Regular Exercise and Weight-Control Behavior Are Protective Factors against Osteoporosis for General Population: A Propensity Score-Matched Analysis from Taiwan Biobank Participants
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Participants
2.2. Study Design and Data Collection
2.3. Taiwan Biobank (TWB)
2.4. BMD Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Assessment of osteoporosis at the primary health care level. Summ. Rep. A WHO Sci. Group. WHO Geneva 2004. Available online: https://www.who.int/chp/topics/Osteoporosis.pdf (accessed on 20 January 2022).
- Glaser, D.L.; Kaplan, F.S. Osteoporosis. Definition and clinical presentation. Spine 1997, 22, 12s–16s. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, A.; Luyten, F.P.; Flamaing, J.; Gielen, E. Pharmacological treatment of osteoporosis in the oldest old. Clin. Interv Aging 2017, 12, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Javaid, M.K.; et al. Fragility fractures in Europe: Burden, management and opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Ortendahl, J.D.; Vanderpuye-Orgle, J.; Grauer, A.; Arellano, J.; Lemay, J.; Harmon, A.L.; Broder, M.S.; Singer, A.J. Healthcare Policy Changes in Osteoporosis Can Improve Outcomes and Reduce Costs in the United States. JBMR Plus 2019, 3, e10192. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Chan, D.-C.; Chen, J.-F.; Cheng, T.-T.; Wu, C.-H.; Soong, Y.-K.; Tsai, K.-S.; Yang, R.-S. Clinical practice guidelines for the prevention and treatment of osteoporosis in Taiwan: Summary. J. Bone Miner. Metab. 2014, 32, 10–16. [Google Scholar] [CrossRef]
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; Muratore, M.; Quarta, E.; Paola, M.D.; Casciaro, S. Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World J. Radiol. 2013, 5, 398–410. [Google Scholar] [CrossRef] [PubMed]
- The Royal Australian College of General Practitioners and Osteoporosis Australia. Osteoporosis Prevention, Diagnosis and Management in Postmenopausal Women and Men Over 50 Years of Age; RACGP: East Melbourne, Canada, 2017. [Google Scholar]
- Chen, F.-P.; Huang, T.-S.; Fu, T.-S.; Sun, C.-C.; Chao, A.-S.; Tsai, T.-L. Secular trends in incidence of osteoporosis in Taiwan: A nationwide population-based study. Biomed. J. 2018, 41, 314–320. [Google Scholar] [CrossRef]
- Yi-Chin, L.; Wen-Harn, P. Bone Mineral Density in Adults in Taiwan: Results of the Nutrition and Health Survey in Taiwan 2005-2008 (NAHSIT 2005-2008). Asia Pac. J. Clin. Nutr. 2011, 20, 283–291. [Google Scholar] [CrossRef]
- Lyles, C.R.; Schafer, A.L.; Seligman, H.K. Income, food insecurity, and osteoporosis among older adults in the 2007-2008 National Health and Nutrition Examination Survey (NHANES). J. Health Care Poor Underserved 2014, 25, 1530–1541. [Google Scholar] [CrossRef]
- Packard, P.T.; Heaney, R.P. Medical nutrition therapy for patients with osteoporosis. J. Am. Diet. Assoc. 1997, 97, 414–417. [Google Scholar] [CrossRef]
- Brennan, R.M.; Wactawski-Wende, J.; Crespo, C.J.; Dmochowski, J. Factors associated with treatment initiation after osteoporosis screening. Am. J. Epidemiol. 2004, 160, 475–483. [Google Scholar] [CrossRef][Green Version]
