Development of a Novel Nutrition-Related Multivariate Biomarker for Mild Cognitive Impairment Based on the Plasma Free Amino Acid Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
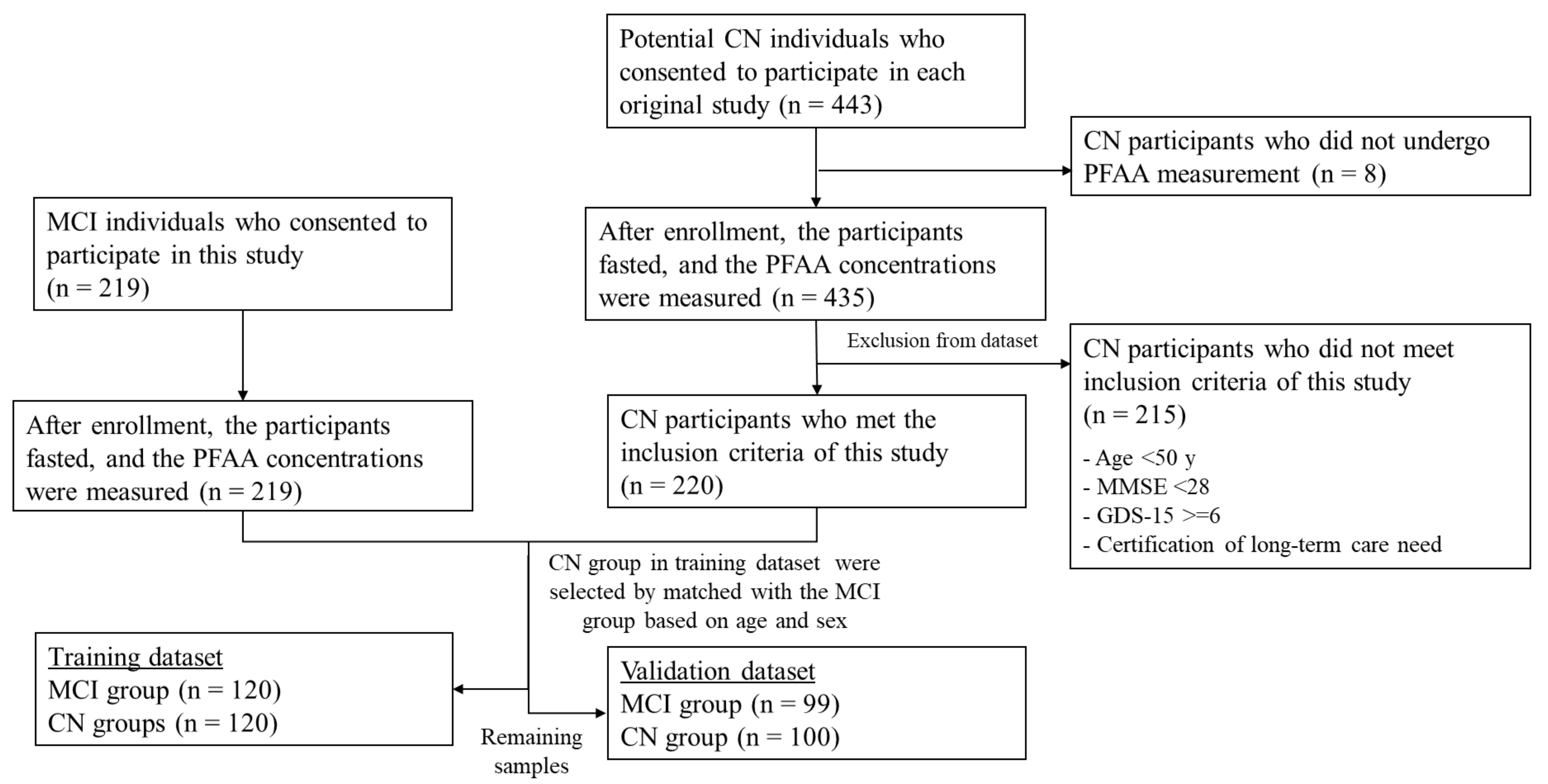
2.2. Participants
2.3. Cognitive Assessment and Inclusion and Exclusion Criteria
2.4. PFAA Analysis
2.5. Blood Biochemistry and APOE Genotyping
2.6. Dataset Preparation
2.7. Statistical Analysis
2.7.1. Characteristics and PFAA Profiles
2.7.2. Receiver Operating Characteristic (ROC) Curve Analysis
2.7.3. PFAA Index Model Development
2.7.4. Model Selection
- 1
- Generate a bootstrap sample by sampling 120 paired participants from the original sample (training dataset).
