Fibroblast Growth Factor 19 and Fibroblast Growth Factor 21 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Gastric Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Surgical Technique of Gastric Bypass (GB)
2.3. Metabolic Profiles and Blood Sampling
2.4. Measurement of the Plasma FGF 19, FGF 21, and Serum Total Bile Acid Levels
2.5. Definition of DM Complete Remission and Insulin Resistance
2.6. Definition of NAFLD Based on the Hepatic Steatosis Index (HSI)
2.7. Statistical Analysis
3. Results
3.1. Changes in Metabolic Profiles and Laboratory Data after GB
3.2. Characteristic Differences between DM-CR and DM-Non-CR Subjects
3.3. Characteristic Differences between HSI-I and HSI-Non-I Subjects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Chiang, D.J.; Pritchard, M.T.; Nagy, L.E. Obesity, diabetes mellitus, and liver fibrosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 300, G697–G702. [Google Scholar] [CrossRef] [PubMed]
- Clamp, L.D.; Hume, D.J.; Lambert, E.V.; Kroff, J. Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history. Nutr. Diabetes 2017, 7, e282. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Ishigami, M.; Watanabe, Y.; Sumi, H.; Doisaki, M.; Yamaguchi, T.; Ito, T.; Ishizu, Y.; Kuzuya, T.; Honda, T.; et al. Effect of weight change and lifestyle modifications on the development or remission of nonalcoholic fatty liver disease: Sex-specific analysis. Sci. Rep. 2020, 10, 481. [Google Scholar] [CrossRef]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Jurowich, C.; Thalheimer, A.; Hartmann, D.; Bender, G.; Seyfried, F.; Germer, C.T.; Wichelmann, C. Improvement of type 2 diabetes mellitus (T2DM) after bariatric surgery--who fails in the early postoperative course? Obes. Surg. 2012, 22, 1521–1526. [Google Scholar] [CrossRef]
- Huang, H.H.; Lee, W.J.; Chen, S.C.; Chen, T.F.; Lee, S.D.; Chen, C.Y. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. J. Clin. Med. 2019, 8, 815. [Google Scholar] [CrossRef]
- Bastos, E.C.; Barbosa, E.M.; Soriano, G.M.; dos Santos, E.A.; Vasconcelos, S.M. Determinants of weight regain after bariatric surgery. Arq. Bras. De Cir. Dig. 2013, 26, 26–32. [Google Scholar] [CrossRef]
- Wu, W.C.; Lee, W.J.; Lee, T.H.; Chen, S.C.; Chen, C.Y. Do different bariatric surgical procedures influence plasma levels of matrix metalloproteinase-2, -7, and -9 among patients with type 2 diabetes mellitus? World J. Diabetes 2020, 11, 252–260. [Google Scholar] [CrossRef]
