Mokko Lactone Alleviates Doxorubicin-Induced Cardiotoxicity in Rats via Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Toxicity Study
2.4. Experimental Protocol
2.5. Electrocardiography
2.6. Biochemical Assays and Measurements of Cardiac Enzymes
2.7. Histopathological Study
2.8. Assessment of Oxidative and Inflammatory Markers
2.9. Tissue Staining for Immunohistochemistry
2.10. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.11. Statistics
3. Results
3.1. Assessment of Acute Toxicity of ML
3.2. Assessment of Heart Electrical Activities
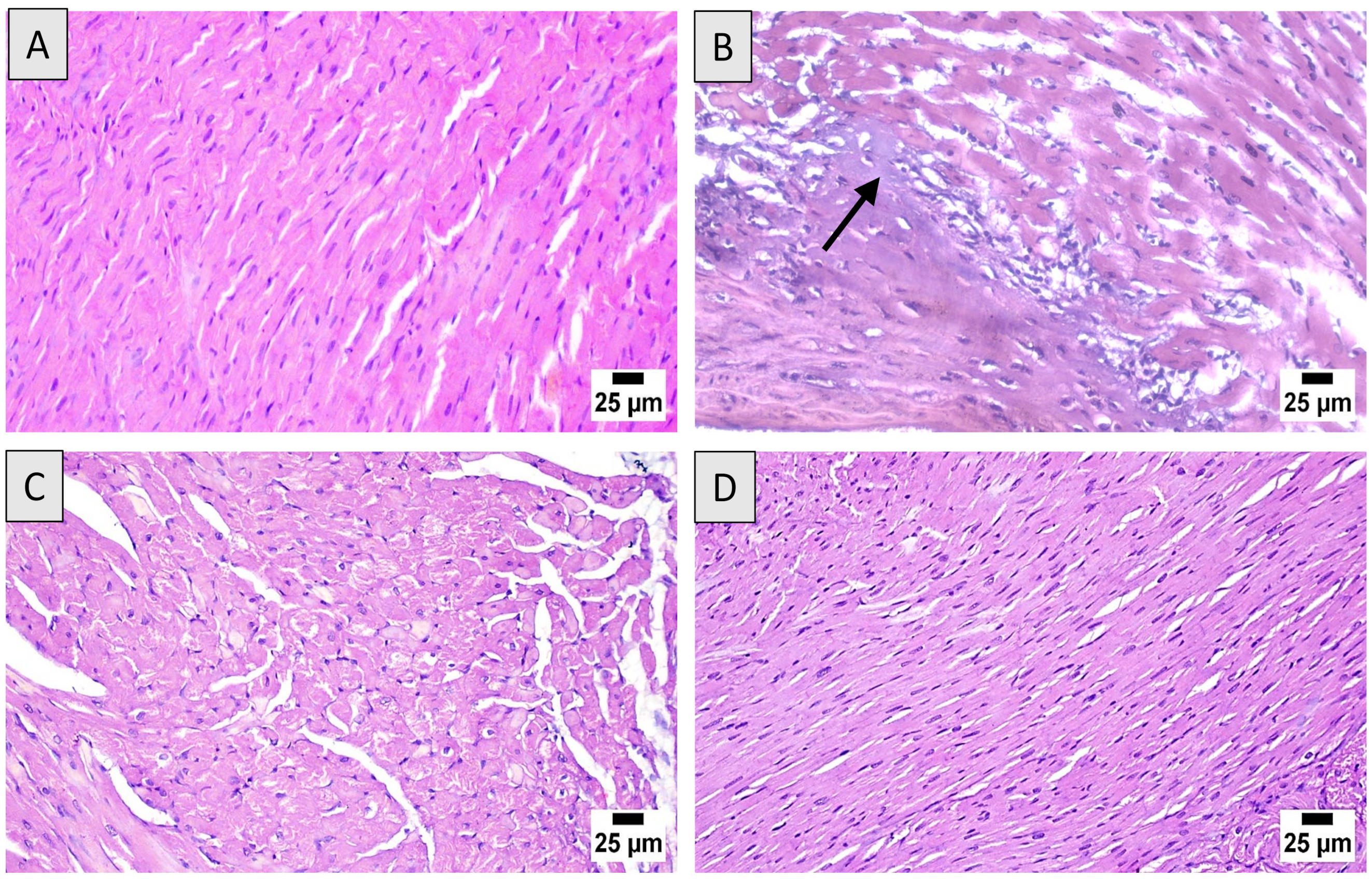
3.3. Histopathological Assessment
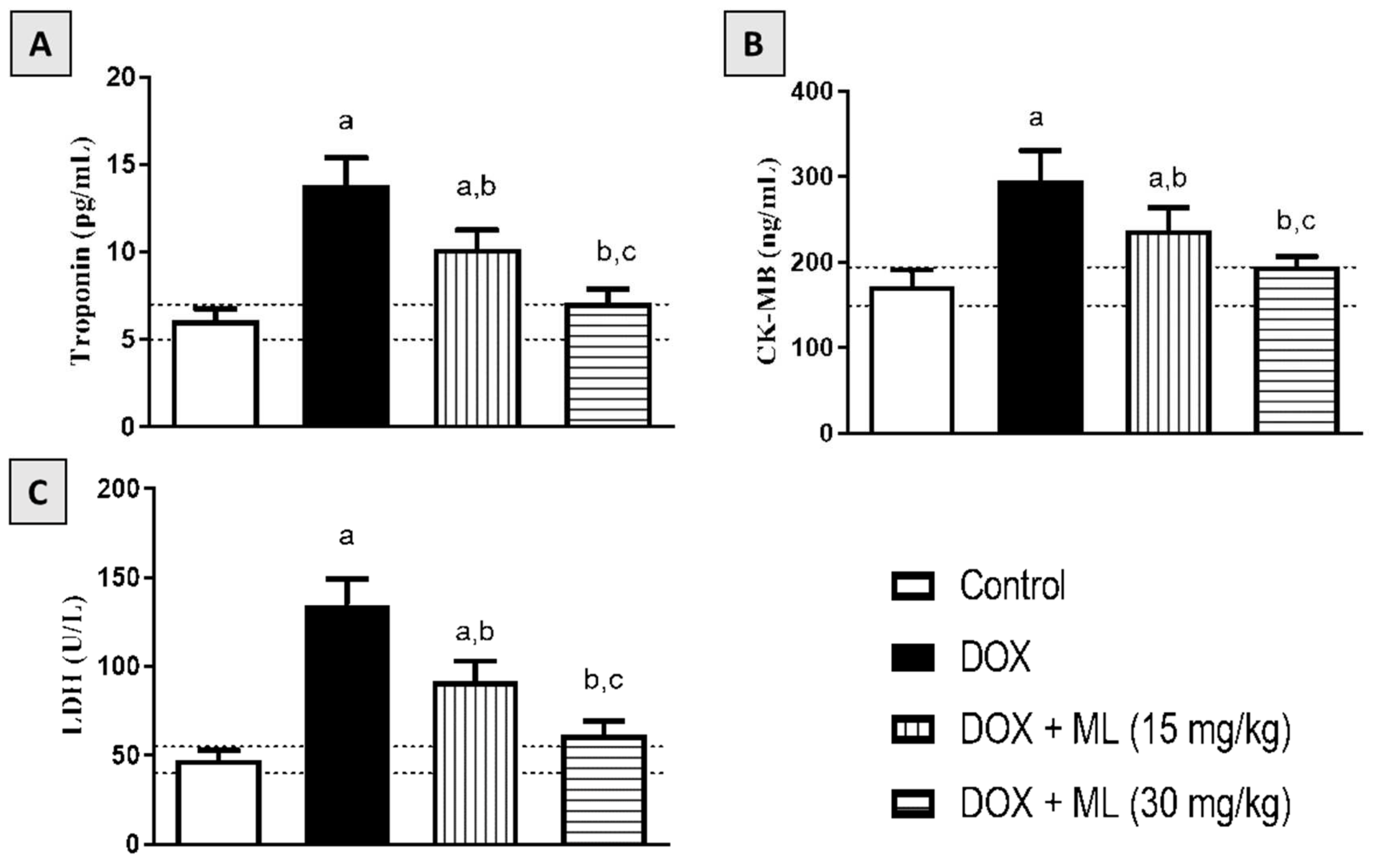
3.4. Effect of ML on Serum Cardiac Markers
3.5. Effect of ML on Cardiac Oxidative Stress
3.6. Effect of ML on Nrf2 and HO-1 Expression
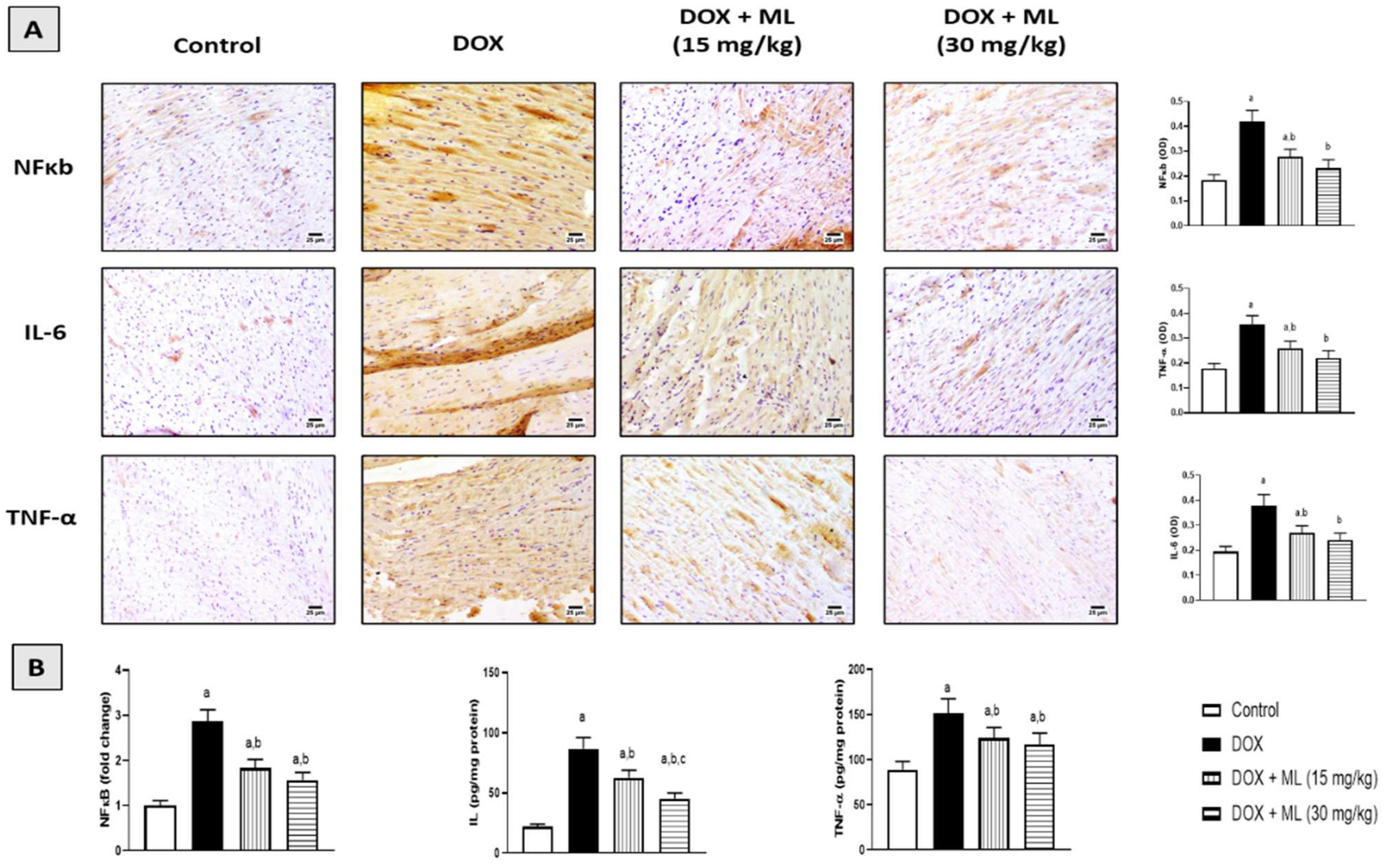
3.7. Effect of ML on Expression of Heart Inflammatory Markers
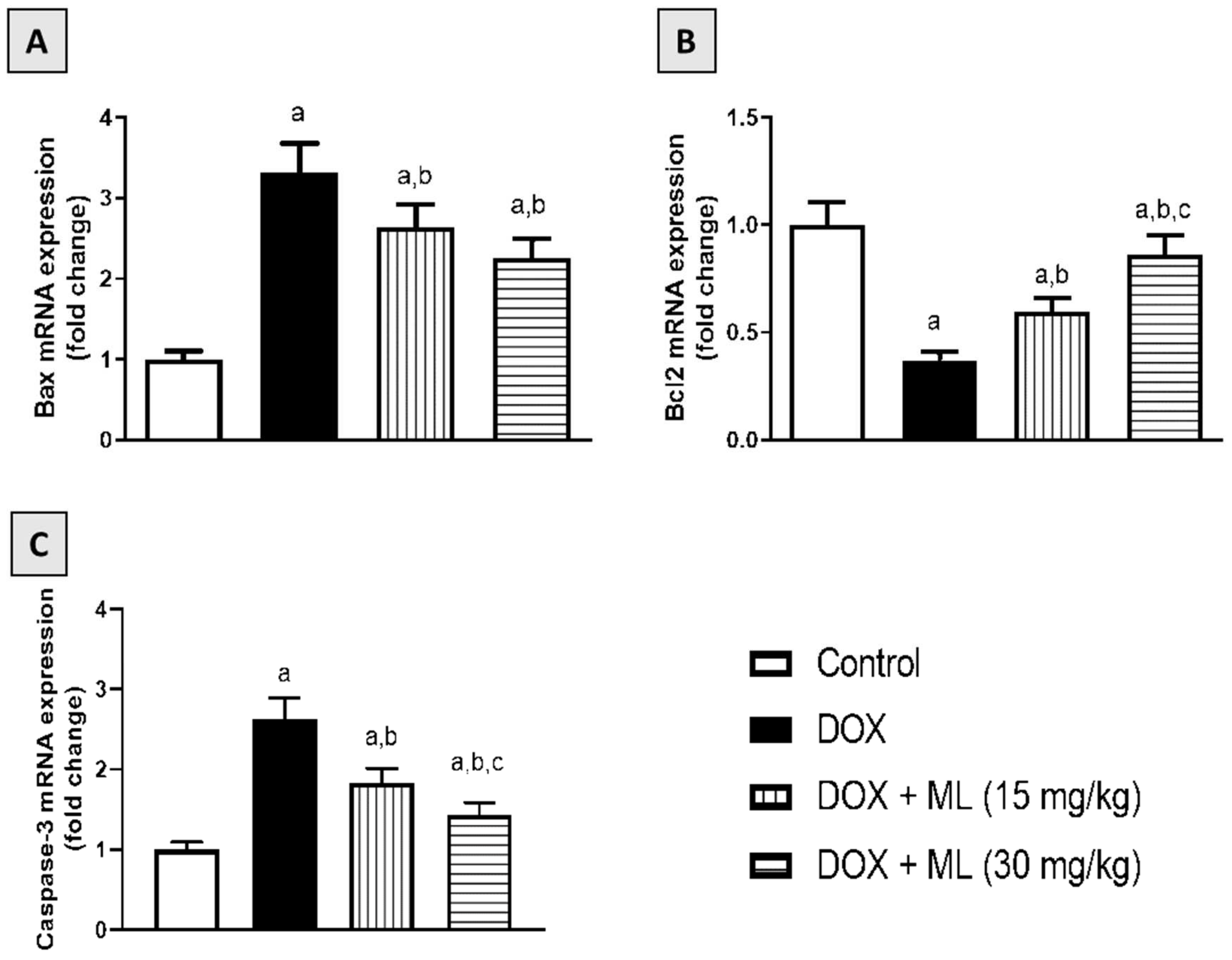
3.8. Effect of ML on Bax, Bcl-2, and Caspase-3 mRNA Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, D.; Aronow, W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp. Pract. 2019, 47, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment (Review). Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Pandapotan, M.; Satria, D. Protective effect of Vernonia amygdalina Delile against doxorubicin-induced cardiotoxicity. Heliyon 2021, 7, e07434. [Google Scholar] [CrossRef] [PubMed]
