits for Photostress Recovery, Disability Glare, and Visual Discomfort. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7406–7415. [Google Scholar] [CrossRef]
- National Institutes of Health. Facts about Age-Related Macular Degeneration. 2019. Available online: https://Nei.nih.gov/health/maculardegen/armd_facts (accessed on 15 September 2020).
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Klaver, C.C.W. The EYE-RISK consortium and theEuropean Eye Epidemiology (E3) consortium Prevalence of Age-Related Macular Degeneration in Europe. The Past and the Future. Ophthamology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Paradowska, D.; Kucharska, E. Nutrition assessment of patients with AMD. World Sci. News 2020, 139, 221–232. [Google Scholar]
- Jonasson, F.; Fisher, D.E.; Eiriksdottir, G.; Sigurdsson, S.; Klein, R.; Launer, L.J.; Harris, T.; Gudnason, V.; Cotch, M.F. Five-year incidence, progression and risk factors for age-related macular degeneration: The Age, Gene/Environment Susceptibility Study. Ophthalmology 2014, 121, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Hubbell, M.; Jairam, P.; Ambati, B. Neovascular Macular Degeneration: A Review of Etiology, Risk Factors, and Recent Advances in Research and Therapy. Int. J. Mol. Sci. 2021, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Finley, E.L.; Patat, S.A.; Schey, K.L. Photooxidation of lens proteins with xanthurenic acid: A putative chromophore for cataractogenesis. Photochem. Photobiol. 2001, 74, 740–744. [Google Scholar] [CrossRef]
- Suárez-Barrio, C.; Del Olmo-Aguado, S.; García-Pérez, E.; de la Fuente, M.; Muruzabal, F.; Anitua, E.; Baamonde-Arbaiza, B.; Fernández-Vega-Cueto, L.; Fernández-Vega, L.; Merayo-Lloves, J. Antioxidant Role of PRGF on RPE Cells after Blue Light Insult as a Therapy for Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 1021. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.A.; Lenaers, G.; et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef]
- Mettu, P.S.; Wielgus, A.R.; Ong, S.S.; Cousins, S.W. Retinal pigment epithelium response to oxidant injury in the pathogenesis of early age-related macular degeneration. Mol. Asp. Med. 2012, 33, 376–398. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Bao, X.; Cong, Y.; Fan, B.; Li, G. Autophagy in Age-Related Macular Degeneration: A Regulatory Mechanism of Oxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 2896036. [Google Scholar] [CrossRef]
- Trevithick-Sutton, C.C.; Foote, C.S.; Collins, M.; Trevithick, J.R. The Retinal Carotenoids Zeaxanthin and Lutein Scavenge Superoxide and Hydroxyl Radicals: A Chemiluminescence and ESR Study. Mol. Vis. 2006, 12, 1127–1135. [Google Scholar] [PubMed]
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Scavenging Capacity of Marine Carotenoids against Reactive Oxygen and Nitrogen Species in a Membrane-Mimicking System. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.; Wisniewska, A.; Widomska, J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch. Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kuijk, E.F.V.; Hurst, J.S.; Praisan, J.K.; Moore, R.; Barnes, S. Electrospray Tandem Mass Spectrometric Analysis of Macular Pigment Oxidation Products in Human Eyes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1787. [Google Scholar]
- Lakshminarayana, R.; Aruna, G.; Sathisha, U.V.; Dharmesh, S.M.; Baskaran, V. Structural elucidation of possible lutein oxidation products mediated through peroxyl radical inducer 2,2′-Azobis (2-methylpropionamidine) dihydrochloride: Antioxidant and cytotoxic influence of oxidized lutein in HeLa cells. Chem. Biol. Interact. 2013, 203, 448–455. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Augustynska, D.; Jemioła-Rzemińska, M.; Burda, K.; Strzałka, K. Influence of polar and nonpolar carotenoids on structural and adhesive properties of model membranes. Chem. Biol. Interact. 2015, 239, 19–25. [Google Scholar] [CrossRef]
- Böhm, F.; Edge, R.; Truscott, T.G. Interactions of dietary carotenoids with singlet oxygen (1O2) and free radicals: Potential effects for human health. Acta Biochim. Pol. 2012, 59, 27–30. [Google Scholar] [CrossRef]
- El-Agamey, A.; McGarvey, D.J. The reactivity of carotenoid radicals with oxygen. Free Radic. Res. 2007, 41, 295–302. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids—A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 15, 2005–2015. [Google Scholar] [CrossRef]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. Biomed. Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Davey, P.G.; Roman, B.O.; Evans, D.W. Efficacy of commercially available nutritional supplements: Analysis of Serum uptake, macular pigment optical density and visual functional response. Nutrients 2020, 12, 1321. [Google Scholar] [CrossRef]