- Bijelic, R.; Balaban, J.; Milicevic, S. Correlation of the Lipid Profile, BMI and Bone Mineral Density in Postmenopausal Women. Mater. Sociomed. 2016, 28, 412–415. [Google Scholar] [CrossRef]
- Cheung, A.M.; Giangregorio, L. Mechanical stimuli and bone health: What is the evidence? Curr. Opin. Rheumatol. 2012, 24, 561–566. [Google Scholar] [CrossRef]
- Zhu, K.; Prince, R.L. Lifestyle and Osteoporosis. Curr. Osteoporos. Rep. 2015, 13, 52–59. [Google Scholar] [CrossRef]
- Lau, E.M.; Cooper, C. The epidemiology of osteoporosis. The oriental perspective in a world context. Clin. Orthop. Relat. Res. 1996, 323, 65–74. [Google Scholar] [CrossRef]
- Ho, S.C. Body measurements, bone mass, and fractures. Does the East differ from the West? Clin. Orthop. Relat. Res. 1996, 323, 75–80. [Google Scholar] [CrossRef]
- Nakamura, T.; Turner, C.H.; Yoshikawa, T.; Slemenda, C.W.; Peacock, M.; Burr, D.B.; Mizuno, Y.; Orimo, H.; Ouchi, Y.; Johnston, C.C., Jr. Do variations in hip geometry explain differences in hip fracture risk between Japanese and white Americans? J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1994, 9, 1071–1076. [Google Scholar] [CrossRef]
- Sugimoto, T.; Sato, M.; Dehle, F.C.; Brnabic, A.J.; Weston, A.; Burge, R. Lifestyle-Related Metabolic Disorders, Osteoporosis, and Fracture Risk in Asia: A Systematic Review. Value Health Reg. Issues 2016, 9, 49–56. [Google Scholar] [CrossRef]
- Khoo, C.C.; Woo, J.; Leung, P.C.; Kwok, A.; Kwok, T. Determinants of bone mineral density in older postmenopausal Chinese women. Climacteric J. Int. Menopause Soc. 2011, 14, 378–383. [Google Scholar] [CrossRef]
- Chen, H.-l.; Deng, L.-l.; Li, J.-f. Prevalence of Osteoporosis and Its Associated Factors among Older Men with Type 2 Diabetes. Int. J. Endocrinol. 2013, 2013, 285729. [Google Scholar] [CrossRef]
- Anaforoglu, I.; Nar-Demirer, A.; Bascil-Tutuncu, N.; Ertorer, M.E. Prevalence of osteoporosis and factors affecting bone mineral density among postmenopausal Turkish women with type 2 diabetes. J. Diabetes Complicat. 2009, 23, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Warensjö, E.; Byberg, L.; Melhus, H.; Gedeborg, R.; Mallmin, H.; Wolk, A.; Michaëlsson, K. Dietary calcium intake and risk of fracture and osteoporosis: Prospective longitudinal cohort study. BMJ 2011, 342, d1473. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Hsu, S.Y.; Leung, P.C.; Chan, C.; Swaminathan, R.; Fan, Y.K.; Chan, S.S. A longitudinal study of the determinants of bone mass in Chinese women aged 21 to 40. I. Baseline association of anthropometric measurements with bone mineral density. Ann. Epidemiol. 1993, 3, 256–263. [Google Scholar] [CrossRef]
- Yan, L.; Crabtree, N.J.; Reeve, J.; Zhou, B.; Dequeker, J.; Nijs, J.; Falch, J.A.; Prentice, A. Does hip strength analysis explain the lower incidence of hip fracture in the People’s Republic of China? Bone 2004, 34, 584–588. [Google Scholar] [CrossRef]
- Forsén, L.; Bjørndal, A.; Bjartveit, K.; Edna, T.H.; Holmen, J.; Jessen, V.; Westberg, G. Interaction between current smoking, leanness, and physical inactivity in the prediction of hip fracture. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1994, 9, 1671–1678. [Google Scholar] [CrossRef]
