Efficacy of Vitamin D Supplements in Prevention of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods and Materials
2.1. Data Sources and Search
2.2. Data Selection and Quality
2.3. Assessment of Risk of Bias
2.4. Main and Subgroup Meta-Analysis
2.5. Statistical Analysis
3. Results
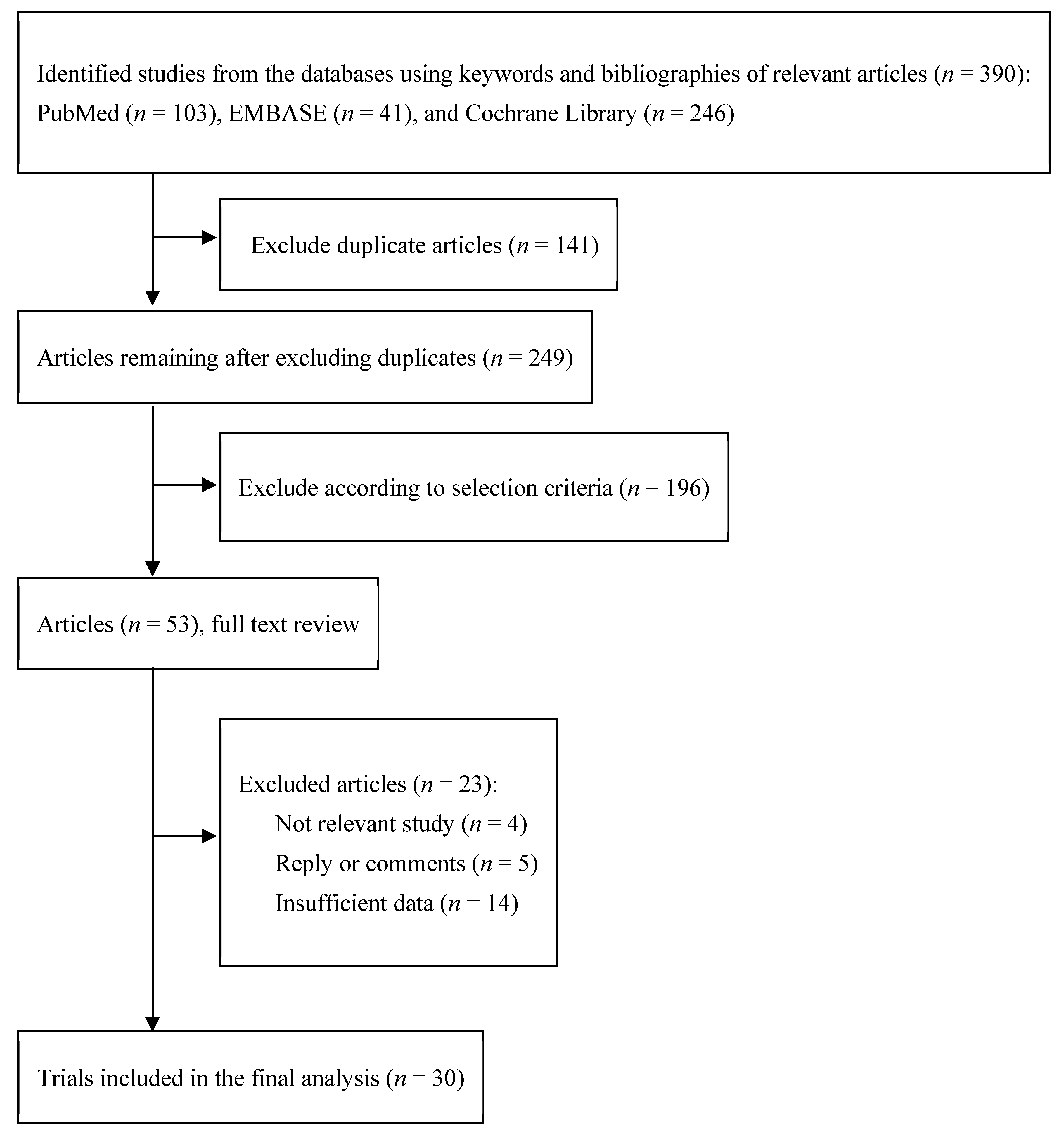
3.1. Identification of Relevant Studies
3.2. General Characteristics of Trials
3.3. Association between Vitamin D Supplementation and Prevention of ARIs
3.4. Quality Assessment
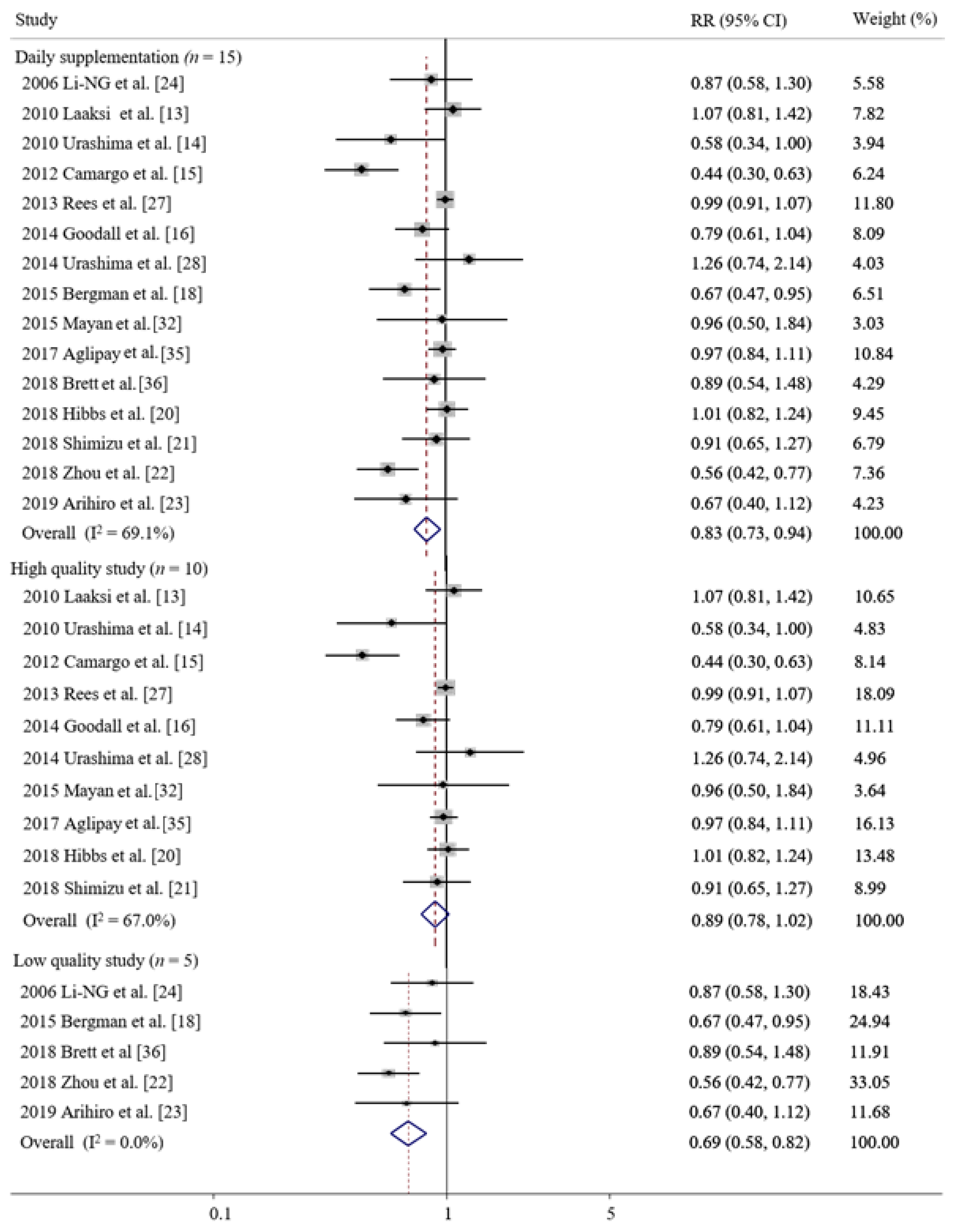
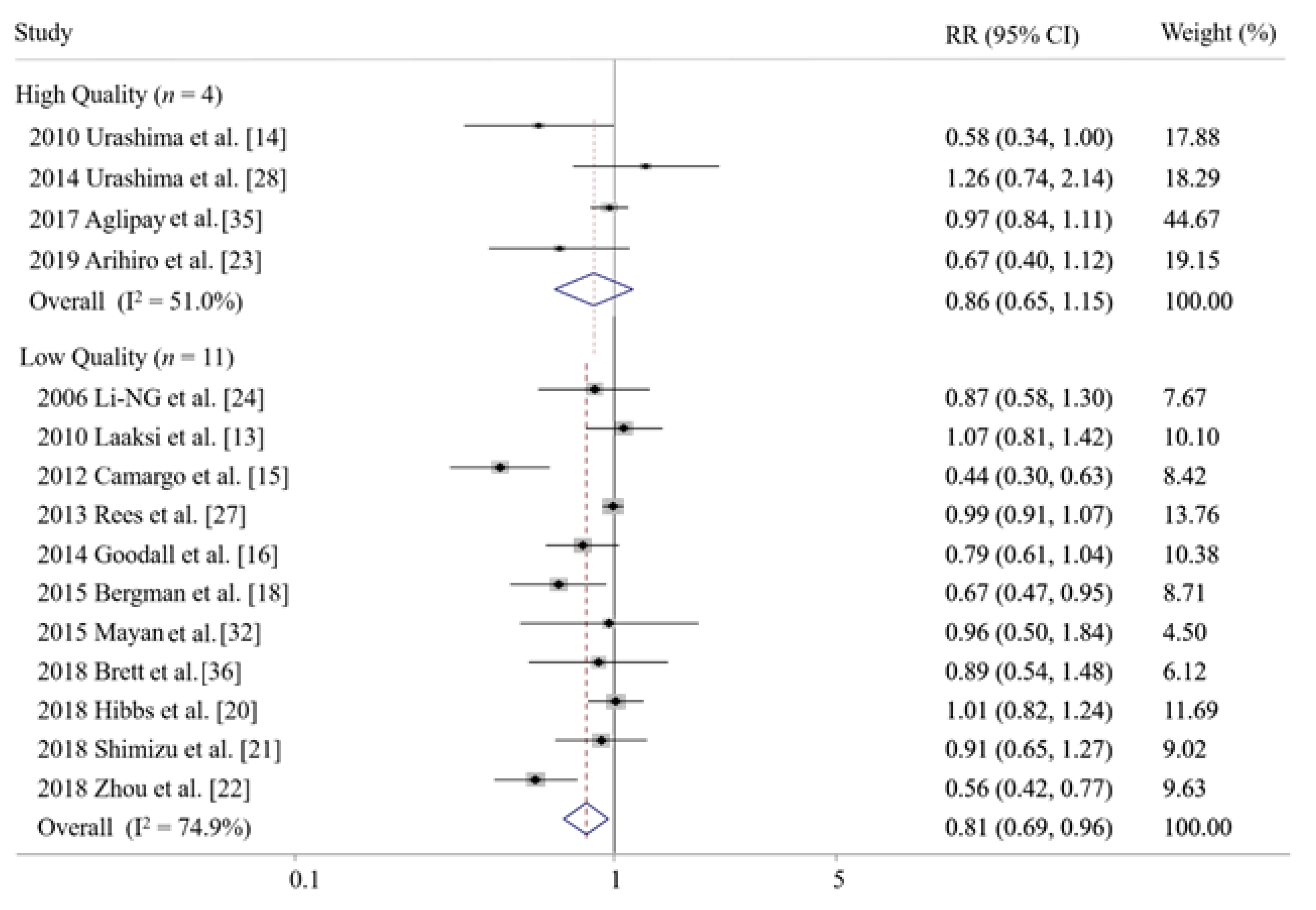
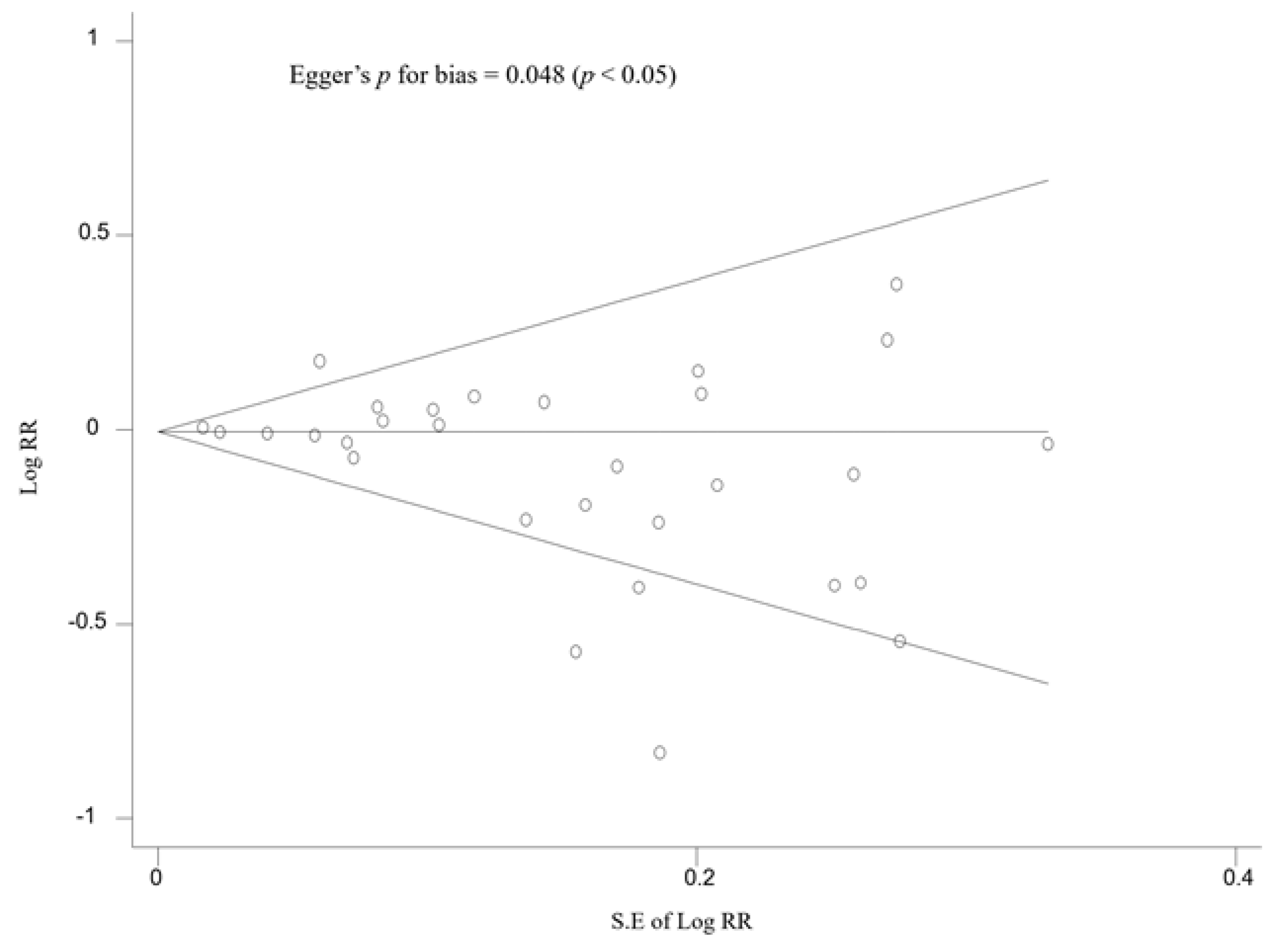
3.5. Subgroup Meta-Analysis and the Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grief, S.N. Upper respiratory infections. Prim. Care Clin. Off. Pract. 2013, 40, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.T.; Goldman, R.B. Chapter 337: The common cold. In Cecil Medicine, 26th ed.; Goldman, L., Schafer, A.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 2150–2152. [Google Scholar]
- Musher, D.M. Chapter 91: Overview of pneumonia. In Cecil Medicine, 26th ed.; Goldman, L., Schafer, A.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 592–603. [Google Scholar]
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Swartz, S.; Fullman, N.; Mosser, J.; Thompson, R.L.; Reiner, R.C., Jr.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 1133–1161. [Google Scholar] [CrossRef] [Green Version]
- Mousa, H.A. Prevention and Treatment of Influenza, Influenza-Like Illness, and Common Cold by Herbal, Complementary, and Natural Therapies. J. Evid. Based Complementary Altern. Med. 2017, 22, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.I.; Bromage, S.; Fawzi, W. Effect of micronutrient supplements on influenza and other respiratory tract infections among adults: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e003176. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Isonne, C.; Santoro, M.M.; Baccolini, V. The effects of orally administered lactoferrin in the prevention and management of viral infections: A systematic review. Rev. Med. Virol. 2022, 32, e2261. [Google Scholar] [CrossRef] [PubMed]
- Khammissa, R.A.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. BioMed Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [Green Version]
- Christakos, S.; Hewison, M.; Gardner, D.G.; Wagner, C.L.; Sergeev, I.N.; Rutten, E.; Pittas, A.G.; Boland, R.; Ferrucci, L.; Bikle, D.D. Vitamin D: Beyond bone. Ann. N. Y. Acad. Sci. 2013, 1287, 45–58. [Google Scholar] [CrossRef]
- Hayashi, H.; Okamatsu, M.; Ogasawara, H.; Tsugawa, N.; Isoda, N.; Matsuno, K.; Sakoda, Y. Oral Supplementation of the Vitamin D Metabolite 25(OH)D3 Against Influenza Virus Infection in Mice. Nutrients 2020, 12, 2000. [Google Scholar] [CrossRef]
- Adenote, A.; Dumic, I.; Madrid, C.; Barusya, C.; Nordstrom, C.W.; Rueda Prada, L. NAFLD and Infection, a Nuanced Relationship. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5556354. [Google Scholar] [CrossRef]