- 2
- Develop a model using the bootstrap sample:
- a
- Determine the AUC of ROC of this model on the bootstrap sample (bootstrap performance).
- b
- Determine the AUC of ROC of the bootstrap model in the original sample (test performance).
- 3
- Calculate the optimism as the difference between the bootstrap performance and the test performance.Repeat steps 1–3 100 times. Average the estimates of optimism.
- 4
- The optimism-corrected performance was calculated by subtracting the optimism from the apparent performance. We selected models with the optimism-corrected performance (AUC of ROC) in the top 50 for validation.
2.7.5. Model Validation
2.8. Software
3. Results
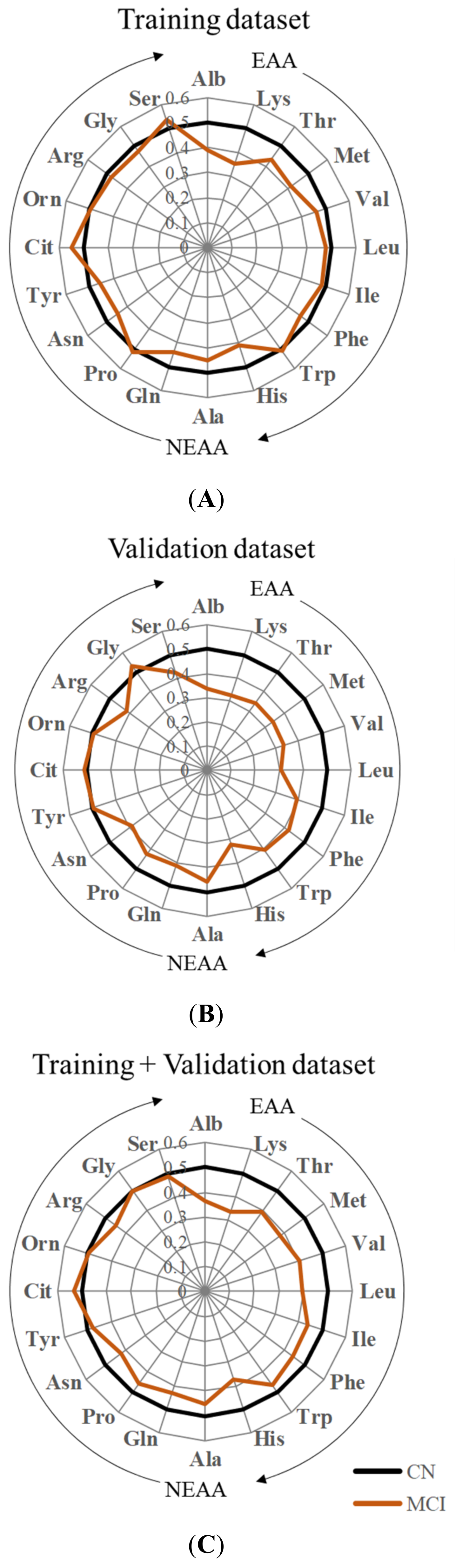
3.1. Characteristics and PFAA Profiles of MCI and CN Participants
3.2. PFAA Index Development
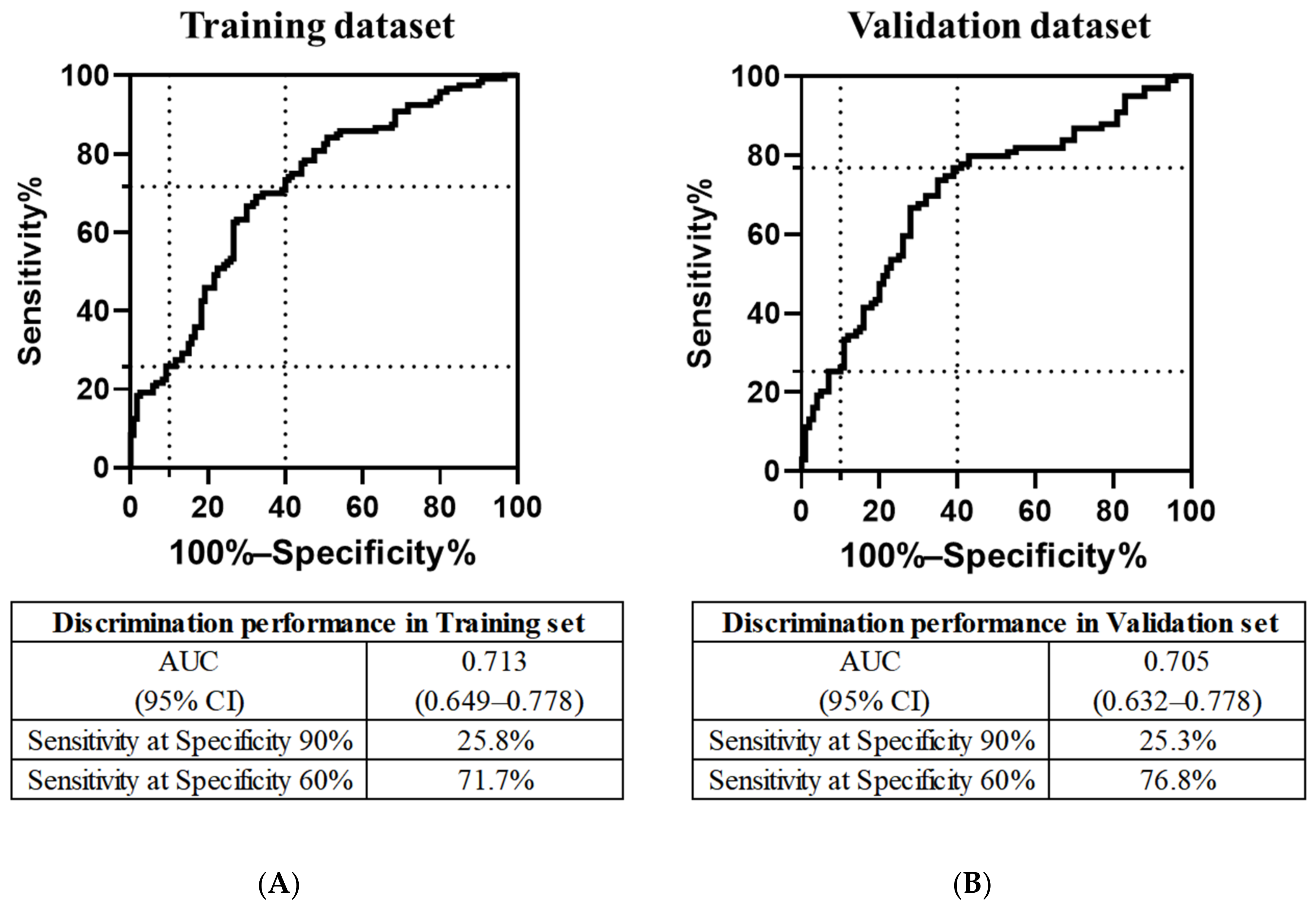
3.3. Discriminatory Performance of the PFAA Index
3.4. Association of the PFAA Index with MCI Risk Factors and Other Variables
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [Green Version]
- El-Hayek, Y.H.; Wiley, R.E.; Khoury, C.P.; Daya, R.P.; Ballard, C.; Evans, A.R.; Karran, M.; Molinuevo, J.L.; Norton, M.; Atri, A. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. J. Alzheimers Dis. 2019, 70, 323–341. [Google Scholar] [CrossRef] [Green Version]
- Leroi, I.; Watanabe, K.; Hird, N.; Sugihara, T. “Psychogeritechnology” in Japan: Exemplars from a super-aged society. Int. J. Geriatr. Psychiatry 2018, 33, 1533–1540. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levalahti, E.; Ahtiluoto, S.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Li, J.; Joshi, P.; Ang, T.F.A.; Liu, C.; Auerbach, S.; Devine, S.; Au, R. Mid- to Late-Life Body Mass Index and Dementia Risk: 38 Years of Follow-up of the Framingham Study. Am. J. Epidemiol. 2021, 190, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Nyunt, M.S.; Gao, Q.; Wee, S.L.; Yap, K.B.; Ng, T.P. Association of Frailty and Malnutrition With Long-term Functional and Mortality Outcomes Among Community-Dwelling Older Adults: Results From the Singapore Longitudinal Aging Study 1. JAMA Netw. Open 2018, 1, e180650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nes, M.; Sem, S.W.; Rousseau, B.; Bjorneboe, G.E.; Engedal, K.; Trygg, K.; Pedersen, J.I. Dietary intakes and nutritional status of old people with dementia living at home in Oslo. Eur. J. Clin. Nutr. 1988, 42, 581–593. [Google Scholar] [PubMed]