- Bennink, R.J.; van der Hoff, M.J.; Van Hemert, F.J.; De Bruin, K.M.; Spijkerboer, A.L.; Vanderheyden, J.-L.; Steinmetz, N.; Van Eck-Smit, B.L. Annexin V imaging of acute doxorubicin cardiotoxicity (apoptosis) in rats. J. Nucl. Med. 2004, 45, 842–848. [Google Scholar]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Autschbach, R. Doxorubicin in experimental and clinical heart failure. Eur. J. Cardio-Thoracic Surg. 2006, 30, 611–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk Factors for Doxorubicin-lnduced Congestive Heart Failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Billingham, M.E.; Mason, J.W.; Bristow, M.R.; Daniels, J.R. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat. Rep. 1978, 62, 865–872. [Google Scholar] [PubMed]
- Buja, L.M.; Ferrans, V.J.; Mayer, R.J.; Roberts, W.C.; Henderson, E.S. Cardiac ultrastructural changes induced by daunorubicin therapy. Cancer 1973, 32, 771–788. [Google Scholar] [CrossRef]
- Zilinyi, R.; Czompa, A.; Czegledi, A.; Gajtko, A.; Pituk, D.; Lekli, I.; Tosaki, A. The Cardioprotective Effect of Metformin in Doxorubicin-Induced Cardiotoxicity: The Role of Autophagy. Molecules 2018, 23, 1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Agamy, D.S.; Ibrahim, S.R.M.; Ahmed, N.; Khoshhal, S.; Abo-Haded, H.M.; Elkablawy, M.A.; Aljuhani, N.; Mohamed, G.A. Aspernolide F, as a new cardioprotective butyrolactone against doxorubicin-induced cardiotoxicity. Int. Immunopharmacol. 2019, 72, 429–436. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Lipshultz, S.E.; Alvarez, J.A.; Scully, R. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart 2007, 94, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Injac, R.; Strukelj, B. Recent Advances in Protection against Doxorubicin-induced Toxicity. Technol. Cancer Res. Treat. 2008, 7, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Huertas, J.R.; Battino, M.; Mataix, J.; Ramirez-Tortosa, M.C. Antioxidant nutrients and adriamycin toxicity. Toxicology 2002, 180, 79–95. [Google Scholar] [CrossRef]
- De La Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2018. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2019, 24, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Far, A.; Shaheen, H.M.; Alsenosy, A.E.-W.; El-Sayed, Y.; Al Jaouni, S.K.; Mousa, S. Costus speciosus: Traditional uses, phytochemistry, and therapeutic potentials. Pharmacogn. Rev. 2018, 12, 120. [Google Scholar] [CrossRef]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones fromArtemisiaGenus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.D.; Wilson, I.D. ChemInform Abstract: Insect Hormones and Insect Chemical Ecology. ChemInform 2001, 32, 263–375. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Mérillon, J.-M. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Al-Attas, A.A.; El-Shaer, N.S.; Mohamed, G.A.; Ibrahim, S.R.; Esmat, A. Anti-inflammatory sesquiterpenes from Costus speciosus rhizomes. J. Ethnopharmacol. 2015, 176, 365–374. [Google Scholar] [CrossRef]