- Seddon, J.M.; Ajani, U.A.; Sperduto, F.L.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, Vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case–Control Study Group. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Qiu, S.; Wei, Y.; Zhou, X.; Jiang, Z.; Zhang, T.; Jiang, X.; Zhang, S. Intravitreal injection of docosahexaenoic acid attenuated photoreceptor cell injury in a NaIO3-induced age-related macular degeneration rat model. Neurosci. Lett. 2017, 14, 53–61. [Google Scholar] [CrossRef]
- Prokopiou, E.; Kolovos, P.; Kalogerou, M.; Neokleous, A.; Papagregoriou, G.; Deltas, C.; Malas, S.; Georgiou, T. Therapeutic potential of omega-3 fatty acids supplementation in a mouse model of dry macular degeneration. BMJ Open Ophthalmol. 2017, 19, e000056. [Google Scholar] [CrossRef]
- Cho, E.; Hankinson, S.E.; Rosner, B.; Willett, W.C.; Colditz, G.A. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am. J. Clin. Nutr. 2008, 87, 1837–1843. [Google Scholar] [CrossRef]
- Moeller, S.M.; Parekh, N.; Tinker, L.; Ritenbaugh, C.; Blodi, B.; Wallace, R.B.; Mares, J.A. CAREDS Research Study Group. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): Ancillary study of the Women’s Health Initiative. Arch. Ophthalmol. 2006, 124, 1151–1162. [Google Scholar] [CrossRef]
- Tan, J.S.; Wang, J.J.; Flood, V.; Rochtchina, E.; Smith, W.; Mitchell, P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2008, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Schnurrbusch, U.E.; Zinkernagel, M.S.; Munk, M.R.; Ebneter, A.; Wolf, S. Oral lutein supplementation enhances macular pigment density and contrast sensitivity but not in combination with polyunsaturated fatty acids. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8069–8074. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Yan, S.F.; Ma, L.; Zou, Z.Y.; Xu, X.R.; Dou, H.L.; Lin, X.M. Serum and macular responses to multiple xanthophyll supplements in patients with early age-related macular degeneration. Nutrition 2013, 29, 387–392. [Google Scholar] [CrossRef]
- Akuffo, K.O.; Nolan, J.M.; Howard, A.N.; Moran, R.; Stack, J.; Klein, R.; Klein, B.E.; Meuer, S.M.; Sabour-Pickett, S.; Thurnham, D.I.; et al. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye 2015, 29, 902–912. [Google Scholar] [CrossRef]
- Akuffo, K.O.; Beatty, S.; Peto, T.; Stack, J.; Stringham, J.; Kelly, D.; Leung, I.; Corcoran, L.; Nolan, J.M. The impact of supplemental antioxidants on visual function in nonadvanced age-related macular degeneration: A head-to-head randomized clinical trial. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5347–5360. [Google Scholar] [CrossRef]
- Arnold, C.; Winter, L.; Frohlich, K.; Jentsch, S.; Dawczynski, J.; Jahreis, G.; Bohm, V. Macular xanthophylls and omega-3 long-chain polyunsaturated fatty acids in age-related macular degeneration: A randomized trial. JAMA Ophthalmol. 2013, 131, 564–572. [Google Scholar] [CrossRef]
- Sawa, M.; Shunto, T.; Nishiyama, I.; Yokoyama, A.; Shigeta, R.; Miura, S.; Kawasaki, R. Effects of lutein supplementation in Japanese patients with unilateral age-related macular degeneration: The Sakai lutein Study. Sci. Rep. 2020, 10, 5958. [Google Scholar] [CrossRef]
- Beatty, S.; Chakravarthy, U.; Nolan, J.M.; Muldrew, K.A.; Woodside, J.V.; Denny, F.; Stevenson, M.R. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology 2013, 120, 600–606. [Google Scholar] [CrossRef]
- Ma, L.; Yan, S.F.; Huang, Y.M.; Lu, X.R.; Qian, F.; Pang, H.L.; Xu, X.R.; Zou, Z.Y.; Dong, P.C.; Xiao, X.; et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 2012, 119, 2290–2297. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef]
- Weigert, G.; Kaya, S.; Pemp, B.; Sacu, S.; Lasta, M.; Werkmeister, R.M.; Dragostinoff, N.; Simader, C.; Garhofer, G.; Schmidt-Erfurth, U.; et al. Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8174–8178. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; Nolan, J.M.; Howard, A.N.; Beatty, S. Macular response to supplementation with differing xanthophyll formulations in subjects with and without age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Layana, A.; Recalde, S.; Alaman, A.S.; Robredo, P.F. Effects of lutein and docosahexaenoic Acid supplementation on macular pigment optical density in a randomized controlled trial. Nutrients 2013, 5, 543–551. [Google Scholar] [CrossRef]