- Laaksi, I.; Ruohola, J.P.; Mattila, V.; Auvinen, A.; Ylikomi, T.; Pihlajamaki, H. Vitamin D supplementation for the prevention of acute respiratory tract infection: A randomized, double-blinded trial among young Finnish men. J. Infect. Dis. 2010, 202, 809–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, C.A., Jr.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012, 130, 561–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodall, E.C.; Granados, A.C.; Luinstra, K.; Pullenayegum, E.; Coleman, B.L.; Loeb, M.; Smieja, M. Vitamin D3 and gargling for the prevention of upper respiratory tract infections: A randomized controlled trial. BMC Infect. Dis. 2014, 273, 1471–2334. [Google Scholar] [CrossRef] [Green Version]
- Slow, S.; Priest, P.; Chambers, S.; Stewart, A.; Jennings, L.; Florkowski, C.; Livesey, J.; Camargo, C.; Scragg, R.; Murdoch, D. Effect of vitamin D3 supplementation on Staphylococcus aureus nasal carriage: A randomized, double-blind, placebo-controlled trial in healthy adults. Clin. Microbiol. Infect. 2014, 20, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Bergman, P.; Norlin, A.C.; Hansen, S.; Bjorkhem-Bergman, L. Vitamin D supplementation to patients with frequent respiratory tract infections: A post hoc analysis of a randomized and placebo-controlled trial. BMC Res. Notes 2015, 8, 391. [Google Scholar] [CrossRef] [Green Version]
- Ginde, A.A.; Blatchford, P.; Breese, K.; Zarrabi, L.; Linnebur, S.A.; Wallace, J.I.; Schwartz, R.S. High-Dose Monthly Vitamin D for Prevention of Acute Respiratory Infection in Older Long-Term Care Residents: A Randomized Clinical Trial. J. Am. Geriatr. Soc. 2017, 65, 496–503. [Google Scholar] [CrossRef]
- Hibbs, A.M.; Ross, K.; Kerns, L.A.; Wagner, C.; Fuloria, M.; Groh-Wargo, S.; Zimmerman, T.; Minich, N.; Tatsuoka, C. Effect of Vitamin D Supplementation on Recurrent Wheezing in Black Infants Who Were Born Preterm: The D-Wheeze Randomized Clinical Trial. JAMA 2018, 319, 2086–2094. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ito, Y.; Yui, K.; Egawa, K.; Orimo, H. Intake of 25-Hydroxyvitamin D3 Reduces Duration and Severity of Upper Respiratory Tract Infection: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Comparison Study. J. Nutr. Health Aging 2018, 22, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Du, J.; Huang, L.; Wang, Y.; Shi, Y.; Lin, H. Preventive Effects of Vitamin D on Seasonal Influenza A in Infants: A Multicenter, Randomized, Open, Controlled Clinical Trial. Pediatr. Infect. Dis. J. 2018, 37, 749–754. [Google Scholar] [CrossRef]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1088–1095. [Google Scholar] [CrossRef]
- Li-Ng, M.; Aloia, J.F.; Pollack, S.; Cunha, B.A.; Mikhail, M.; Yeh, J.; Berbari, N. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol. Infect. 2009, 137, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Manaseki-Holland, S.; Maroof, Z.; Bruce, J.; Mughal, M.Z.; Masher, M.I.; Bhutta, Z.A.; Walraven, G.; Chandramohan, D. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: A randomised controlled superiority trial. Lancet 2012, 379, 1419–1427. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, D.R.; Slow, S.; Chambers, S.T.; Jennings, L.C.; Stewart, A.W.; Priest, P.C.; Florkowski, C.M.; Livesey, J.H.; Camargo, C.A.; Scragg, R. Effect of Vitamin D3 Supplementation on Upper Respiratory Tract Infections in Healthy Adults: The VIDARIS Randomized Controlled Trial. JAMA 2012, 308, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, J.R.; Hendricks, K.; Barry, E.L.; Peacock, J.L.; Mott, L.A.; Sandler, R.S.; Bresalier, R.S.; Goodman, M.; Bostick, R.M.; Baron, J.A. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin. Infect. Dis. 2013, 57, 1384–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urashima, M.; Mezawa, H.; Noya, M.; Camargo, C.A., Jr. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: A randomized controlled trial. Food Funct. 2014, 5, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Hanifa, Y.; Witt, K.D.; Barnes, N.C.; Hooper, R.; Patel, M.; Stevens, N.; Enayat, Z.; Balayah, Z.; Syed, A.; et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax 2015, 70, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Martineau, A.R.; James, W.Y.; Hooper, R.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.; et al. Vitamin D 3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Martineau, A.R.; MacLaughlin, B.D.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Kilpin, K.; McLaughlin, D.; Fletcher, G.; Mein, C.A.; et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015, 70, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Mayan, I.; Somech, R.; Lev, A.; Cohen, A.H.; Constantini, N.W.; Dubnov-Raz, G. Thymus Activity, Vitamin D, and Respiratory Infections in Adolescent Swimmers. Isr. Med. Assoc. J. 2015, 17, 571–575. [Google Scholar]
- Denlinger, L.C.; King, T.S.; Cardet, J.C.; Craig, T.; Holguin, F.; Jackson, D.J.; Kraft, M.; Peters, S.P.; Ross, K.; Sumino, K.; et al. Vitamin D Supplementation and the Risk of Colds in Patients with Asthma. Am. J. Respir. Crit. Care Med. 2016, 193, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Dewan, P.; Shah, D.; Sharma, N.; Bedi, N.; Kaur, I.R.; Bansal, A.K.; Madhu, S.V. Vitamin D Supplementation for Treatment and Prevention of Pneumonia in Under-five Children: A Randomized Double-blind Placebo Controlled Trial. Indian Pediatr. 2016, 53, 967–976. [Google Scholar] [CrossRef]
- Aglipay, M.; Birken, C.S.; Parkin, P.C.; Loeb, M.B.; Thorpe, K.; Chen, Y.; Laupacis, A.; Mamdani, M.; MacArthur, C.; Hoch, J.S.; et al. Effect of High-Dose vs Standard-Dose Wintertime Vitamin D Supplementation on Viral Upper Respiratory Tract Infections in Young Healthy Children. JAMA 2017, 318, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Brett, N.R.; Lavery, P.; Agellon, S.; Vanstone, C.A.; Goruk, S.; Field, C.J.; Weiler, H.A. Vitamin D Status and Immune Health Outcomes in a Cross-Sectional Study and a Randomized Trial of Healthy Young Children. Nutrients 2018, 10, 680. [Google Scholar] [CrossRef] [Green Version]
- Loeb, M.; Dang, A.D.; Thiem, V.D.; Thanabalan, V.; Wang, B.; Nguyen, N.B.; Tran, H.T.M.; Luong, T.M.; Singh, P.; Smieja, M.; et al. Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influenza Other Respir. Viruses 2019, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kamble, D.; Mahantshetti, N.S. Effect of Vitamin D Supplementation in the Prevention of Recurrent Pneumonia in Under-Five Children. Indian J. Pediatr. 2019, 86, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Sluyter, J.; Stewart, A.W.; Khaw, K.T.; Lawes, C.M.; Toop, L.; Waayer, D.; Scragg, R. Effect of Monthly High-Dose Vitamin D Supplementation on Acute Respiratory Infections in Older Adults: A Randomized Controlled Trial. Clin. Infect. Dis. 2020, 71, 311–317. [Google Scholar] [CrossRef]