- Júnior, W.S.; Nonino-Borges, C.B. Clinical predictors of different grades of nonalcoholic fatty liver disease. Obes. Surg. 2012, 22, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Chen, J.C.; Chen, C.Y. Is there any useful surrogate to evaluate metabolic fatty liver disease? J. Chin. Med. Assoc. 2021, 84, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015, 26, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Kemper, J.K. MicroRNA-34a and Impaired FGF19/21 Signaling in Obesity. Vitam. Horm. 2016, 101, 175–196. [Google Scholar] [CrossRef]
- Fu, L.; John, L.M.; Adams, S.H.; Yu, X.X.; Tomlinson, E.; Renz, M.; Williams, P.M.; Soriano, R.; Corpuz, R.; Moffat, B.; et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004, 145, 2594–2603. [Google Scholar] [CrossRef]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Adams, A.C.; Coskun, T.; Rovira, A.R.; Schneider, M.A.; Raches, D.W.; Micanovic, R.; Bina, H.A.; Dunbar, J.D.; Kharitonenkov, A. Fundamentals of FGF19 & FGF21 action in vitro and in vivo. PLoS ONE 2012, 7, e38438. [Google Scholar] [CrossRef]
- Tomlinson, E.; Fu, L.; John, L.; Hultgren, B.; Huang, X.; Renz, M.; Stephan, J.P.; Tsai, S.P.; Powell-Braxton, L.; French, D.; et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002, 143, 1741–1747. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef]
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef]
- Lundåsen, T.; Gälman, C.; Angelin, B.; Rudling, M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 2006, 260, 530–536. [Google Scholar] [CrossRef]
- Barutcuoglu, B.; Basol, G.; Cakir, Y.; Cetinkalp, S.; Parildar, Z.; Kabaroglu, C.; Ozmen, D.; Mutaf, I.; Bayindir, O. Fibroblast growth factor-19 levels in type 2 diabetic patients with metabolic syndrome. Ann. Clin. Lab. Sci. 2011, 41, 390–396. [Google Scholar] [PubMed]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Garza, Ú.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges. Int. J. Mol. Sci. 2019, 20, 4692. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Mai, K.; Andres, J.; Biedasek, K.; Weicht, J.; Bobbert, T.; Sabath, M.; Meinus, S.; Reinecke, F.; Möhlig, M.; Weickert, M.O.; et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 2009, 58, 1532–1538. [Google Scholar] [CrossRef]
- Iroz, A.; Montagner, A.; Benhamed, F.; Levavasseur, F.; Polizzi, A.; Anthony, E.; Régnier, M.; Fouché, E.; Lukowicz, C.; Cauzac, M.; et al. A Specific ChREBP and PPARα Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep. 2017, 21, 403–416. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- Dushay, J.; Chui, P.C.; Gopalakrishnan, G.S.; Varela-Rey, M.; Crawley, M.; Fisher, F.M.; Badman, M.K.; Martinez-Chantar, M.L.; Maratos-Flier, E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 456–463. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Gallego-Escuredo, J.M.; Catalán, V.; Rodríguez, A.; Domingo, P.; Moncada, R.; Valentí, V.; Salvador, J.; Giralt, M.; Villarroya, F.; et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin. Nutr. 2017, 36, 861–868. [Google Scholar] [CrossRef]