- Garcia-Layana, A.; Recalde, S.; Hernandez, M.; Abraldes, M.J.; Nascimento, J.; Hernandez-Galilea, E.; Olmedilla-Alonso, B.; Escobar-Barranco, J.J.; Zapata, M.A.; Silva, R.; et al. A randomized study of nutritional supplementation in patients with unilateral wet age-related macular degeneration. Nutrients 2021, 13, 1253. [Google Scholar] [CrossRef] [PubMed]
- Sabour-Pickett, S.; Beatty, S.; Connolly, E.; Loughman, J.; Stack, J.; Howard, A.; Klein, R.; Klein, B.E.; Meuer, S.M.; Myers, C.E.; et al. Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. Retina 2014, 34, 1757–1766. [Google Scholar] [CrossRef]
- Murray, I.J.; Makridaki, M.; van der Veen, R.L.; Carden, D.; Parry, N.R.; Berendschot, T.T. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: The CLEAR study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1781–1788. [Google Scholar] [CrossRef]
- Thakkinstian, A.; Han, P.; McEvoy, M.; Smith, W.; Hoh, J.; Magnusson, K.; Zhang, K.; Attia, J. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum. Mol. Genet. 2006, 15, 2784–2790. [Google Scholar] [CrossRef]
- Ho, L.; van Leeuwen, R.; Witteman, J.C.; van Duijn, C.M.; Uitterlinden, A.G.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Klaver, C.C. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: The Rotterdam study. Arch. Ophthalmol. 2011, 129, 758–766. [Google Scholar] [CrossRef]
- Jabbarpoor Bonyadi, M.H.; Yaseri, M.; Nikkhah, H.; Bonyadi, M.; Soheilian, M. Association of risk genotypes of ARMS2/LOC387715 A69S and CFH Y402H with age-related macular degeneration with and without reticular pseudodrusen: A meta-analysis. Acta Ophthalmol. 2018, 96, e105–e110. [Google Scholar] [CrossRef]
- Vavvas, D.G.; Small, K.W.; Awh, C.C.; Zanke, B.W.; Tibshirani, R.J.; Kustra, R. CFH and ARMS2 genetic risk determines progression to neovascular age-related macular degeneration after antioxidant and zinc supplementation. Proc. Natl. Acad. Sci. USA 2018, 115, E696–E704. [Google Scholar] [CrossRef]
- Bonyadi, M.; Foruzandeh, Z.; Mohammadian, T.; Fotouhi, N.; Soheilian, M.; Jabbarpoor Bonyadi, M.H.; Javadzadeh, A.; Moein, H.; Yaseri, M. Evaluation of CC-cytokine ligand 2 and complementary factor H Y402H polymorphisms and their interactional association with age-related macular degeneration. Acta Ophthalmol. 2016, 94, e779–e785. [Google Scholar] [CrossRef] [PubMed]
- Guymer, R.H.; Wu, Z.; Hodgson, L.A.B.; Caruso, E.; Brassington, K.H.; Tindill, N.; Aung, K.Z.; McGuinness, M.B.; Fletcher, E.L.; Chen, F.K.; et al. Laser Intervention in Early Stages of Age-Related Macular Degeneration Study Group. Subthreshold Nanosecond Laser Intervention in Age-Related Macular Degeneration: The LEAD Randomized Controlled Clinical Trial. Ophthalmology 2018, 126, 829–838. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Querques, G.; Cicinelli, M.V.; Rabiolo, A.; de Vitis, L.; Sacconi, R.; Querques, L.; Bandello, F. Laser photocoagulation as treatment of non-exudative age-related macular degeneration: State-of-the-art and future perspectives. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1–9. [Google Scholar] [CrossRef]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2014, 8, CD005139. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Kuppermann, B.D.; Bandello, F.; Loewenstein, A. Faricimab: Expanding horizon beyond VEGF. Eye 2020, 34, 802–804. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. [Google Scholar] [CrossRef]
- Al-Khersan, H.; Hussain, R.M.; Ciulla, T.A.; Dugel, P.U. Innovative therapies for neovascular age-related macular degeneration. Expert. Opin. Pharmacother. 2019, 20, 1879–1891. [Google Scholar] [CrossRef]

| Food Product | Lutein/Zeaxanthin mg/100 g |
|---|---|
| kale | 39.55 |
| spinach | 11.93 |
| lettuce | 2.63 |
| broccoli | 2.44 |
| brussels sprouts | 1.59 |
| green peas (canned) | 1.35 |
| corn (canned) | 0.88 |
| green beans | 0.64 |
| carrot | 0.35 |
| cabbage | 0.31 |
| melon | 0.04 |
| AREDS1 Dose/Day | AREDS2 Dose/Day | |
|---|---|---|
| Vitamin C | 500 mg | 500 mg |
| Vitamin E | 400 IU | 400 IU |
| Beta-carotene | 15 mg | - |
| Zinc | 80 mg | 25 mg |
| Copper | 2 mg | 2 mg |
| Lutein | - | 10 mg |
| Zeaxanthin | - | 2 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. https://doi.org/10.3390/nu14040827
Mrowicka M, Mrowicki J, Kucharska E, Majsterek I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients. 2022; 14(4):827. https://doi.org/10.3390/nu14040827
Chicago/Turabian StyleMrowicka, Małgorzata, Jerzy Mrowicki, Ewa Kucharska, and Ireneusz Majsterek. 2022. "Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease" Nutrients 14, no. 4: 827. https://doi.org/10.3390/nu14040827
APA StyleMrowicka, M., Mrowicki, J., Kucharska, E., & Majsterek, I. (2022). Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients, 14(4), 827. https://doi.org/10.3390/nu14040827