- LaMonte, M.J.; Wactawski-Wende, J.; Larson, J.C.; Mai, X.; Robbins, J.A.; LeBoff, M.S.; Chen, Z.; Jackson, R.D.; LaCroix, A.Z.; Ockene, J.K.; et al. Association of Physical Activity and Fracture Risk Among Postmenopausal Women. JAMA Netw. Open 2019, 2, e1914084. [Google Scholar] [CrossRef]
- Gómez-Cabello, A.; Ara, I.; González-Agüero, A.; Casajús, J.A.; Vicente-Rodríguez, G. Effects of Training on Bone Mass in Older Adults. Sports Med. 2012, 42, 301–325. [Google Scholar] [CrossRef]
- Zehnacker, C.H.; Bemis-Dougherty, A. Effect of Weighted Exercises on Bone Mineral Density in Post Menopausal Women A Systematic Review. J. Geriatr. Phys. Ther. 2007, 30, 79–88. [Google Scholar] [CrossRef]
- Bolam, K.A.; van Uffelen, J.G.Z.; Taaffe, D.R. The effect of physical exercise on bone density in middle-aged and older men: A systematic review. Osteoporos. Int. 2013, 24, 2749–2762. [Google Scholar] [CrossRef]
- Miko, I.; Szerb, I.; Szerb, A.; Bender, T.; Poor, G.J.J.o.r.m. Effect of a balance-training programme on postural balance, aerobic capacity and frequency of falls in women with osteoporosis: A randomized controlled trial. J. Rehabil. Med. 2018, 50, 542–547. [Google Scholar] [CrossRef]
- Ferrari, S.L.; Bianchi, M.L.; Eisman, J.A.; Foldes, A.J.; Adami, S.; Wahl, D.A.; Stepan, J.; De Vernejoul, M.-C.; Kaufman, J.-M. Osteoporosis in young adults: Pathophysiology, diagnosis, and management. Osteoporos. Int. 2012, 23, 2735–2748. [Google Scholar] [CrossRef]
- Bonjour, J.P.; Chevalley, T.; Rizzoli, R.; Ferrari, S. Gene-environment interactions in the skeletal response to nutrition and exercise during growth. Med. Sport Sci. 2007, 51, 64–80. [Google Scholar] [CrossRef]
- Ferrari, S.; Rizzoli, R.; Slosman, D.; Bonjour, J.P. Familial resemblance for bone mineral mass is expressed before puberty. J. Clin. Endocrinol. Metab. 1998, 83, 358–361. [Google Scholar] [CrossRef]
- Fan, C.-T.; Lin, J.-C.; Lee, C.-H. Taiwan Biobank: A project aiming to aid Taiwan’s transition into a biomedical island. Pharmacogenomics 2008, 9, 235–246. [Google Scholar] [CrossRef]
- Lu, Y.; Genant, H.K.; Shepherd, J.; Zhao, S.; Mathur, A.; Fuerst, T.P.; Cummings, S.R. Classification of Osteoporosis Based on Bone Mineral Densities. J. Bone Miner. Res. 2001, 16, 901–910. [Google Scholar] [CrossRef]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef]
- Behringer, M.; Gruetzner, S.; McCourt, M.; Mester, J. Effects of Weight-Bearing Activities on Bone Mineral Content and Density in Children and Adolescents: A Meta-Analysis. J. Bone Miner. Res. 2014, 29, 467–478. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Kohrt, W.M. Exercise and Bone Mineral Density in Premenopausal Women: A Meta-Analysis of Randomized Controlled Trials. Int. J. Endocrinol. 2013, 2013, 741639. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, M.; Xu, Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: A meta-analysis. Osteoporos. Int. 2015, 26, 1605–1618. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Kohrt, W.M. Exercise and bone mineral density in men: A meta-analysis of randomized controlled trials. Bone 2013, 53, 103–111. [Google Scholar] [CrossRef]