- Yeh, C.; Huang, H.H.; Chen, S.C.; Chen, T.F.; Ser, K.H.; Chen, C.Y. Comparison of consumption behavior and appetite sensations among patients with type 2 diabetes mellitus after bariatric surgery. PeerJ 2017, 5, e3090. [Google Scholar] [CrossRef]
- Lee, W.J.; Chen, C.Y.; Chong, K.; Lee, Y.C.; Chen, S.C.; Lee, S.D. Changes in postprandial gut hormones after metabolic surgery: A comparison of gastric bypass and sleeve gastrectomy. Surg. Obes. Relat. Dis. 2011, 7, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Chong, K.; Ser, K.H.; Lee, Y.C.; Chen, S.C.; Chen, J.C.; Tsai, M.H.; Chuang, L.M. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: A randomized controlled trial. Arch. Surg. 2011, 146, 143–148. [Google Scholar] [CrossRef]
- Wittgrove, A.C.; Clark, G.W. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: Technique and results, with 3-60 month follow-up. Obes. Surg. 2000, 10, 233–239. [Google Scholar] [CrossRef]
- Chiu, C.C.; Lee, W.J.; Wang, W.; Wei, P.L.; Huang, M.T. Prevention of trocar-wound hernia in laparoscopic bariatric operations. Obes. Surg. 2006, 16, 913–918. [Google Scholar] [CrossRef]
- Captieux, M.; Prigge, R.; Wild, S.; Guthrie, B. Defining remission of type 2 diabetes in research studies: A systematic scoping review. PLoS Med. 2020, 17, e1003396. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, P.Y.; Huang, H.H.; Yeh, C.; Chen, S.C.; Lee, W.J.; Chen, C.Y. Change of plasma amylin after bariatric surgery challenged by oral glucose is associated with remission of type 2 diabetes mellitus. J. Chin. Med. Assoc. 2021, 84, 1001–1006. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Lok, A.S. Use of Liver Imaging and Biopsy in Clinical Practice. N. Engl. J. Med. 2017, 377, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R.; Goldin, R.D.; Main, J.; Murray-Lyon, I.; Hargreaves, S.; Thomas, H.C. Management of chronic hepatitis C: Clinical audit of biopsy based management algorithm. BMJ 1997, 315, 453–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, T.Y.; Kim, M.S.; Hong, H.P.; Kang, K.A.; Jun, D.W. Comparative Assessment and External Validation of Hepatic Steatosis Formulae in a Community-Based Setting. J. Clin. Med. 2020, 9, 2851. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Haluzíková, D.; Lacinová, Z.; Kaválková, P.; Drápalová, J.; Křížová, J.; Bártlová, M.; Mráz, M.; Petr, T.; Vítek, L.; Kasalický, M.; et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity 2013, 21, 1335–1342. [Google Scholar] [CrossRef]