- Sirwi, A.; Shaik, R.A.; Alamoudi, A.J.; Eid, B.G.; Kammoun, A.K.; Ibrahim, S.R.M.; Mohamed, G.A.; Abdallah, H.M.; Abdel-Naim, A.B. Mokko Lactone Attenuates Doxorubicin-Induced Hepatotoxicity in Rats: Emphasis on Sirt-1/FOXO1/NF-κB Axis. Nutrients 2021, 13, 4142. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin Cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Iliskovic, N. Doxorubicin-Induced Cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef]
- Kalivendi, S.V.; Konorev, E.A.; Cunningham, S.; Vanamala, S.K.; Kaji, E.H.; Joseph, J.; Kalyanaraman, B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: Role of mitochondrial reactive oxygen species and calcium. Biochem. J. 2005, 389, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Ahmed El-Shaer, N.S.A.; Asfour, H.Z.; Elshali, K.Z.; Awad Shaaban, M.I.; Al-Attas, A.A.M.; Allah Mo-hamed, G.A. Antimicrobial, antiquorum sensing, and antiproliferative activities of sesquiterpenes from Costus speciosus rhizomes. Pak. J. Pharm. Sci. 2019, 32, 109–115. [Google Scholar] [PubMed]
- Lim, K.H.; Ko, D.; Kim, J.-H. Cardioprotective potential of Korean Red Ginseng extract on isoproterenol-induced cardiac injury in rats. J. Ginseng Res. 2013, 37, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaul, N.; Siveski-Iliskovic, N.; Hill, M.; Slezak, J.; Singal, P.K. Free radicals and the heart. J. Pharmacol. Toxicol. Methods 1993, 30, 55–67. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, B.Y. Costunolide—A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Yan, M.; Chen, L.; Fang, P.; Li, Z.; Wan, Z.; Cao, S.; Hou, Z.; Wei, S.; Li, W.; et al. Nrf2-dependent antioxidant response mediated the protective effect of tanshinone IIA on doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 2018, 16, 3333–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, W.; Niu, T.; Wang, H.; Li, B.; Shao, L.; Lai, Y.; Li, H.; Janicki, J.S.; Wang, X.L.; et al. Nrf2 Deficiency Exaggerates Doxorubicin-Induced Cardiotoxicity and Cardiac Dysfunction. Oxidative Med. Cell. Longev. 2014, 2014, 748524. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.-L.; Bai, X.; Zhang, L. rhTNFR:Fc increases Nrf2 expression via miR-27a mediation to protect myocardium against sepsis injury. Biochem. Biophys. Res. Commun. 2015, 464, 855–861. [Google Scholar] [CrossRef]
- Zhang, S.; You, Z.-Q.; Yang, L.; Li, L.-L.; Wu, Y.-P.; Gu, L.-Q.; Xin, Y.-F. Protective effect of Shenmai injection on doxorubicin-induced cardiotoxicity via regulation of inflammatory mediators. BMC Complement. Altern. Med. 2019, 19, 317. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Shi, J.; Li, Y.-J.; Wei, L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp. 2009, 57, 435–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niture, S.K.; Jaiswal, A.K. Nrf2 Protein Up-regulates Antiapoptotic Protein Bcl-2 and Prevents Cellular Apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2 by costunolide provides neuroprotective effect in PC12 cells. Food Funct. 2019, 10, 4143–4152. [Google Scholar] [CrossRef]