- Jensen, L.B.; Kollerup, G.; Quaade, F.; SØRensen, O.H. Bone Mineral Changes in Obese Women During a Moderate Weight Loss With and Without Calcium Supplementation. J. Bone Miner. Res. 2001, 16, 141–147. [Google Scholar] [CrossRef]
- Villareal, D.T.; Fontana, L.; Weiss, E.P.; Racette, S.B.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Holloszy, J.O. Bone Mineral Density Response to Caloric Restriction–Induced Weight Loss or Exercise-Induced Weight Loss: A Randomized Controlled Trial. Arch. Intern. Med. 2006, 166, 2502–2510. [Google Scholar] [CrossRef]
- Radak, T.L. Caloric Restriction and Calcium’s Effect on Bone Metabolism and Body Composition in Overweight and Obese Premenopausal Women. Nutr. Rev. 2004, 62, 468–481. [Google Scholar] [CrossRef]
- Qiao, D.; Li, Y.; Liu, X.; Zhang, X.; Qian, X.; Zhang, H.; Zhang, G.; Wang, C. Association of obesity with bone mineral density and osteoporosis in adults: A systematic review and meta-analysis. Public Health 2020, 180, 22–28. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Fang, W.-H.; Wang, C.-C.; Kao, T.-W.; Chang, Y.-W.; Wu, C.-J.; Zhou, Y.-C.; Sun, Y.-S.; Chen, W.-L. Body fat has stronger associations with bone mass density than body mass index in metabolically healthy obesity. PLoS ONE 2018, 13, e0206812. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Lee, J.G.; Kim, S.S.; Kim, B.H.; Kim, S.-J.; Kim, Y.K.; Kim, I.J. Association between bone mineral density and metabolic syndrome in pre- and postmenopausal women. Endocr. J. 2011, 58, 87–93. [Google Scholar] [CrossRef]
- Chain, A.; Crivelli, M.; Faerstein, E.; Bezerra, F.F. Association between fat mass and bone mineral density among Brazilian women differs by menopausal status: The Pró-Saúde Study. Nutrition 2017, 33, 14–19. [Google Scholar] [CrossRef]
- Madeira, E.; Mafort, T.T.; Madeira, M.; Guedes, E.P.; Moreira, R.O.; de Mendonça, L.M.C.; Lima, I.C.B.; de Pinho, P.R.A.; Lopes, A.J.; Farias, M.L.F. Lean mass as a predictor of bone density and microarchitecture in adult obese individuals with metabolic syndrome. Bone 2014, 59, 89–92. [Google Scholar] [CrossRef]
- Pasco, J.A.; Gould, H.; Brennan, S.L.; Nicholson, G.C.; Kotowicz, M.A. Musculoskeletal deterioration in men accompanies increases in body fat. Obesity 2014, 22, 863–867. [Google Scholar] [CrossRef]
- Lin, H.-H.; Hsu, H.-Y.; Tsai, M.-C.; Hsu, L.-Y.; Chien, K.-L.; Yeh, T.-L. Association between type 2 diabetes and osteoporosis risk: A representative cohort study in Taiwan. PLoS ONE 2021, 16, e0254451. [Google Scholar] [CrossRef]
- Asokan, A.G.; Jaganathan, J.; Philip, R.; Soman, R.R.; Sebastian, S.T.; Pullishery, F. Evaluation of bone mineral density among type 2 diabetes mellitus patients in South Karnataka. J. Nat. Sci. Biol. Med. 2017, 8, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen Inhibits Bone Resorption by Directly Inducing Apoptosis of the Bone-resorbing Osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Ohlsson, C.; Mellström, D.; Andersson, E.M. Smoking-Induced Risk of Osteoporosis Is Partly Mediated by Cadmium from Tobacco Smoke: The MrOS Sweden Study. J. Bone Miner. Res. 2020, 35, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.A.; Brown, R.B.; Razzaque, M.S. Osteoporosis in Populations with High Calcium Intake: Does Phosphate Toxicity Explain the Paradox? Indian J. Clin. Biochem. 2015, 30, 365–367. [Google Scholar] [CrossRef]