- Gerhard, G.S.; Styer, A.M.; Wood, G.C.; Roesch, S.L.; Petrick, A.T.; Gabrielsen, J.; Strodel, W.E.; Still, C.D.; Argyropoulos, G. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care 2013, 36, 1859–1864. [Google Scholar] [CrossRef]
- Ryan, P.M.; Hayward, N.E.; Sless, R.T.; Garwood, P.; Rahmani, J. Effect of bariatric surgery on circulating FGF-19: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13038. [Google Scholar] [CrossRef]
- Tucker, B.; Li, H.; Long, X.; Rye, K.A.; Ong, K.L. Fibroblast growth factor 21 in non-alcoholic fatty liver disease. Metabolism 2019, 101, 153994. [Google Scholar] [CrossRef]
- Zhang, J.; Gupte, J.; Gong, Y.; Weiszmann, J.; Zhang, Y.; Lee, K.J.; Richards, W.G.; Li, Y. Chronic Over-expression of Fibroblast Growth Factor 21 Increases Bile Acid Biosynthesis by Opposing FGF15/19 Action. EBioMedicine 2017, 15, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Rusli, F.; Deelen, J.; Andriyani, E.; Boekschoten, M.V.; Lute, C.; van den Akker, E.B.; Müller, M.; Beekman, M.; Steegenga, W.T. Fibroblast growth factor 21 reflects liver fat accumulation and dysregulation of signalling pathways in the liver of C57BL/6J mice. Sci. Rep. 2016, 6, 30484. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, H.; Fang, Q.; Zhang, J.; Zhang, M.; Zhang, L.; Wu, L.; Hou, X.; Lu, J.; Bao, Y.; et al. Complementary Role of Fibroblast Growth Factor 21 and Cytokeratin 18 in Monitoring the Different Stages of Nonalcoholic Fatty Liver Disease. Sci. Rep. 2017, 7, 5095. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, P.; Martin, R.C.; Cui, G.; Wang, G.; Tan, Y.; Cai, L.; Lv, G.; Li, Y. Lack of fibroblast growth factor 21 accelerates metabolic liver injury characterized by steatohepatities in mice. Am. J. Cancer Res. 2016, 6, 1011–1025. [Google Scholar]
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Grønbæk, H. Bariatric surgery in patients with non-alcoholic fatty liver disease-from pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–149. [Google Scholar] [CrossRef]
- Park, J.M.; Chiu, C.F.; Chen, S.C.; Lee, W.J.; Chen, C.Y. Changes in post-oral glucose challenge pancreatic polypeptide hormone levels following metabolic surgery: A comparison of gastric bypass and sleeve gastrectomy. Neuropeptides 2020, 81, 102032. [Google Scholar] [CrossRef]