- Ganmaa, D.; Uyanga, B.; Zhou, X.; Gantsetseg, G.; Delgerekh, B.; Enkhmaa, D.; Khulan, D.; Ariunzaya, S.; Sumiya, E.; Bolortuya, B.; et al. Vitamin D Supplements for Prevention of Tuberculosis Infection and Disease. N. Engl. J. Med. 2020, 383, 359–368. [Google Scholar] [CrossRef]
- Sudfeld, C.R.; Mugusi, F.; Muhihi, A.; Aboud, S.; Nagu, T.J.; Ulenga, N.; Hong, B.; Wang, M.; Fawzi, W.W. Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: A randomised, double-blind, placebo-controlled trial. Lancet HIV 2020, 7, 463–471. [Google Scholar] [CrossRef]
- Pham, H.; Waterhouse, M.; Baxter, C.; Romero, B.D.; McLeod, D.S.; Armstrong, B.K.; Ebeling, P.R.; English, D.R.; Hartel, G.; Kimlin, M.G.; et al. The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: An analysis of data from the D-Health Trial. Lancet Diabetes Endocrinol. 2021, 9, 69–81. [Google Scholar] [CrossRef]
- Xiao, L.; Xing, C.; Yang, Z.; Xu, S.; Wang, M.; Du, H.; Liu, K.; Huang, Z. Vitamin D supplementation for the prevention of childhood acute respiratory infections: A systematic review of randomised controlled trials. Br. J. Nutr. 2015, 114, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; Goodall, E.C.; et al. Vitamin D supplementation to prevent acute respiratory infections: Individual participant data meta-analysis. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute Respiratory Tract Infection and 25-Hydroxyvitamin D Concentration: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savovic, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Wiley-Blackwell: Chichester, UK, 2020. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Ames, R.W.; Evans, M.C.; Gamble, G.D.; Sharpe, S.J. Long-term effects of calcium supplementation on bone loss and fractures in postmenopausal women: A randomized controlled trial. Am. J. Med. 1995, 98, 331–335. [Google Scholar] [CrossRef]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the anti-viral state. J. Clin. Virol. 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Wei, R.; Christakos, S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 2015, 7, 8251–8260. [Google Scholar] [CrossRef] [Green Version]
- White, J.H. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]

| Study | Region | Study Design (Type of Prevention) | Participants (Average Age, y; Women, %) | Duration of Supplementation, w (Follow-Up Period, w) | Intervention vs. Control | Main Outcome Measures | No. of Patients with Acute Respiratory Infection /No. of Study Participants | ||
|---|---|---|---|---|---|---|---|---|---|
| Supplement Group | Control Group | ||||||||
| 1 | 2009, Li-Ng et al. [24] | U.S. | RDBPCT | 148 healthy adults (59; 80) | 3 (3) | Vitamin D (2000 IU/d) vs. placebo | URI symptoms | 28/78 | 29/70 |
| 2 | 2010, Laaksi et al. [13] | Finland | RDBPCT | 164 healthy young men with military training (n.a.; 0) | 6 (6) | Vitamin D (400 IU/d) vs. placebo | common cold symptoms | 45/80 | 44/84 |