- Sanders, C.L.; Wengreen, H.J.; Schwartz, S.; Behrens, S.J.; Corcoran, C.; Lyketsos, C.G.; Tschanz, J.T.; Cache County, I. Nutritional Status is Associated with Severe Dementia and Mortality: The Cache County Dementia Progression Study. Alzheimer Dis. Assoc. Disord. 2018, 32, 298–304. [Google Scholar] [CrossRef]
- Thomas, D.E.; Chung, A.O.K.O.; Dickerson, J.W.; Tidmarsh, S.F.; Shaw, D.M. Tryptophan and nutritional status of patients with senile dementia. Psychol. Med. 1986, 16, 297–305. [Google Scholar] [CrossRef]
- Goodwin, J.S.; Goodwin, J.M.; Garry, P.J. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA 1983, 249, 2917–2921. [Google Scholar] [CrossRef] [PubMed]
- La Rue, A.; Koehler, K.M.; Wayne, S.J.; Chiulli, S.J.; Haaland, K.Y.; Garry, P.J. Nutritional status and cognitive functioning in a normally aging sample: A 6-y reassessment. Am. J. Clin. Nutr. 1997, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Roberts, L.A.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; O’Connor, H.M.; Knopman, D.S.; Petersen, R.C. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J. Alzheimers Dis. 2012, 32, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, W.; Rainey-Smith, S.R.; Gardener, S.L.; Villemagne, V.L.; Burnham, S.C.; Macaulay, S.L.; Brown, B.M.; Gupta, V.B.; Sohrabi, H.R.; Weinborn, M.; et al. Associations of Dietary Protein and Fiber Intake with Brain and Blood Amyloid-beta. J. Alzheimers Dis. 2018, 61, 1589–1598. [Google Scholar] [CrossRef]
- Licher, S.; Ahmad, S.; Karamujic-Comic, H.; Voortman, T.; Leening, M.J.G.; Ikram, M.A.; Ikram, M.K. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat. Med. 2019, 25, 1364–1369. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, A.; Imaizumi, A.; Yoshida, H. Methods for Absolute Quantification of Human Plasma Free Amino Acids by High-Performance Liquid Chromatography/Electrospray Ionization Mass Spectrometry Using Precolumn Derivatization. Methods Mol. Biol. 2019, 2030, 207–218. [Google Scholar] [CrossRef]
- Yoshida, H.; Kondo, K.; Yamamoto, H.; Kageyama, N.; Ozawa, S.; Shimbo, K.; Muramatsu, T.; Imaizumi, A.; Mizukoshi, T.; Masuda, J.; et al. Validation of an analytical method for human plasma free amino acids by high-performance liquid chromatography ionization mass spectrometry using automated precolumn derivatization. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 998–999, 88–96. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kondo, K.; Tanaka, T.; Muramatsu, T.; Yoshida, H.; Imaizumi, A.; Nagao, K.; Noguchi, Y.; Miyano, H. Reference intervals for plasma-free amino acid in a Japanese population. Ann. Clin. Biochem. 