- Shih-Ying, C.; Jia-Rong, L.; Tzu-Hsiu, C.; Shiou-Guei, G.; Mei-Ding, K.; Wen-Harn, P. Dietary Supplements Usage Among Elderly Taiwanese During 2005-2008. Asia Pac. J. Clin. Nutr. 2011, 20, 327–336. [Google Scholar] [CrossRef]
- Evans, R.G.; Stoddart, G.L. Consuming Research, Producing Policy? Am. J. Public Health 2003, 93, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.E.; Henriksen, C.; Falch, J.A.; Pedersen, J.I.; Tverdal, A. Risk factors for hip fracture in a high incidence area: A case-control study from Oslo, Norway. Osteoporos. Int. 1995, 5, 239–246. [Google Scholar] [CrossRef]
- Bacon, W.E.; Hadden, W.C. Occurrence of Hip Fractures and Socioeconomic Position. J. Aging Health 2000, 12, 193–203. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Shin, J.Y.; Park, B.J. Socioeconomic disparities in osteoporosis prevalence: Different results in the overall Korean adult population and single-person households. J. Prev. Med. Public Health Yebang Uihakhoe Chi 2015, 48, 84–93. [Google Scholar] [CrossRef][Green Version]
- Yu, W.-S.; Wang, C.-H.; Kuo, N.-W. Impact of Urbanization and Sunlight Exposure on Cataract Incidence. Appl. Sci. 2021, 11, 8137. [Google Scholar] [CrossRef]
| Variable | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| T-Score > −2.5 | T-Score ≤ −2.5 | p- | T-Score > −2.5 | T-Score ≤ −2.5 | p- | |
| (n = 1660) | (n = 1660) | Value | (n = 1107) | (n = 1107) | Value | |
| Age | 57.08 ± 7.13 | 58.14 ± 7.03 | <0.001 | 57.61 ± 6.87 | 57.79 ± 7.03 | 0.553 |
| Male sex (%) | 665(40.1) | 665(40.1) | >0.999 | 435(39.3) | 430(38.8) | 0.862 |
| Height (cm) | 160.81 ± 8.06 | 160.28 ± 8.60 | 0.070 | 160.42 ± 7.91 | 160.56 ± 8.52 | 0.677 |
| Weight (kg) | 64.07 ± 11.01 | 61.14 ± 11.42 | <0.001 | 62.47 ± 10.35 | 62.14 ± 11.51 | 0.478 |
| Fat body rate (%) | 28.91 ± 7.67 | 27.62 ± 7.26 | <0.001 | 28.20 ± 7.24 | 28.17 ± 7.22 | 0.931 |
| Waistline | 85.64 ± 9.37 | 83.85 ± 9.41 | <0.001 | 84.46 ± 8.77 | 84.59 ± 9.38 | 0.733 |
| Hipline | 96.65 ± 6.59 | 95.02 ± 6.33 | <0.001 | 95.79 ± 6.21 | 95.75 ± 6.25 | 0.862 |
| Allergic history (%) | 165(9.9) | 177(10.7) | 0.530 | 109(9.8) | 112(10.1) | 0.887 |
| Arthritis (%) | 127(7.7) | 139(8.4) | 0.482 | 82(7.4) | 89(8.0) | 0.633 |
| Gout (%) | 89(5.4) | 70(4.2) | 0.143 | 55(5.0) | 51(4.6) | 0.765 |
| Asthma (%) | 55(3.3) | 53(3.2) | 0.922 | 33(3.0) | 31(2.8) | 0.899 |
| Rheumatic heart (%) | 47(2.8) | 52(3.1) | 0.683 | 27(2.4) | 38(3.4) | 0.208 |
| CAD (%) | 33(2.0) | 40(2.4) | 0.478 | 19(1.7) | 29(2.6) | 0.189 |
| Arrhythmia (%) | 104(6.3) | 97(5.8) | 0.662 | 76(6.9) | 72(6.5) | 0.799 |
| Hyperlipidemia (%) | 159(9.6) | 156(9.4) | 0.906 | 113(10.2) | 94(8.5) | 0.189 |
| Hypertension (%) | 293(17.7) | 301(18.1) | 0.751 | 193(17.4) | 203(18.3) | 0.618 |
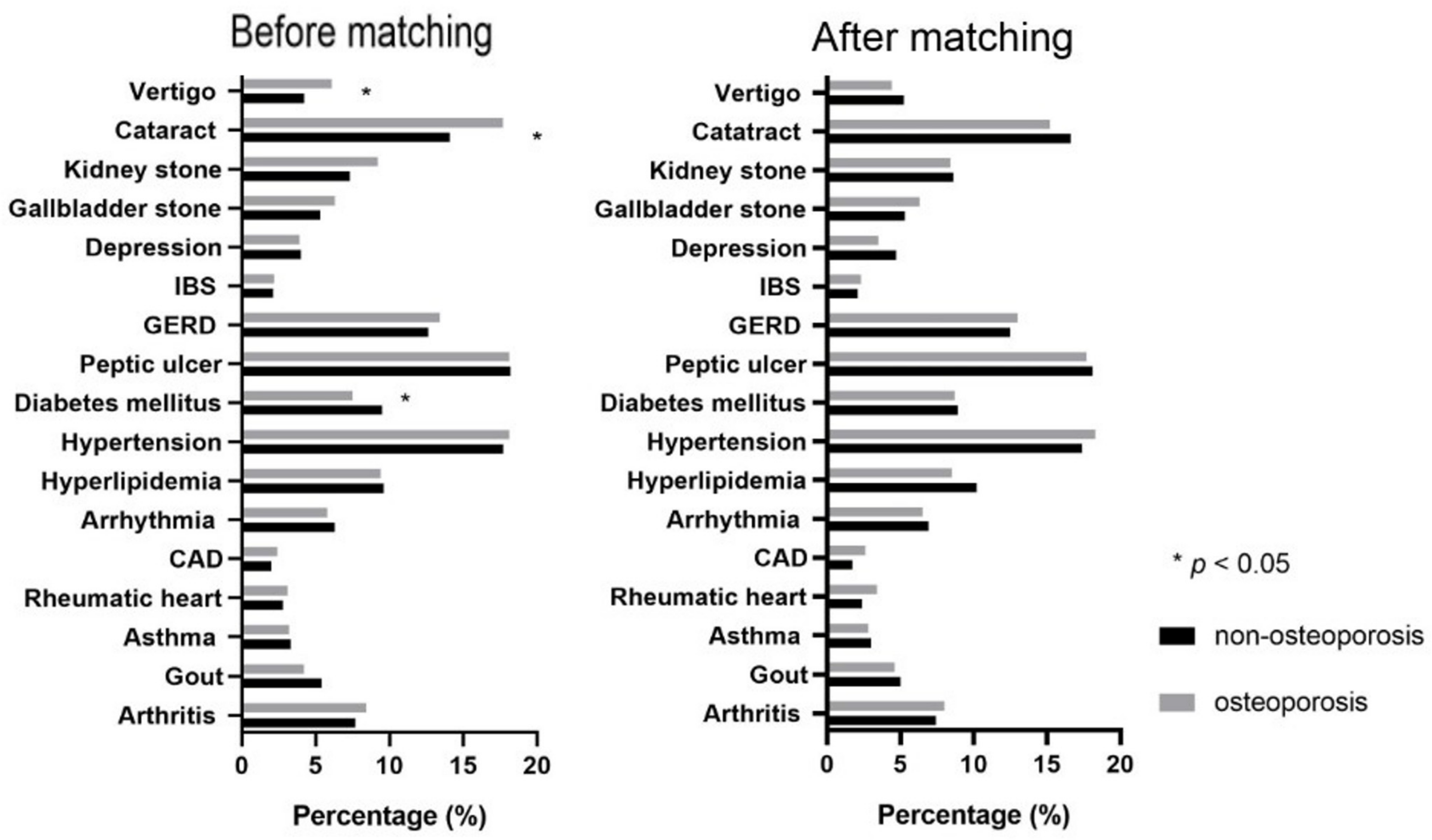
| Diabetes mellitus (%) | 158(9.5) | 125(7.5) | 0.047 | 99(8.9) | 96(8.7) | 0.881 |