- Chen, M.M.; Hale, C.; Stanislaus, S.; Xu, J.; Véniant, M.M. FGF21 acts as a negative regulator of bile acid synthesis. J. Endocrinol. 2018, 237, 139–152. [Google Scholar] [CrossRef]
- Harrison, S.A.; Neff, G.; Guy, C.D.; Bashir, M.R.; Paredes, A.H.; Frias, J.P.; Younes, Z.; Trotter, J.F.; Gunn, N.T.; Moussa, S.E.; et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology 2021, 160, 219–231. [Google Scholar] [CrossRef]
- Henriksson, E.; Andersen, B. FGF19 and FGF21 for the Treatment of NASH-Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human. Front. Endocrinol. 2020, 11, 601349. [Google Scholar] [CrossRef]
- Charles, E.D.; Neuschwander-Tetri, B.A.; Pablo Frias, J.; Kundu, S.; Luo, Y.; Tirucherai, G.S.; Christian, R. Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity 2019, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Sviklāne, L.; Olmane, E.; Dzērve, Z.; Kupčs, K.; Pīrāgs, V.; Sokolovska, J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J. Gastroenterol. Hepatol. 2018, 33, 270–276. [Google Scholar] [CrossRef]
- Kahl, S.; Straßburger, K.; Nowotny, B.; Livingstone, R.; Klüppelholz, B.; Keßel, K.; Hwang, J.H.; Giani, G.; Hoffmann, B.; Pacini, G.; et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS ONE 2014, 9, e94059. [Google Scholar] [CrossRef] [PubMed]


| n = 35 | M0 | M3 | M12 | p Value |
|---|---|---|---|---|
| Metabolic profile | ||||
| Body weight (kg) | 84.78 ± 14.12 | 69.64 ± 9.68 | 63.86 ± 6.75 | <0.001 |
| BMI (kg/m2) | 31.63 ± 4.62 | 26.14 ± 3.19 | 24.41 ± 2.58 | <0.001 |
| Waist circumference (cm) | 103.60 ± 10.25 | 90.40 ± 7.96 | 82.33 ± 5.30 | <0.001 |
| Excess weight loss (%) | 19.00 ± 22.99 | 18.43 ± 10.83 | 0.346 | |
| ABSI | 0.081 ± 0.005 | 0.077 ± 0.017 | 0.077 ± 0.004 | <0.001 |
| Systolic blood pressure (mmHg) | 136.09 ± 14.84 | 132.54 ± 21.34 | 126.86 ± 16.31 | 0.076 |
| Diastolic blood pressure (mmHg) | 85.54 ± 10.73 | 81.23 ± 16.30 | 79.67 ± 14.15 | 0.277 |
| Laboratory data | ||||
| Creatinine (mg/dL) | 0.79 ± 0.32 | 0.76 ± 0.28 | 0.71 ± 0.29 | <0.001 |
| Fasting blood glucose (mg/dL) | 176.66 ± 70.64 | 127.03 ± 46.69 | 114.09 ± 31.11 | <0.001 |
| HbA1c (%) | 9.29 ± 1.52 | 7.07 ± 1.62 | 6.50 ± 1.16 | <0.001 |
| C-peptide (mg/dL) | 2.65 ± 1.19 | 1.69 ± 0.59 | 1.39 ± 0.50 | <0.001 |
| Insulin (mU/L) | 23.16 ± 28.90 | 7.10 ± 6.89 | 5.83 ± 6.86 | 0.007 |
| HOMA-IR index | 9.91 ± 13.39 | 2.03 ± 1.99 | 1.65 ± 2.02 | <0.001 |
| HOMA-β index | 1.15 ± 1.95 | 0.54 ± 0.59 | 0.50 ± 0.67 | 0.060 |
| ALT (U/L) | 41.60 ± 33.46 | 35.43 ± 33.42 | 33.29 ± 33.20 | 0.251 |