2016, 53, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Iwatsubo, T.; Iwata, A.; Suzuki, K.; Ihara, R.; Arai, H.; Ishii, K.; Senda, M.; Ito, K.; Ikeuchi, T.; Kuwano, R.; et al. Japanese and North American Alzheimer’s Disease Neuroimaging Initiative studies: Harmonization for international trials. Alzheimers Dement. 2018, 14, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L.; Jagust, W.J.; Dulberg, C.; Becker, J.T.; DeKosky, S.T.; Fitzpatrick, A.; Breitner, J.; Lyketsos, C.; Jones, B.; Kawas, C.; et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 2. Arch. Neurol. 2003, 60, 1394–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, G.W.; Ercoli, L.M.; Silverman, D.H.; Huang, S.C.; Komo, S.; Bookheimer, S.Y.; Lavretsky, H.; Miller, K.; Siddarth, P.; Rasgon, N.L.; et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 6037–6042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Craney, T.A.; Surles, J.G. Model-Dependent Variance Inflation Factor Cutoff Values. Qual. Eng. 2002, 14, 391–403. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Van der Lee, S.J.; Teunissen, C.E.; Pool, R.; Shipley, M.J.; Teumer, A.; Chouraki, V.; Melo van Lent, D.; Tynkkynen, J.; Fischer, K.; Hernesniemi, J.; et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement. 2018, 14, 707–722. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Bianchi, G.; Martelli, M.; Talerico, T.; Servadei, L.; Zoli, M.; Mariani, E. Plasma amino acid concentrations in patients with amnestic mild cognitive impairment or Alzheimer disease. Am. J. Clin. Nutr. 2004, 80, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Tynkkynen, J.; Chouraki, V.; van der Lee, S.J.; Hernesniemi, J.; Yang, Q.; Li, S.; Beiser, A.; Larson, M.G.; Saaksjarvi, K.; Shipley, M.J.; et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: A prospective study in eight cohorts. Alzheimers Dement. 2018, 14, 723–733. [Google Scholar] [CrossRef]
- Takehana, S.; Yoshida, H.; Ozawa, S.; Yamazaki, J.; Shimbo, K.; Nakayama, A.; Mizukoshi, T.; Miyano, H. The effects of pre-analysis sample handling on human plasma amino acid concentrations. Clin. Chim. Acta 2016, 455, 68–74. [Google Scholar] [CrossRef]
- Yamakado, M.; Nagao, K.; Imaizumi, A.; Tani, M.; Toda, A.; Tanaka, T.; Jinzu, H.; Miyano, H.; Yamamoto, H.; Daimon, T.; et al. Plasma Free Amino Acid Profiles Predict Four-Year Risk of Developing Diabetes, Metabolic Syndrome, Dyslipidemia, and Hypertension in Japanese Population. Sci. Rep. 2015, 5, 11918. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, M.; Tanaka, T.; Nagao, K.; Ishizaka, Y.; Mitushima, T.; Tani, M.; Toda, A.; Toda, E.; Okada, M.; Miyano, H.; et al. Plasma amino acid profile is associated with visceral fat accumulation in obese Japanese subjects. Clin. Obes. 2012, 2, 29–40. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Nishio, N.; Tanaka, T.; Ohji, S.; Otobe, Y.; Koyama, S.; Sato, A.; Suzuki, M.; et al. Plasma Amino Acid Concentrations Are Associated with Muscle Function in Older Japanese Women. J. Nutr. Health Aging 2018, 22, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamamoto, T.; Rikimaru, T.; Inoue, G. Effect of low protein diets on free amino acids in plasma of young men: Effect of wheat gluten diet. J. Nutr. Sci. Vitam. 1979, 25, 427–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, Y.; Yoshimura, Y.; Inoue, G. Effect of low-protein diets on free amino acids in plasma of young men: Effect of protein quality with maintenance or excess energy intake. J. Nutr. Sci. Vitam. 1978, 24, 297–309. [Google Scholar] [CrossRef]
- Sato, H.; Tsukamoto-Yasui, M.; Takado, Y.; Kawasaki, N.; Matsunaga, K.; Ueno, S.; Kanda, M.; Nishimura, M.; Karakawa, S.; Isokawa, M.; et al. Protein Deficiency-Induced Behavioral Abnormalities and Neurotransmitter Loss in Aged Mice Are Ameliorated by Essential Amino Acids. Front. Nutr. 2020, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Takado, Y.; Toyoda, S.; Tsukamoto-Yasui, M.; Minatohara, K.; Takuwa, H.; Urushihata, T.; Takahashi, M.; Shimojo, M.; Ono, M.; et al. Neurodegenerative processes accelerated by protein malnutrition and decelerated by essential amino acids in a tauopathy mouse model. Sci. Adv. 2021, 7, eabd5046. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Paddon-Jones, D.; Leidy, H. Dietary protein and muscle in older persons. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Pelchat, M.L.; Schaefer, S. Dietary monotony and food cravings in young and elderly adults. Physiol. Behav. 2000, 68, 353–359. [Google Scholar] [CrossRef]
- Pilgrim, A.L.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. An overview of appetite decline in older people. Nurs. Older People 2015, 27, 29–35. [Google Scholar] [CrossRef]
- Kawashima, K.; Motohashi, Y.; Fujishima, I. Prevalence of dysphagia among community-dwelling elderly individuals as estimated using a questionnaire for dysphagia screening. Dysphagia 2004, 19, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Akagi, J. Decreased tongue pressure is associated with sarcopenia and sarcopenic dysphagia in the elderly. Dysphagia 2015, 30, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Tohara, H.; Hara, K.; Nakane, A.; Kajisa, E.; Yoshimi, K.; Minakuchi, S. Relationship of aging, skeletal muscle mass, and tooth loss with masseter muscle thickness. BMC Geriatr. 