| 3 | 2010, Urashima et al. [14] | Japan | RDBPCT | 334 Children (10; 44) | 4 (4) | Vitamin D (1200 IU/d) vs. placebo | Influenza A infection | 18/167 | 31/167 |
| 4 | 2012, Camargo et al. [15] | Mongolia | RDBPCT | 244 Children (10; 48) | 3 (3) | Vitamin D (300 IU/d) + milk vs. milk | Acute respiratory infection | 31/141 | 52/103 |
| 5 | 2012, Manaseki et al. [25] | Afghanistan | RDBPCT | 3046 healthy infants (n.a.; 48) | 18 (18) | Vitamin D (100,000 IU/3 m) vs. placebo | pneumonia with CXR | 260/1524 | 245/1522 |
| 6 | 2012, Murdoch et al. [26] | New Zealand | RDBPCT | 322 healthy adults (48; 75) | 18 (18) | Vitamin D (100,000 IU/m) vs. placebo | URI symptoms | 154/161 | 155/161 |
| 7 | 2013, Rees et al. [27] | n.a. | RDBPCT | 759 healthy adults with history of colorectal adenoma (58; 42) | n.a. | Vitamin D (1000 IU/d) vs. placebo | URI symptoms | 303/399 | 276/360 |
| 8 | 2014, Goodall et al. [16] | U.S. | RDBPCT | 492 healthy students (19; 64) | 1 (1) | Vitamin D (10,000 IU/d) vs. placebo | URI symptoms | 70/258 | 80/234 |
| 9 | 2014, Slow et al. [17] | New Zealand | RDBPCT | 207 non-S. aureus nasal carriage adults (48; 75) | 18 (18) | Vitamin D (100,000 IU/m) vs. placebo | S. aureus nasal carriage, culture positive | 28/110 | 17/97 |
| 10 | 2014, Urashima et al. [28] | Japan | RDBPCT | 247 adolescents never have Influenza A (n.a.; 34) | 2 (2) | Vitamin D (2000 IU/d) vs. placebo | Influenza-like illness | 32/148 | 17/99 |
| 11 | 2015, Bergman et al. [18] | Sweden | RDBPCT | 124 patients with primary immunodeficiency (n.a.; n.a.) | 12 (12) | Vitamin D (4000 IU/d) vs. placebo | URI symptoms | 26/62 | 39/62 |
| 12 | 2015, Martineau et al. A (ViDiFlu) [29] | U.K. | RDBPCT | 217 residents of sheltered accommodation housing blocks (67; 66) | 12 (12) | Vitamin D (120,000 IU/2 m + 400 IU/d) vs. Vitamin D ( 400 IU/d) + placebo | ARI symptoms | 83/125 | 58/92 |
| 13 | 2015, Martineau et al. B (ViDiCO) [30] | U.K. | RDBPCT | 205 patients with COPD, emphysema, chronic bronchitis (65; 40) | 12 (12) | Vitamin D (120,000 IU/2 m) vs. placebo | URI symptoms | 76/102 | 75/103 |
| 14 | 2015, Martineau et al. C (ViDiAs) [31] | U.K. | RDBPCT | 232 patients with asthma (48; 57) | 12 (12) | Vitamin D (120,000 IU/2 m) vs. placebo | URI symptoms | 85/115 | 93/117 |
| 15 | 2015, Mayan et al. [32] | Israel | RDBPCT | 55 adolescent swimmers (15; 36) | 12 (12) | Vitamin D (2000 IU/d) vs. placebo | URI symptoms | 11/28 | 11/27 |
| 16 | 2016, Denlinger et al. [33] | n.a. | RDBPCT | 408 patients with asthma (n.a.) | 28 (28) | Vitamin D (4000 IU/d) vs. placebo | URI symptoms | 161/201 | 139/207 |
| 17 | 2016, Gupta et al. [34] | India | RDBPCT | 314 children with pneumonia (12 m; 30) | once (6) | Vitamin D (100,000 IU) vs. placebo | pneumonia | 39/156 | 36/158 |
| 18 | 2017, Aglipay et al. [35] | Canada | RDBPCT | 703 healthy children (3; 42) | 4–8 (4–8) | Vitamin D (2000 IU/d + 400 IU/d) vs. Vitamin D (400 IU/d) | URI | 184/349 | 193/354 |
| 19 | 2017, Ginde et al. [19] | U.S. | RDBPCT | 107 long term care residents (81; 58) | 12 (12) | Vitamin D (100,000 IU/m) vs. Vitamin D (1200 IU/m) | URI symptoms | 17/55 | 24/52 |