| AST (U/L) | 32.17 ± 28.09 | 29.61 ± 20.95 | 28.90 ± 26.14 | 0.364 |
| Alk-p (U/L) | 61.46 ± 19.09 | 81.04 ± 40.46 | 69.81 ± 19.46 | 0.268 |
| γ-GT (U/L) | 42.29 ± 27.87 | 33.37 ± 42.45 | 25.10 ± 22.18 | 0.006 |
| Total cholesterol (mg/dL) | 193.60 ± 42.93 | 176.21 ± 34.03 | 168.09 ± 33.23 | 0.063 |
| Triglyceride (mg/dL) | 231.37 ± 217.51 | 127.36 ± 57.10 | 105.25 ± 45.93 | 0.006 |
| HDL-C (mg/dL) | 41.57 ± 7.80 | 37.93 ± 7.83 | 46.31 ± 10.23 | <0.001 |
| LDL-C (mg/dL) | 117.89 ± 33.89 | 117.14 ± 31.26 | 105.69 ± 30.31 | 0.254 |
| Uric acid (mg/dL) | 5.64 ± 1.63 | 5.43 ± 1.46 | 5.11 ± 1.50 | 0.019 |
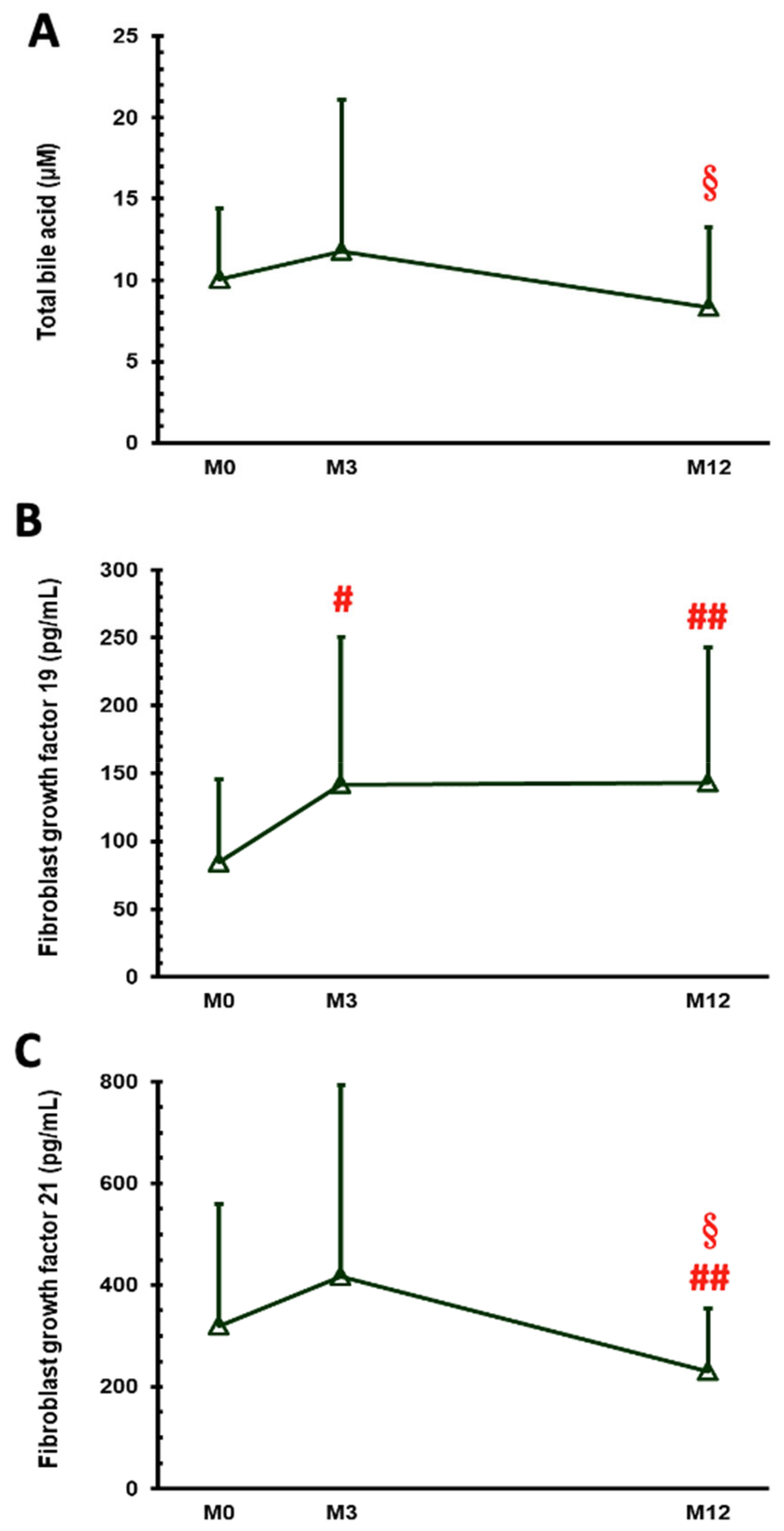
| Total bile acid (µM) | 10.07 ± 4.33 | 11.78 ± 9.32 | 8.31 ± 4.95 | 0.010 |
| FGF 19 (pg/mL) | 84.20 ± 61.31 | 141.76 ± 108.70 | 142.69 ± 100.21 | 0.024 |
| FGF 21 (pg/mL) | 320.06 ± 238.96 | 416.99 ± 375.86 | 230.24 ± 123.71 | 0.005 |
| HSI | 45.89 ± 6.39 | 38.96 ± 4.16 | 36.25 ± 2.61 | <0.001 |
| M0 | M12 | |||||
|---|---|---|---|---|---|---|
| DM-CR (n = 13) | DM-Non-CR (n = 22) | p Value | DM-CR (n = 13) | DM-Non-CR (n = 22) | p Value | |
| Metabolic profile | ||||||
| Body weight (kg) | 93.85 ± 16.25 | 79.42 ± 9.53 | 0.010 | 62.6 ± 7.78 | 64.43 ± 6.38 | 0.511 |
| BMI (kg/m2) | 34.73 ± 5.00 | 29.79 ± 3.27 | 0.001 | 24.88 ± 4.08 | 24.20 ± 1.64 | 0.641 |
| Waist circumference (cm) | 108.69 ± 10.22 | 100.45 ± 9.13 | 0.020 | 80.56 ± 4.61 | 83.13 ± 5.51 | 0.234 |
| ABSI | 0.080 ± 0.006 | 0.082 ± 0.004 | 0.246 | 0.076 ± 0.006 | 0.078 ± 0.003 | 0.584 |
| Systolic blood pressure (mmHg) | 130.92 ± 13.45 | 139.14 ± 15.07 | 0.115 | 124.00 ± 20.70 | 128.00 ± 14.91 | 0.624 |
| Diastolic blood pressure (mmHg) | 83.54 ± 10.82 | 86.73 ± 10.75 | 0.404 | 75.33 ± 14.07 | 81.0 ± 14.29 | 0.389 |
| Laboratory data | ||||||
| Creatinine (mg/dL) | 0.67 ± 0.15 | 0.86 ± 0.37 | 0.051 | 0.60 ± 0.11 | 0.76 ± 0.33 | 0.063 |
| Fasting blood glucose (mg/dL) | 164.69 ± 64.95 | 183.73 ± 74.34 | 0.449 | 89.70 ± 10.22 | 125.18 ± 31.18 | <0.001 |
| HbA1c (%) | 8.51 ± 1.42 | 9.76 ± 1.41 | 0.016 | 5.42 ± 0.38 | 7.09 ± 0.99 | <0.001 |
| C-peptide (mg/dL) | 3.23 ± 1.01 | 2.31 ± 1.18 | 0.026 | 1.35 ± 0.34 | 1.41 ± 0.57 | 0.791 |
| Insulin (mU/L) | 18.05 ± 9.24 | 26.19 ± 35.75 | 0.321 | 4.99 ± 3.07 | 6.24 ± 8.11 | 0.645 |
| HOMA-IR index | 7.21 ± 3.95 | 11.51 ± 16.55 | 0.256 | 1.06 ± 0.67 | 1.93 ± 2.38 | 0.152 |
| HOMA-β index | 0.81 ± 0.59 | 1.34 ± 2.41 | 0.331 | 0.61 ± 0.64 | 0.44 ± 0.69 | 0.479 |
| ALT (U/L) | 56.08 ± 40.45 | 33.05 ± 25.90 | 0.047 | 24.40 ± 17.56 | 37.52 ± 38.16 | 0.200 |