2018, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Polzer, I.; Schimmel, M.; Muller, F.; Biffar, R. Edentulism as part of the general health problems of elderly adults. Int. Dent. J. 2010, 60, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Droogsma, E.; van Asselt, D.Z.; Scholzel-Dorenbos, C.J.; van Steijn, J.H.; van Walderveen, P.E.; van der Hooft, C.S. Nutritional status of community-dwelling elderly with newly diagnosed Alzheimer’s disease: Prevalence of malnutrition and the relation of various factors to nutritional status. J. Nutr. Health Aging 2013, 17, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Grodstein, F.; Proust-Lima, C.; Samieri, C. Long-Term Trajectories of Body Weight, Diet, and Physical Activity From Midlife Through Late Life and Subsequent Cognitive Decline in Women. Am. J. Epidemiol. 2020, 189, 305–313. [Google Scholar] [CrossRef]
- Kinoshita, K.; Otsuka, R.; Takada, M.; Tsukamoto-Yasui, M.; Nishita, Y.; Tange, C.; Tomida, M.; Shimokata, H.; Kuzuya, M.; Imaizumi, A.; et al. The Association between Dietary Amino Acid Intake and Cognitive Decline 8 Years Later in Japanese Community-Dwelling Older Adults. J. Nutr. Health Aging 2021, 25, 165–171. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamashiro, D.; Ogawa, S.; Kobayashi, M.; Cho, D.; Iizuka, A.; Tsukamoto-Yasui, M.; Takada, M.; Isokawa, M.; Nagao, K.; et al. Intake of Seven Essential Amino Acids Improves Cognitive Function and Psychological and Social Function in Middle-Aged and Older Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Front. Nutr. 2020, 7, 586166. [Google Scholar] [CrossRef] [PubMed]
| Training Dataset | Validation Dataset | ||||||
|---|---|---|---|---|---|---|---|
| MCI (N = 120) | CN (N = 120) | p-Value 1 | MCI (N = 99) | CN (N = 100) | p-Value 1 | ||
| Sex | 0.683 | <0.001 | |||||
| Male | N (%) | 39 (32.5) | 43 (35.8) | 35 (35.4) | 60 (60.0) | ||
| Female | N (%) | 81 (67.5) | 77 (64.2) | 64 (64.6) | 40 (40.0) | ||
| Age, years | Mean ± SD | 80.3 ± 5.5 | 79.3 ± 5.4 | 0.159 | 78.6 ± 5.8 | 72.8 ± 6.2 | <0.001 |
| (range) | (67–96) | (64–91) | (63–89) | (51–80) | |||
| BMI, kg/m2 | Mean ± SD | 22.6 ± 3.8 2 | 22.6 ± 2.9 | 0.717 | 22.6 ± 3.4 2 | 23.1 ± 2.9 | 0.403 |
| MMSE | Mean ± SD | 26.9 ± 2.0 | 29.3 ± 0.8 | <0.001 | 26.7 ± 2.1 | 29.4 ± 0.7 | <0.001 |
| GDS-15 | Mean ± SD | 1.5 ± 1.4 | 1.7 ± 1.6 | 0.624 | 1.8 ± 1.4 | 1.7 ± 1.7 | 0.280 |
| Educational background, years | Mean ± SD | 11.2 ± 2.4 | 12.7 ± 2.4 2 | <0.001 | 11.9 ± 2.5 | 13.2 ± 2.7 2 | 0.004 |
| APOE genotype | |||||||