- Cheong, C.-U.; Yeh, C.-S.; Hsieh, Y.-W.; Lee, Y.-R.; Lin, M.-Y.; Chen, C.-Y.; Lee, C.-H. Protective Effects of Costunolide against Hydrogen Peroxide-Induced Injury in PC12 Cells. Molecules 2016, 21, 898. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Yi, M.; Wang, R.; Huang, Y.; Chen, M. Protective Effects of Costunolide Against D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Front. Pharmacol. 2018, 9, 1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Control | DOX | DOX + ML (15 mg/kg) | DOX + ML (30 mg/kg) | |
|---|---|---|---|---|
| P wave (duration, ms) | 30 ± 1 (29–30) | 22 ± 5 a (17–27) | 26 ± 1 (25–27) | 34± 1 b (33–35) |
| QRS complex (duration, ms) | 63 ± 2 (61–65) | 30 ± 1 a (29–31) | 40 ± 2 (38–42) | 65 ± 1 b (64–66) |
| QRS amplitude (µV) | 82 ± 2 (80–84) | 140 ± 10 a (130–150) | 60 ± 2 b (58–62) | 80± 1 b (79–81) |
| QT interval (duration, ms) | 50 ± 1 (49–51) | 72 ± 3 a (69–75) | 60 ± 1 (59–61) | 54± 1 b (53–55) |
| PR interval (duration, ms) | 20 ± 1 (19–21) | 25 ± 1 a (24–26) | 22 ± 1 (21–23) | 19 ± 1 b (18–20) |
| RR interval (duration, ms) | 150 ± 1 (149–151) | 260 ± 1 a (259–261) | 220 ± 3 (217–223) | 130 ± 3 b (127–133) |
| ST segment amplitude (µV) | 51 ± 3 (48–54) | 180 ± 3 a (177–183) | 100 ± 3 (97–103) | 55± 1 b (54–56) |
| Control | DOX | DOX + ML (15 mg/kg) | DOX + ML (30 mg/kg) | |
|---|---|---|---|---|
| Disruption of cardiac muscles | - | +++ | ++ | + |
| Interstitial edema | - | +++ | ++ | + |
| Inflammatory cellular infiltrate | - | +++ | ++ | + |