| AST (U/L) | 37.00 ± 27.61 | 29.32 ± 28.61 | 0.443 | 22.70 ± 12.05 | 31.86 ± 30.52 | 0.371 |
| Alk-p (U/L) | 64.46 ± 24.37 | 59.68 ± 15.55 | 0.482 | 62.70 ± 16.57 | 73.19 ± 20.18 | 0.164 |
| γ-GT (U/L) | 51.58 ± 34.04 | 36.42 ± 22.19 | 0.143 | 12.40 ± 5.30 | 31.45 ± 24.71 | 0.003 |
| Total cholesterol (mg/dL) | 186.62 ± 49.00 | 197.73 ± 39.54 | 0.468 | 155.50 ± 24.65 | 173.82 ± 35.50 | 0.151 |
| Triglyceride (mg/dL) | 179.08 ± 133.37 | 262.27 ± 252.47 | 0.212 | 74.00 ± 24.10 | 119.45 ± 46.79 | 0.001 |
| HDL-C (mg/dL) | 39.85 ± 7.71 | 42.59 ± 7.85 | 0.322 | 45.80 ± 7.94 | 46.55 ± 11.28 | 0.852 |
| LDL-C (mg/dL) | 119.46 ± 39.61 | 116.95 ± 31.00 | 0.836 | 95.10 ± 19.71 | 110.50 ± 33.34 | 0.187 |
| Uric acid (mg/dL) | 5.69 ± 1.58 | 5.61 ± 1.69 | 0.886 | 4.63 ± 1.18 | 5.33 ± 1.61 | 0.230 |
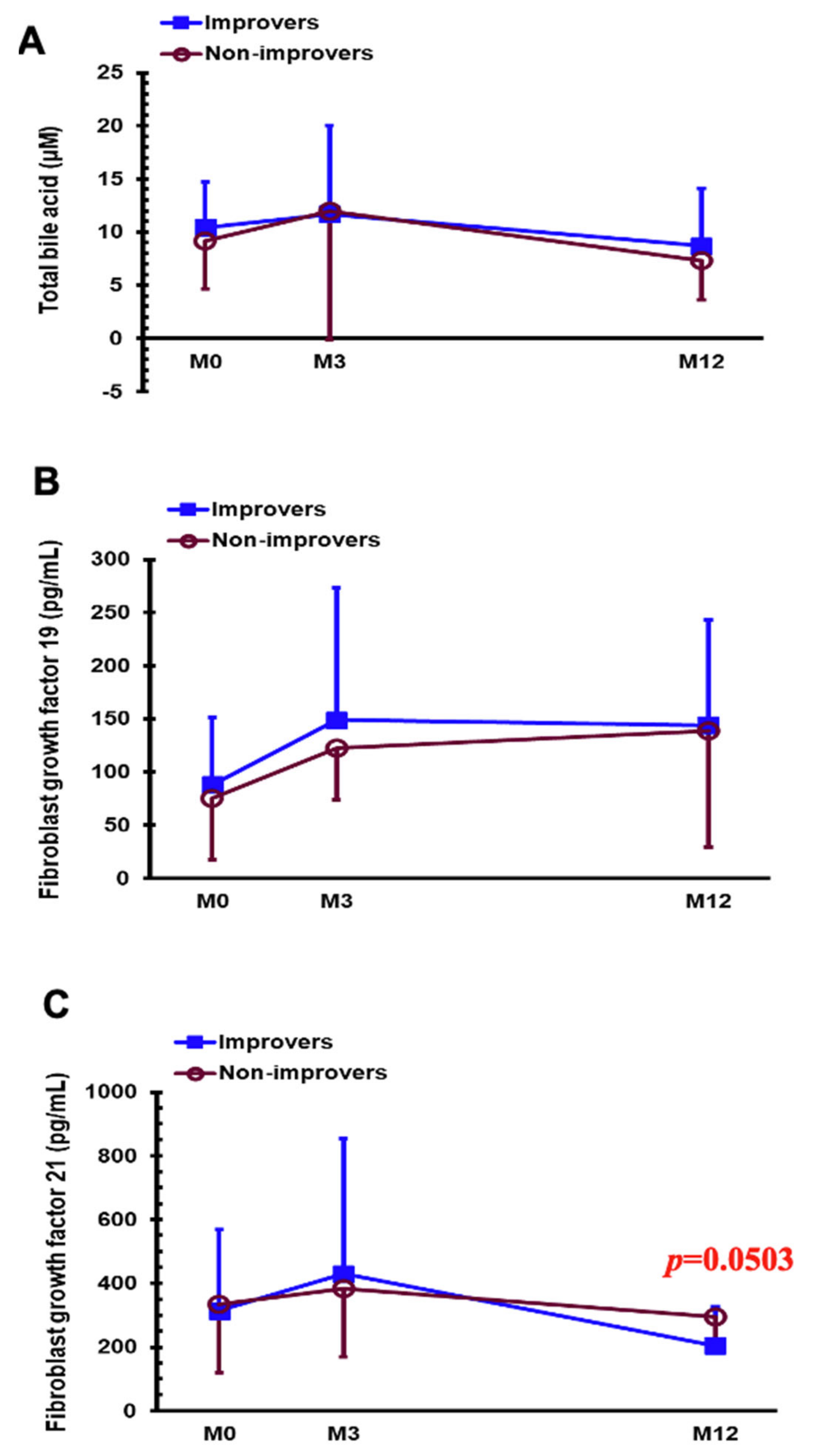
| Total bile acid (µM) | 9.97 ± 3.63 | 10.13 ± 4.77 | 0.919 | 7.61 ± 2.91 | 8.72 ± 5.86 | 0.462 |
| FGF 19 (pg/mL) | 63.73 ± 40.17 | 96.29 ± 68.93 | 0.131 | 162.41 ± 131.83 | 131.03 ± 77.10 | 0.445 |
| FGF 21 (pg/mL) | 370.14 ± 300.53 | 290.47 ± 195.89 | 0.348 | 221.17 ± 93.30 | 235.60 ± 140.44 | 0.744 |
| HSI | 51.30 ± 5.05 | 42.69 ± 4.75 | <0.001 | 36.08 ± 3.10 | 36.33 ± 2.44 | 0.831 |
| M0 | M12 | |||||
|---|---|---|---|---|---|---|
| HSI-I (n = 25) | HSI-Non-I (n = 10) | p Value | HSI-I (n = 25) | HSI-Non-I (n = 10) | p Value | |
| Metabolic profile | ||||||
| Body weight (kg) | 86.19 ± 15.56 | 81.26 ± 9.38 | 0.358 | 62.42 ± 6.57 | 66.59 ± 6.55 | 0.116 |
| BMI (kg/m2) | 31.98 ± 5.14 | 30.73 ± 2.98 | 0.476 | 23.96 ± 2.78 | 25.27 ± 2.02 | 0.199 |
| Waist circumference (cm) | 104.04 ± 11.09 | 102.55 ± 8.31 | 0.705 | 81.11 ± 4.30 | 84.65 ± 6.43 | 0.087 |
| ABSI | 0.081 ± 0.005 | 0.082 ± 0.004 | 0.606 | 0.077 ± 0.004 | 0.077 ± 0.005 | 0.995 |
| Systolic blood pressure (mmHg) | 139.60 ± 15.11 | 127.30 ± 10.14 | 0.024 | 129.58 ± 15.08 | 123.22 ± 18.07 | 0.390 |
| Diastolic blood pressure (mmHg) | 87.00 ± 11.60 | 81.90 ± 7.46 | 0.209 | 80.92 ± 14.49 | 78.00 ± 14.37 | 0.652 |
| Laboratory data | ||||||
| Creatinine (mg/dL) | 0.82 ± 0.36 | 0.72 ± 0.13 | 0.438 | 0.72 ± 0.35 | 0.68 ± 0.070 | 0.542 |
| Fasting blood glucose (mg/dL) | 161.44 ± 66.11 | 214.70 ± 70.31 | 0.042 | 108.91 ± 25.25 | 125.50 ± 40.45 | 0.166 |
| HbA1c (%) | 9.11 ± 1.70 | 9.76 ± 0.83 | 0.258 | 6.41 ± 1.11 | 6.72 ± 1.30 | 0.482 |
| C-peptide (mg/dL) | 2.51 ± 1.17 | 3.02 ± 1.22 | 0.255 | 1.27 ± 0.50 | 1.63 ± 0.44 | 0.062 |
| Insulin (mU/L) | 21.70 ± 24.92 | 26.82 ± 38.45 | 0.643 | 3.43 ± 1.78 | 10.88 ± 10.38 | 0.050 |