| positive (with ε4 allele) | N (%) | 42 (35) | - | 33 (33.3) | - | ||
| negative (without ε4 allele) | N (%) | 78 (65) | - | 60 (60.6) | - | ||
| missing | N (%) | 0 (0) | - | 6 (6.1) | - | ||
| Training Set | Validation Set | Training + Validation Set | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MCI (N = 120) | CN (N = 120) | MCI (N = 99) | CN (N = 100) | MCI (N = 219) | CN (N = 220) | ||||
| Mean ± SD | Mean ± SD | p-Value 1 | Mean ± SD | Mean ± SD | p-Value 1 | Mean ± SD | Mean ± SD | p-Value 1 | |
| Alb | 4.2 ± 0.3 | 4.3 ± 0.3 | 0.003 | 4.2 ± 0.3 | 4.4 ± 0.3 | <0.001 | 4.2 ± 0.3 | 4.4 ± 0.3 | <0.001 |
| Lys | 178.4 ± 29.8 | 192.2 ± 28.4 | <0.001 | 182.1 ± 29.4 | 200.2 ± 32.6 | <0.001 | 180.1 ± 29.6 | 195.8 ± 30.6 | <0.001 |
| Thr | 110.1 ± 22.4 | 115.2 ± 22.2 | 0.080 | 111.3 ± 24.6 | 124.2 ± 26.7 | <0.001 | 110.7 ± 23.4 | 119.3 ± 24.7 | <0.001 |
| Met | 23.7 ± 4.9 | 24.7 ± 4.2 | 0.024 | 23.7 ± 5.4 | 26.1 ± 4.8 | <0.001 | 23.7 ± 5.1 | 25.3 ± 4.5 | <0.001 |
| Val | 204.8 ± 42.5 | 207.9 ± 32.5 | 0.284 | 202.1 ± 43.0 | 225.3 ± 42.2 | <0.001 | 203.6 ± 42.7 | 215.8 ± 38.1 | <0.001 |
| Leu | 107.5 ± 24.0 | 109.0 ± 20.9 | 0.540 | 107.0 ± 26.9 | 122.6 ± 24.9 | <0.001 | 107.3 ± 25.3 | 115.2 ± 23.7 | <0.001 |
| Ile | 57.8 ± 14.7 | 57.9 ± 12.8 | 0.670 | 57.9 ± 15.8 | 63.0 ± 15.5 | 0.007 | 57.8 ± 15.2 | 60.2 ± 14.3 | 0.030 |
| Phe | 61.6 ± 12.4 | 62.0 ± 8.8 | 0.334 | 59.2 ± 10.4 | 61.5 ± 9.2 | 0.045 | 60.5 ± 11.6 | 61.8 ± 9.0 | 0.040 |
| Trp | 50.2 ± 9.9 | 49.9 ± 8.4 | 0.765 | 49.6 ± 8.9 | 52.0 ± 7.9 | 0.023 | 49.9 ± 9.4 | 50.9 ± 8.2 | 0.177 |
| His | 76.7 ± 9.2 | 78.9 ± 8.7 | 0.018 | 76.1 ± 10.4 | 82.1 ± 9.5 | <0.001 | 76.4 ± 9.7 | 80.4 ± 9.2 | <0.001 |
| Ala | 345.2 ± 90.4 | 356.0 ± 77.6 | 0.161 | 351.2 ± 90.2 | 359.1 ± 77.9 | 0.328 | 348.0 ± 90.2 | 357.4 ± 77.5 | 0.093 |
| Gln | 595.8 ± 71.4 | 606.4 ± 58.0 | 0.107 | 596.1 ± 67.9 | 613.3 ± 54.0 | 0.032 | 595.9 ± 69.7 | 609.5 ± 56.2 | 0.009 |
| Pro | 144.1 ± 56.1 | 142.1 ± 43.5 | 0.651 | 141.9 ± 50.3 | 149.8 ± 45.1 | 0.072 | 143.1 ± 53.4 | 145.6 ± 44.3 | 0.117 |
| Asn | 45.1 ± 7.6 | 46.6 ± 7.8 | 0.139 | 44.9 ± 6.9 | 47.5 ± 7.5 | 0.007 | 45.0 ± 7.3 | 47.0 ± 7.6 | 0.004 |
| Tyr | 63.7 ± 14.3 | 64.1 ± 11.0 | 0.221 | 64.0 ± 12.9 | 64.1 ± 12.8 | 0.989 | 63.9 ± 13.6 | 64.1 ± 11.8 | 0.400 |
| Cit | 39.5 ± 10.9 | 37.6 ± 10.1 | 0.185 | 37.5 ± 10.9 | 36.4 ± 8.9 | 0.774 | 38.6 ± 10.9 | 37.1 ± 9.6 | 0.200 |
| Orn | 58.6 ± 15.8 | 58.9 ± 18.5 | 0.936 | 57.5 ± 13.6 | 58.0 ± 13.3 | 0.915 | 58.1 ± 14.8 | 58.5 ± 16.4 | 0.970 |
| Arg | 93.6 ± 18.8 | 94.6 ± 18.7 | 0.569 | 92.4 ± 18.2 | 97.3 ± 19.7 | 0.044 | 93.1 ± 18.5 | 95.8 ± 19.1 | 0.079 |