| Apoptosis | - | ++ | + | ± |
| Necrosis (nuclear pyknosis, karyolysis, karyorrhexis) | - | ++ | + | ± |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirwi, A.; Shaik, R.A.; Alamoudi, A.J.; Eid, B.G.; Elfaky, M.A.; Ibrahim, S.R.M.; Mohamed, G.A.; Abdallah, H.M.; Abdel-Naim, A.B. Mokko Lactone Alleviates Doxorubicin-Induced Cardiotoxicity in Rats via Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Nutrients 2022, 14, 733. https://doi.org/10.3390/nu14040733
Sirwi A, Shaik RA, Alamoudi AJ, Eid BG, Elfaky MA, Ibrahim SRM, Mohamed GA, Abdallah HM, Abdel-Naim AB. Mokko Lactone Alleviates Doxorubicin-Induced Cardiotoxicity in Rats via Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Nutrients. 2022; 14(4):733. https://doi.org/10.3390/nu14040733
Chicago/Turabian StyleSirwi, Alaa, Rasheed A. Shaik, Abdulmohsin J. Alamoudi, Basma G. Eid, Mahmoud A. Elfaky, Sabrin R. M. Ibrahim, Gamal A. Mohamed, Hossam M. Abdallah, and Ashraf B. Abdel-Naim. 2022. "Mokko Lactone Alleviates Doxorubicin-Induced Cardiotoxicity in Rats via Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities" Nutrients 14, no. 4: 733. https://doi.org/10.3390/nu14040733
APA StyleSirwi, A., Shaik, R. A., Alamoudi, A. J., Eid, B. G., Elfaky, M. A., Ibrahim, S. R. M., Mohamed, G. A., Abdallah, H. M., & Abdel-Naim, A. B. (2022). Mokko Lactone Alleviates Doxorubicin-Induced Cardiotoxicity in Rats via Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Nutrients, 14(4), 733. https://doi.org/10.3390/nu14040733