| Peptic ulcer (%) | 302(18.2) | 301(18.1) | >0.999 | 200(18.1) | 196(17.7) | 0.868 |
| GERD (%) | 209(12.6) | 223(13.4) | 0.503 | 138(12.5) | 144(13.0) | 0.750 |
| IBS (%) | 35(2.1) | 36(2.2) | >0.999 | 23(2.1) | 25(2.3) | 0.884 |
| Depression disorder (%) | 66(4.0) | 64(3.9) | 0.929 | 52(4.7) | 39(3.5) | 0.199 |
| Gallbladder stone (%) | 88(5.3) | 105(6.3) | 0.235 | 59(5.3) | 70(6.3) | 0.364 |
| Kidney stone (%) | 121(7.3) | 152(9.2) | 0.058 | 95(8.6) | 93(8.4) | 0.939 |
| Vertigo (%) | 70(4.2) | 102(6.1) | 0.015 | 58(5.2) | 49(4.4) | 0.428 |
| Joint stiffness (%) | 421(25.4) | 467(28.1) | 0.078 | 272(24.6) | 304(27.5) | 0.133 |
| Neck pain (%) | 501(30.2) | 529(31.9) | 0.311 | 342(30.9) | 342(30.9) | >0.999 |
| Sciatic pain (%) | 164(9.9) | 163(9.8) | >0.999 | 104(9.4) | 112(10.1) | 0.616 |
| Headache (%) | 304(18.3) | 341(20.5) | 0.114 | 206(18.6) | 221(20.0) | 0.451 |
| Cataract (%) | 234(14.1) | 294(17.7) | 0.005 | 184(16.6) | 168(15.2) | 0.383 |
| Glaucoma (%) | 41(2.5) | 33(2.0) | 0.411 | 23(2.1) | 23(2.1) | >0.999 |
| Dry-eye syndrome (%) | 238(14.3) | 263(15.8) | 0.245 | 164(14.8) | 171(15.4) | 0.722 |
| Retinal detachment (%) | 33(2.0) | 48(2.9) | 0.115 | 26(2.3) | 34(3.1) | 0.360 |
| Myodesopsia (%) | 258(15.5) | 277(16.7) | 0.396 | 178(16.1) | 181(16.4) | 0.908 |
| Variable | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| T-Score > −2.5 | T-Score ≤ −2.5 | p- | T-Score > −2.5 | T-Score ≤ −2.5 | p- | |
| (n = 1660) | (n = 1660) | Value | (n = 1107) | (n = 1107) | Value | |
| SBP (mmHg) | 122.01 ± 17.48 | 123.02 ± 18.78 | 0.110 | 122.77 ± 17.98 | 122.27 ± 18.97 | 0.532 |
| DBP (mmHg) | 73.15 ± 10.70 | 73.40 ± 10.96 | 0.500 | 73.21 ± 10.85 | 72.99 ± 10.91 | 0.629 |
| Heart rate | 34.90 ± 4.59 | 35.58 ± 4.83 | <0.001 | 35.32 ± 4.73 | 35.09 ± 4.58 | 0.239 |
| RBC | 4.77 ± 0.51 | 4.68 ± 0.51 | <0.001 | 4.73 ± 0.51 | 4.71 ± 0.49 | 0.592 |
| WBC | 5.75 ± 1.46 | 5.81 ± 1.57 | 0.226 | 5.77 ± 1.50 | 5.70 ± 1.44 | 0.249 |
| Hb | 14.00 ± 1.46 | 13.79 ± 1.47 | <0.001 | 13.89 ± 1.48 | 13.87 ± 1.43 | 0.823 |
| Hct | 43.40 ± 4.33 | 42.78 ± 4.28 | <0.001 | 43.03 ± 4.38 | 43.06 ± 4.17 | 0.855 |
| Plt | 229.18 ± 55.06 | 228.35 ± 54.89 | 0.663 | 229.63 ± 55.87 | 227.70 ± 54.20 | 0.409 |
| HbA1c (%) | 5.99 ± 0.96 | 5.96 ± 0.91 | 0.268 | 5.97 ± 0.89 | 5.98 ± 0.93 | 0.794 |
| Fasting blood sugar (mg/dL) | 101.10 ± 26.65 | 99.33 ± 24.38 | 0.047 | 100.54 ± 25.14 | 99.88 ± 24.97 | 0.536 |
| Total cholesterol (mg/dL) | 200.76 ± 36.95 | 200.11 ± 36.90 | 0.615 | 200.21 ± 36.99 | 200.54 ± 36.36 | 0.832 |
| TG (mg/dL) | 120.21 ± 86.33 | 123.99 ± 102.88 | 0.251 | 119.57 ± 75.25 | 118.61 ± 79.84 | 0.770 |
| HDL (mg/dL) | 54.14 ± 13.73 | 54.90 ± 13.99 | 0.119 | 54.62 ± 14.38 | 54.85 ± 13.47 | 0.693 |
| LDL (mg/dL) | 125.53 ± 32.10 | 123.23 ± 32.08 | 0.039 | 124.41 ± 32.49 | 124.57 ± 32.46 | 0.906 |
| Total bilirubin (g/dL) | 0.69 ± 0.29 | 0.68 ± 0.27 | 0.248 | 0.69 ± 0.30 | 0.69 ± 0.27 | 0.934 |
| Albumin (g/dL) | 4.54 ± 0.24 | 4.52 ± 0.23 | 0.008 | 4.53 ± 0.24 | 4.53 ± 0.23 | 0.510 |
| GPT (U/L) | 25.24 ± 20.33 | 24.13 ± 17.42 | 0.092 | 25.07 ± 20.26 | 24.06 ± 16.11 | 0.192 |