| 20 | 2018, Brett et al. [36] | Canada | OLRCT | 49 healthy children (6; 47) | 3 (3) | Vitamin D fortified food (600 IU/d) vs. placebo | common cold symptoms | 13/25 | 14/24 |
| 21 | 2018, Hibbs et al. [20] | U.S. | RDBPCT | 306 preterm black infants (n.a.; 67) | 6 (12) | Vitamin D (400 IU/d) vs. placebo | URI | 84/153 | 83/153 |
| 22 | 2018, Shimizu et al. [21] | Japan | RDBPCT | 215 healthy adults (54; 69) | 16 (16) | Vitamin D (400 IU/d) vs. placebo | URI symptoms | 41/110 | 43/105 |
| 23 | 2018, Zhou et al. [22] | China | OLRCT | 332 healthy infants (8; 48) | 4 (4) | Vitamin D (1200 IU/d + 400 IU/d) vs. Vitamin D (400 IU/d) | Influenza A | 43/164 | 78/168 |
| 24 | 2019, Arihiro et al. [23] | Japan | RDBPCT | 223 patients with IBD (45; 39) | 6 (6) | Vitamin D (500 IU/d) vs. placebo | URI symptoms | 19/108 | 30/115 |
| 25 | 2019, Loeb et al. [37] | Vietnam | RDBPCT | 1300 healthy children and adolescent (9; 52) | 8 (8) | Vitamin D (14,000 IU/w) vs. placebo | Influenza A or B | 50/650 | 43/650 |
| 26 | 2019, Singh et al. [38] | n.a. | OLRCT | 100 children with pneumonia (n.a.; 42) | 8 (12) | Vitamin D (300,000/3 m) + milk vs. placebo + milk | LRI symptoms | 28/50 | 34/50 |
| 27 | 2020, Camargo et al. [39] | New Zealand | RDBPCT | 5056 healthy adults (66; 42) | 19.2 (19.2) | Vitamin D (100,000 IU/m) vs. placebo | ARI symptoms | 1882/2539 | 1855/2517 |
| 28 | 2020, Ganmaa et al. [40] | Mongolia | RDBPCT | 8117 children without TB (9; 49) | 36 (36) | Vitamin D (14,000 IU/w) vs. placebo | Pulmonary TB, QFT results | 147/4074 | 134/4043 |
| 29 | 2020, Sudfeld et al. [41] | Tanzania | RDBPCT | 3639 patients with HIV with ART (39; 32) | 12 (12) | Vitamin D (50,000 IU/w than 2000 IU/d) vs. placebo | Pulmonary TB | 50/1812 | 64/1827 |
| 30 | 2021, Pham et al. [42] | Australia | RDBPCT | 2598 healthy adults (n.a.; 51) | 60 (60) | Vitamin D (60,000 IU/m) vs. placebo | ARI symptoms | 410/1318 | 404/1280 |
| Study | Randomization | Description of Randomization Methods | DOUBLE-BLIND | Using Identical Placebo | Follow-Up Reporting | Total Score | |
|---|---|---|---|---|---|---|---|
| 1 | 2009, Li-Ng et al. [24] | 1 | 1 | 1 | 0 | 1 | 4 |
| 2 | 2010, Laaksi et al. [13] | 1 | 1 | 1 | 1 | 1 | 5 |
| 3 | 2010, Urashima et al. [14] | 1 | 1 | 1 | 1 | 1 | 5 |
| 4 | 2012, Camargo et al. [15] | 1 | 1 | 1 | 1 | 1 | 5 |
| 5 | 2012, Manaseki et al. [25] | 1 | 1 | 1 | 1 | 1 | 5 |
| 6 | 2012, Murdoch et al. [26] | 1 | 1 | 1 | 1 | 1 | 5 |
| 7 | 2013, Rees et al. [27] | 1 | 1 | 1 | 1 | 1 | 5 |
| 8 | 2014, Goodall et al. [16] | 1 | 1 | 1 | 1 | 1 | 5 |
| 9 | 2014, Slow et al. [17] | 1 | 0 | 1 | 1 | 1 | 4 |
| 10 | 2014, Urashima et al. [28] | 1 | 1 | 1 | 1 | 1 | 5 |
| 11 | 2015, Bergman et al. [18] | 1 | 1 | 1 | 0 | 1 | 4 |
| 12 | 2015, Martineau et al. A (ViDiFlu) [29] | 1 | 0 | 1 | 0 | 1 | 3 |
| 13 | 2014, Martineau et al. B (ViDiCO) [30] | 1 | 1 | 1 | 1 | 1 | 5 |
| 14 | 2015, Martineau et al. C (ViDiAs) [31] | 1 | 1 | 1 | 1 | 1 | 5 |
| 15 | 2015, Mayan et al. [32] | 1 | 0 | 1 | 1 | 1 | 4 |
| 16 | 2016, Denlinger et al. [33] | 1 | 0 | 1 | 0 | 1 | 3 |
| 17 | 2016, Gupta et al. [34] | 1 | 1 | 1 | 1 | 1 | 5 |
| 18 | 2017, Aglipay et al. [35] | 1 | 1 | 1 | 1 | 1 | 5 |
| 19 | 2017, Ginde et al. [19] | 1 | 1 | 1 | 1 | 1 | 5 |