| HOMA-IR index | 7.94 ± 7.82 | 14.83 ± 21.82 | 0.353 | 0.81 ± 0.49 | 3.16 ± 2.80 | 0.027 |
| HOMA-β index | 1.33 ± 2.23 | 0.70 ± 0.88 | 0.397 | 0.33 ± 0.38 | 0.93 ± 1.01 | 0.098 |
| ALT (U/L) | 37.32 ± 32.73 | 52.30 ± 34.57 | 0.237 | 35.81 ± 39.21 | 28.00 ± 14.54 | 0.428 |
| AST (U/L) | 27.36 ± 21.08 | 44.20 ± 39.62 | 0.110 | 32.00 ± 33.78 | 22.40 ± 10.20 | 0.209 |
| Alk-p (U/L) | 64.20 ± 20.51 | 54.60 ± 13.49 | 0.183 | 70.33 ± 20.21 | 68.70 ± 18.79 | 0.831 |
| γ-GT (U/L) | 43.32 ± 31.22 | 39.78 ± 18.53 | 0.754 | 24.75 ± 19.72 | 25.80 ± 27.64 | 0.905 |
| Total cholesterol (mg/dL) | 193.40 ± 40.09 | 194.10 ± 51.72 | 0.966 | 168.18 ± 34.42 | 167.90 ± 32.25 | 0.983 |
| Triglyceride (mg/dL) | 189.52 ± 115.25 | 336.00 ± 355.37 | 0.231 | 90.18 ± 32.01 | 138.40 ± 55.67 | 0.026 |
| HDL-C (mg/dL) | 41.92 ± 7.04 | 40.70 ± 9.83 | 0.682 | 47.82 ± 9.13 | 43.00 ± 12.18 | 0.223 |
| LDL-C (mg/dL) | 122.44 ± 29.39 | 106.50 ± 42.84 | 0.214 | 105.36 ± 33.32 | 106.40 ± 23.94 | 0.930 |
| Uric acid (mg/dL) | 5.84 ± 1.57 | 5.13 ± 1.75 | 0.682 | 5.27 ± 1.67 | 4.77 ± 1.08 | 0.399 |
| Total bile acid (µM) | 10.43 ± 4.30 | 9.17 ± 4.50 | 0.444 | 8.71 ± 5.38 | 7.31 ± 3.71 | 0.457 |
| FGF 19 (pg/mL) | 87.83 ± 63.63 | 75.11 ± 57.23 | 0.587 | 144.15 ± 98.45 | 139.04 ± 109.87 | 0.894 |
| FGF 21 (pg/mL) | 314.54 ± 252.60 | 333.85 ± 212.77 | 0.833 | 204.06 ± 122.68 | 295.67 ± 104.96 | 0.046 |
| HSI | 46.29 ± 6.80 | 44.88 ± 5.42 | 0.562 | 34.49 ± 1.25 | 38.70 ± 1.93 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-Y.; Chen, H.-H.; Lee, W.-J.; Chen, S.-C.; Lee, S.-D.; Chen, C.-Y. Fibroblast Growth Factor 19 and Fibroblast Growth Factor 21 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Gastric Bypass. Nutrients 2022, 14, 645. https://doi.org/10.3390/nu14030645
Guo J-Y, Chen H-H, Lee W-J, Chen S-C, Lee S-D, Chen C-Y. Fibroblast Growth Factor 19 and Fibroblast Growth Factor 21 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Gastric Bypass. Nutrients. 2022; 14(3):645. https://doi.org/10.3390/nu14030645
Chicago/Turabian StyleGuo, Jiun-Yu, Hsin-Hung Chen, Wei-Jei Lee, Shu-Chun Chen, Shou-Dong Lee, and Chih-Yen Chen. 2022. "Fibroblast Growth Factor 19 and Fibroblast Growth Factor 21 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Gastric Bypass" Nutrients 14, no. 3: 645. https://doi.org/10.3390/nu14030645
APA StyleGuo, J.-Y., Chen, H.-H., Lee, W.-J., Chen, S.-C., Lee, S.-D., & Chen, C.-Y. (2022). Fibroblast Growth Factor 19 and Fibroblast Growth Factor 21 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Gastric Bypass. Nutrients, 14(3), 645. https://doi.org/10.3390/nu14030645