| α-fetoprotein (U/L) | 3.35 ± 3.83 | 3.48 ± 3.31 | 0.312 | 3.42 ± 4.55 | 3.37 ± 1.75 | 0.703 |
| γ-GT (U/L) | 25.63 ± 25.62 | 28.32 ± 52.69 | 0.062 | 26.19 ± 28.05 | 25.68 ± 27.43 | 0.662 |
| BUN (mg/dL) | 14.36 ± 3.93 | 14.17 ± 4.87 | 0.223 | 14.38 ± 3.88 | 14.37 ± 4.46 | 0.957 |
| Creatinine (mg/dL) | 0.75 ± 0.21 | 0.74 ± 0.39 | 0.169 | 0.75 ± 0.21 | 0.74 ± 0.36 | 0.333 |
| Uric acid (mg/dL) | 5.72 ± 1.37 | 5.47 ± 1.39 | <0.001 | 5.60 ± 1.35 | 5.55 ± 1.38 | 0.345 |
| Urine microalbumin (mg/dL) | 32.86 ± 195.62 | 34.07 ± 142.98 | 0.838 | 28.14 ± 108.75 | 37.40 ± 163.67 | 0.117 |
| Variable | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| T-Score > −2.5 | T-Score ≤ −2.5 | p- | T-Score > −2.5 | T-Score ≤ −2.5 | p- | |
| (n = 1660) | (n = 1660) | Value | (n = 1107) | (n = 1107) | Value | |
| Income (per month) | 5.21 ± 3.44 | 4.97 ± 3.29 | 0.045 | 5.03 ± 3.41 | 5.10 ± 3.34 | 0.600 |
| Education | <0.001 | 0.478 | ||||
| College | 685(41.3) | 595(35.8) | 429(38.8) | 440(39.7) | ||
| Senior high school | 541(32.6) | 561(33.8) | 367(33.2) | 365(33.0) | ||
| Junior high school | 200(12.0) | 228(13.7) | 139(12.6) | 127(11.5) | ||
| Elementary school | 218(13.1) | 254(15.3) | 158(14.3) | 160(14.5) | ||
| Illiteracy | 16(1.0) | 22(1.3) | 14(1.3) | 15(1.4) | ||
| Marital status | <0.001 | 0.239 | ||||
| Married | 1338(80.6) | 1273(76.7) | 870(78.6) | 867(78.3) | ||
| Single | 64(3.9) | 86(5.2) | 51(4.6) | 48(4.3) | ||
| Divorced/separated/widowed | 258(15.5) | 301(18.1) | 186(16.8) | 192(17.3) | ||
| Residence (%) | <0.001 | 0.855 | ||||
| Northern Taiwan | 320(19.3) | 392(23.6) | 226(20.4) | 248(22.4) | ||
| Central Taiwan | 401(24.2) | 439(26.4) | 278(25.1) | 273(24.7) | ||
| Southern Taiwan | 911(54.9) | 797(48.0) | 584(52.8) | 568(51.3) | ||
| Eastern Taiwan | 28(1.7) | 32(1.9) | 19(1.7) | 18(1.6) | ||
| Variable | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| T-Score > −2.5 | T-Score ≤ −2.5 | p- | T-Score > −2.5 | T-Score ≤ −2.5 | p- | |
| (n = 1660) | (n = 1660) | Value | (n = 1107) | (n = 1107) | Value | |
| Cook at home | 1034(62.3) | 1001(60.3) | 0.254 | 679(61.3) | 685(61.9) | 0.827 |
| Eat late-night supper | 378(22.8) | 425(25.6) | 0.062 | 252(22.8) | 276(24.9) | 0.251 |
| Eat out | 0.579 | 0.963 | ||||
| 1 meal per day | 290(17.5) | 264(15.9) | 183(16.5) | 173(15.6) | ||
| 2–3 meal per day | 197(11.9) | 219(13.2) | 132(11.9) | 142(12.8) | ||
| 1–3 meals per week | 424(25.5) | 426(25.7) | 269(24.3) | 280(25.3) | ||
| 4–6 meals per week | 118(7.1) | 112(6.7) | 78(7.0) | 77(7.0) | ||
| 1–3 meals per month | 499(30.1) | 489(29.5) | 347(31.3) | 338(30.5) | ||
| None | 132(8.0) | 150(9.0) | 98(8.9) | 97(8.8) | ||
| Source of drinking-water | 0.637 | 0.700 | ||||
| Well water | 6(0.4) | 11(0.7) | 4(0.4) | 9(0.8) | ||
| Tap water | 256(15.4) | 271(16.3) | 168(15.2) | 171(15.4) | ||
| Purified water | 1191(71.7) | 1161(69.9) | 792(71.5) | 789(71.3) | ||
| Mineral water | 75(4.5) | 77(4.6) | 55(5.0) | 50(4.5) | ||
| Others | 132(8.0) | 140(8.4) | 88(7.9) | 88(7.9) | ||
| Drink tea | 504(30.4) | 510(30.7) | 0.851 | 318(28.7) | 346(31.3) | 0.210 |
| Drink coffee | 545(32.8) | 531(32.0) | 0.630 | 361(32.6) | 353(31.9) | 0.750 |
| Vegetarian | 0.373 | 0.474 | ||||