| 20 | 2018, Brett et al. [36] | 1 | 0 | 0 | 0 | 0 | 1 |
| 21 | 2018, Hibbs et al. [20] | 1 | 1 | 1 | 1 | 1 | 5 |
| 22 | 2018, Shimizu et al. [21] | 1 | 0 | 1 | 1 | 1 | 5 |
| 23 | 2018, Zhou et al. [22] | 1 | 0 | 0 | 0 | 1 | 2 |
| 24 | 2019, Arihiro et al. [23] | 1 | 1 | 1 | 0 | 1 | 4 |
| 25 | 2019, Loeb et al. [37] | 1 | 1 | 1 | 1 | 1 | 5 |
| 26 | 2019, Singh et al. [38] | 1 | 0 | 0 | 1 | 1 | 3 |
| 27 | 2020, Camargo et al. [39] | 1 | 1 | 1 | 1 | 1 | 5 |
| 28 | 2020, Ganmaa et al. [40] | 1 | 0 | 1 | 0 | 1 | 3 |
| 29 | 2020, Sudfeld et al. [41] | 1 | 1 | 1 | 1 | 1 | 5 |
| 30 | 2021, Pham et al. [42] | 1 | 1 | 1 | 1 | 1 | 5 |
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | No. of Low Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| 2009, Li-Ng et al. [24] | Low | Unclear | Low | High | Low | Low | Low | 5 |
| 2010, Laaksi et al. [13] | Low | Low | Unclear | Low | Unclear | Low | Low | 5 |
| 2010, Urashima et al. [14] | Low | Low | Low | Low | Unclear | Low | Low | 6 |
| 2012, Camargo et al. [15] | Low | Low | Low | Unclear | Low | Unclear | Low | 5 |
| 2012, Manaseki et al. [25] | Low | Low | Low | Low | Unclear | Low | Low | 6 |
| 2012, Murdoch et al. [26] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2013, Rees et al. [27] | Low | Low | Low | High | Unclear | Low | Low | 5 |
| 2014, Goodall et al. [16] | Low | Low | Low | Low | Unclear | Unclear | Low | 5 |
| 2014, Slow et al. [17] | Unclear | Unclear | Low | Low | Low | Low | Low | 5 |
| 2014, Urashima et al. [28] | Low | Low | Low | Unclear | Low | Low | Low | 6 |
| 2015, Bergman et al. [18] | Low | Low | Low | Low | Unclear | Unclear | Low | 5 |
| 2015, Martineau et al. A (ViDiFlu) [29] | Low | Unclear | Low | Low | Low | Low | Low | 6 |
| 2014, Martineau et al. B (ViDiCO) [30] | Low | Unclear | Low | Unclear | Low | Low | Low | 5 |
| 2015, Martineau et al. C (ViDiAs) [31] | Low | Low | Low | Unclear | Low | Low | Low | 6 |
| 2015, Mayan et al. [32] | Unclear | High | Unclear | Unclear | Unclear | Low | Low | 2 |
| 2016, Denlinger et al. [33] | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | 2 |
| 2016, Gupta et al. [34] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2017, Aglipay et al. [35] | Low | Low | Low | Low | Unclear | Low | Low | 6 |
| 2017, Ginde et al. [19] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2018, Brett et al. [36] | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | 2 |
| 2018, Hibbs et al. et al. [20] | Low | Unclear | Low | Unclear | Low | Low | Low | 5 |
| 2018, Shimizu et al. [21] | Low | Low | Low | Unclear | Unclear | Low | Low | 5 |
| 2018, Zhou et al. [22] | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Low | 2 |
| 2019, Arihiro et al. [23] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2019, Loeb et al. [37] | Low | Low | Low | Low | Unclear | Low | Low | 6 |
| 2019, Singh et al. [38] | Unclear | Low | Unclear | Unclear | Unclear | Low | Low | 3 |
| 2020, Camargo et al. [39] | Low | Low | Low | Unclear | Low | Low | Low | 6 |
| 2020, Ganmaa et al. [40] | Low | Unclear | Low | Low | Low | Low | Low | 6 |
| 2020, Sudfeld et al. [41] | Low | Low | Low | Low | Low | Low | Low | 7 |