| Gly | 221.7 ± 59.1 | 223.7 ± 52.8 | 0.548 | 230.9 ± 60.2 | 225.3 ± 62.2 | 0.452 | 225.8 ± 59.6 | 224.4 ± 57.1 | 0.974 |
| Ser | 108.5 ± 18.6 | 105.7 ± 18.8 | 0.297 | 104.5 ± 20.3 | 110.3 ± 21.3 | 0.058 | 106.7 ± 19.4 | 107.8 ± 20.1 | 0.629 |
| p-Value for a Variable in Logistic Regression | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Base Model | +Age | +Sex | +MMSE | +BMI | +A, S | +A, M | +A, B | +S, M | +S, B | +M, B | +A, S, M | +A, S, B | +A, M, B | +S, M, B | +A, S, M, B |
| PFAA index | 3.8 × 10−8 | 7.6 × 10−8 | 3.8 × 10−8 | 3.3 × 10−5 | 4.4 × 10−8 | 7.9 × 10−8 | 2.1 × 10−5 | 1.2 × 10−7 | 3.4 × 10−5 | 4.7 × 10−8 | 4.2 × 10−5 | 2.2 × 10−5 | 1.4 × 10−7 | 2.8 × 10−5 | 4.3 × 10−5 | 2.9 × 10−5 |
| Age | 0.84 | 0.79 | 0.19 | 0.65 | 0.21 | 0.60 | 0.24 | 0.26 | ||||||||
| Sex | 0.57 | 0.56 | 0.60 | 0.50 | 0.68 | 0.47 | 0.53 | 0.60 | ||||||||
| MMSE | 3.6 × 10−12 | 6.0 × 10−12 | 3.9 × 10−12 | 2.0 × 10−11 | 6.4 × 10−12 | 3.0 × 10−11 | 2.1 × 10−11 | 3.1 × 10−11 | ||||||||
| BMI | 0.46 | 0.49 | 0.46 | 0.85 | 0.49 | 0.79 | 0.86 | 0.79 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeuchi, T.; Yano, Y.; Sato, W.; Morikawa, F.; Toru, S.; Nishimura, C.; Miyazawa, N.; Kuroha, Y.; Koike, R.; Tanaka, S.; et al. Development of a Novel Nutrition-Related Multivariate Biomarker for Mild Cognitive Impairment Based on the Plasma Free Amino Acid Profile. Nutrients 2022, 14, 637. https://doi.org/10.3390/nu14030637
Ikeuchi T, Yano Y, Sato W, Morikawa F, Toru S, Nishimura C, Miyazawa N, Kuroha Y, Koike R, Tanaka S, et al. Development of a Novel Nutrition-Related Multivariate Biomarker for Mild Cognitive Impairment Based on the Plasma Free Amino Acid Profile. Nutrients. 2022; 14(3):637. https://doi.org/10.3390/nu14030637
Chicago/Turabian StyleIkeuchi, Takeshi, Yuki Yano, Wataru Sato, Fumiyoshi Morikawa, Shuta Toru, Chika Nishimura, Nobuhiko Miyazawa, Yasuko Kuroha, Ryoko Koike, Shin Tanaka, and et al. 2022. "Development of a Novel Nutrition-Related Multivariate Biomarker for Mild Cognitive Impairment Based on the Plasma Free Amino Acid Profile" Nutrients 14, no. 3: 637. https://doi.org/10.3390/nu14030637
APA StyleIkeuchi, T., Yano, Y., Sato, W., Morikawa, F., Toru, S., Nishimura, C., Miyazawa, N., Kuroha, Y., Koike, R., Tanaka, S., Utsumi, K., Kasuga, K., Tokutake, T., Ono, K., Yano, S., Naruse, S., Yajima, R., Hamano, T., Yokoyama, Y., ... Nagao, K. (2022). Development of a Novel Nutrition-Related Multivariate Biomarker for Mild Cognitive Impairment Based on the Plasma Free Amino Acid Profile. Nutrients, 14(3), 637. https://doi.org/10.3390/nu14030637