| Used to be | 65(3.9) | 61(3.7) | 44(4.0) | 34(3.1) | ||
| Yes | 82(4.9) | 100(6.0) | 60(5.4) | 65(5.9) | ||
| No | 1513(91.1) | 1499(90.3) | 1003(90.6) | 1008(91.1) | ||
| Drink alcohol | 0.996 | 0.321 | ||||
| Quit | 58(3.5) | 59(3.6) | 38(3.4) | 35(3.2) | ||
| Frequently | 110(6.6) | 110(6.6) | 73(6.6) | 57(5.1) | ||
| No or rarely | 1492(89.9) | 1491(89.8) | 996(90.0) | 1015(91.7) | ||
| Voluntary smoking | 107(6.4) | 196(11.8) | <0.001 | 92(8.3) | 83(7.5) | 0.529 |
| Involuntary smoking | 159(9.6) | 179(10.8) | 0.275 | 99(8.9) | 103(9.3) | 0.825 |
| Chewing betel nut | 22(1.3) | 33(2.0) | 0.174 | 18(1.6) | 14(1.3) | 0.593 |
| Substance dependence | 27(1.6) | 31(1.9) | 0.691 | 16(1.4) | 19(1.7) | 0.734 |
| Taking dietary Supplement | 0.989 | 0.638 | ||||
| Irregularly | 367(22.1) | 365(22.0) | 247(22.3) | 239(21.6) | ||
| Regularly | 601(36.2) | 605(36.4) | 406(36.7) | 392(35.4) | ||
| None | 692(41.7) | 690(41.6) | 454(41.0) | 476(43.0) | ||
| Behavior of seeking Healthcare | 0.342 | 0.456 | ||||
| Visit Chinese traditional Doctor | 156(9.4) | 161(9.7) | 108(9.8) | 106(9.6) | ||
| Visit doctor | 975(58.7) | 957(57.7) | 649(58.6) | 652(58.9) | ||
| Go to pharmacy | 123(7.4) | 116(7.0) | 76(6.9) | 66(6.0) | ||
| Folk medicine | 28(1.7) | 27(1.6) | 19(1.7) | 18(1.6) | ||
| Observation | 226(13.6) | 208(12.5) | 158(14.3) | 142(12.8) | ||
| Others | 226(13.6) | 208(12.5) | 158(14.3) | 142(12.8) | ||
| Regular exercise | 963(58.0) | 820(49.4) | <0.001 | 658(59.4) | 564(50.9) | <0.001 |
| Weight control | 826(49.8) | 670(40.4) | <0.001 | 547(49.4) | 469(42.4) | 0.001 |
| OR | 95% CI of OR | |
|---|---|---|
| Regular exercise | 0.709 | 0.599–0.839 |
| Weight control | 0.753 | 0.636–0.890 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Huang, C.-Y.; Hsieh, C.-H.; Chien, P.-C.; Chen, C.-C.; Hou, S.-Y.; Wu, S.-C. Regular Exercise and Weight-Control Behavior Are Protective Factors against Osteoporosis for General Population: A Propensity Score-Matched Analysis from Taiwan Biobank Participants. Nutrients 2022, 14, 641. https://doi.org/10.3390/nu14030641
Hsu C-Y, Huang C-Y, Hsieh C-H, Chien P-C, Chen C-C, Hou S-Y, Wu S-C. Regular Exercise and Weight-Control Behavior Are Protective Factors against Osteoporosis for General Population: A Propensity Score-Matched Analysis from Taiwan Biobank Participants. Nutrients. 2022; 14(3):641. https://doi.org/10.3390/nu14030641
Chicago/Turabian StyleHsu, Chih-Yi, Chun-Ying Huang, Ching-Hua Hsieh, Peng-Chen Chien, Chih-Chun Chen, Shao-Yun Hou, and Shao-Chun Wu. 2022. "Regular Exercise and Weight-Control Behavior Are Protective Factors against Osteoporosis for General Population: A Propensity Score-Matched Analysis from Taiwan Biobank Participants" Nutrients 14, no. 3: 641. https://doi.org/10.3390/nu14030641
APA StyleHsu, C.-Y., Huang, C.-Y., Hsieh, C.-H., Chien, P.-C., Chen, C.-C., Hou, S.-Y., & Wu, S.-C. (2022). Regular Exercise and Weight-Control Behavior Are Protective Factors against Osteoporosis for General Population: A Propensity Score-Matched Analysis from Taiwan Biobank Participants. Nutrients, 14(3), 641. https://doi.org/10.3390/nu14030641