| 2021, Pham et al. [42] | Low | Low | Low | Unclear | Unclear | Low | Low | 5 |
| Factors | No. of Trials | Summary RR (95% CI) | Heterogeneity, I2 (%) |
|---|---|---|---|
| All | 30 | 0.96 (0.91–1.01) | 59.0 |
| Duration of Vitamin D supplementation | |||
| Long term | 15 | 1.01 (0.9–1.06) | 38.1 |
| Short term | 13 | 0.83 (0.71–0.97) * | 66.8 |
| Jadad score | |||
| High quality | 9 | 0.88 (0.73–1.05) | 68.7 |
| Low quality | 4 | 0.71 (0.57–0.89) * | 26.7 |
| Cochrane ROB | |||
| High quality | 5 | 0.93 (0.74–1.16) | 43.6 |
| Low quality | 8 | 0.78 (0.64–0.97) * | 72.9 |
| Regimen | |||
| Daily | 15 | 0.83 (0.73–0.95) * | 69.1 |
| Jadad score | |||
| High quality | 10 | 0.89 (0.78–1.02) | 67.0 |
| Low quality | 5 | 0.69 (0.58–0.82) * | 0.0 |
| Cochrane ROB | |||
| High quality | 4 | 0.87 (0.66–1.15) | 51.0 |
| Low quality | 11 | 0.81 (0.69–0.96) * | 74.9 |
| Weekly | 3 | 1.10 (0.95–1.26) | 25.0 |
| Monthly | 10 | 1.00 (0.98–1.02) | 0.0 |
| Dose | |||
| High does (>2000 IU) | 8 | 0.95 (0.88–1.02) | 57.3 |
| Low dose (≤2000 IU) | 20 | 0.92 (0.85–1.00) * (0.997) | 59.5 |
| Type of Disease | |||
| URI | 24 | 0.97 (0.91–1.03) | 53.9 |
| LRI | 7 | 1.00 (0.91–1.11) | 0.0 |
| Number of study participants | |||
| >1000 | 6 | 1.00 (0.98–1.04) | 0.0 |
| ≤1000 | 24 | 0.92 (0.85–0.99) * | 68.7 |
| Region | |||
| America (Canada, U.S.) | 6 | 0.93 (0.84–1.03) | 0.0 |
| Europe (Finland, Sweden, UK) | 5 | 0.97 (0.86–1.09) | 36.9 |
| Asia (Afghanistan, China, India, Israel, Japan, Mongolia, Vietnam) | 11 | 0.85 (0.69–1.05) | 74.3 |
| Oceania (Australia, New Zealand) | 4 | 1.00 (0.98–1.03 | 0.0 |
| Type of prevention | |||
| Primary prevention | 26 | 0.94 (0.89–0.99) * | 58.5 |
| Secondary prevention | 4 | 1.05 (0.92–1.21) | 59.2 |
| Mean age | |||
| Children | 12 | 0.87 (0.75–1.02) | 70.9 |
| Adults | 18 | 0.99 (0.95–1.04) | 41.1 |
| Funding source | |||
| Pharmaceutical company | 8 | 0.99 (0.93–1.04) | 0.0 |
| Not pharmaceutical company | 22 | 0.94 (0.87–1.00) | 69.9 |
| Use of placebo | 29 | 0.98 (0.94–1.02) | 44.2 |
| Quality | |||
| Jadad score | |||
| High quality (≥5) | 18 | 1.00 (0.97–1.02) | 0.0 |
| Low quality (<5) | 12 | 0.85 (0.69–1.04) | 80.6 |
| Cochrane ROB | |||
| High quality (>5) | 14 | 1.00 (0.96–1.03) | 10.9 |
| Low quality (≤5) | 16 | 0.90 (0.81–1.01) | 73.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-E.; Myung, S.-K.; Cho, H. Efficacy of Vitamin D Supplements in Prevention of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials. Nutrients 2022, 14, 818. https://doi.org/10.3390/nu14040818
Cho H-E, Myung S-K, Cho H. Efficacy of Vitamin D Supplements in Prevention of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials. Nutrients. 2022; 14(4):818. https://doi.org/10.3390/nu14040818
Chicago/Turabian StyleCho, Hae-Eun, Seung-Kwon Myung, and Herim Cho. 2022. "Efficacy of Vitamin D Supplements in Prevention of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials" Nutrients 14, no. 4: 818. https://doi.org/10.3390/nu14040818
APA StyleCho, H.-E., Myung, S.-K., & Cho, H. (2022). Efficacy of Vitamin D Supplements in Prevention of Acute Respiratory Infection: A Meta-Analysis for Randomized Controlled Trials. Nutrients, 14(4), 818. https://doi.org/10.3390/nu14040818






