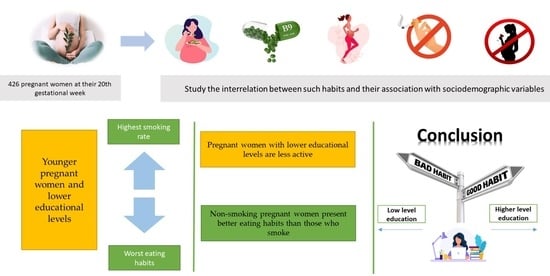
Relationship between Eating Habits, Physical Activity and Tobacco and Alcohol Use in Pregnant Women: Sociodemographic Inequalities
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection and Participants
2.3. Ethics
2.4. Questionnaire
- (a)
- Sociodemographic variables: age, educational level (categorized between groups from the lowest to the highest: (1) Low level of studies, e.g., primary education; (2) Medium level of studies, e.g., compulsory secondary education, professional training; (3) University studies) and work situation (categorized in 5 groups, in descending order: full-time employment, part-time employment, unemployment, housewife—as self-defined employment status—and other employment statuses, such as: student, on sick leave, under legal working age).
- (b)
- Obstetric variables: number of pregnancies (including the current one) and pregnancy planning.
- (c)
- Variables related to alcohol and tobacco consumption during pregnancy: Alcohol: by using selected questions from the Alcohol Use Disorders Identification Test (AUDIT) [32], self-declared alcohol consumption patterns during pregnancy were assessed, resulting in the following categories: never, one time or less a month, from 2 to 4 times a month, from 2 to 3 times a week. Tobacco: the self-declared tobacco consumption frequency during pregnancy was collected, classifying it into the following categories: never, once a month, once a week, 1–3 cigarettes a day, 4–6 cigarettes a day, 7–10 cigarettes a day, 11–14 cigarettes a day, and 15–20 cigarettes a day.
- (d)
- Variables related to the consumption of fruits, vegetables, legumes, rye or wholemeal bread as indicators of the consumption of recommendable food products, with self-declared consumption frequency of: never, not very frequently, 1–3 days a week, 4–6 days a week, and every day.
- (e)
- Variables related to the consumption of coffee, tea, caffeinated soft drinks as indicators of not recommendable or harmful food products, with self-declared consumption frequency of: never, not very frequently, once a month, once a week, and every day.
- (f)
- Number of hours of physical exercise a week (with varied examples of moderate aerobic physical activity), categorized as follows: none, around half an hour a week, around an hour a week, around 2–3 h a week, around 4–6 h a week, and 7 or more hours a week.
- (g)
- Variable related to the consumption of folic acid, with self-declared consumption frequency of: never, since before pregnancy, since the first trimester, since the second trimester.
2.5. Data Analysis Design
3. Results
3.1. Descriptive Statistics
3.2. Associations between Smoking Habit, Physical Activity and Diet during Pregnancy
3.3. Associations between Alcohol Consumption, Physical Activity and Diet during Pregnancy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef] [PubMed]
- World Health organization (WHO). Intermittent Iron and Folic Acid Supplementation during Pregnancy. Available online: http://www.who.int/elena/titles/intermittent_iron_pregnancy/es/ (accessed on 1 June 2021).
- De-Regil, L.M.; Peña-Rosas, J.P.; Fernández-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015, 2015, CD007950. [Google Scholar] [CrossRef] [PubMed]
- Ballestín, S.S.; Campos, M.I.G.; Ballestín, J.B.; Bartolomé, M.J.L. Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients 2021, 13, 3134. [Google Scholar] [CrossRef]
- McKillop, D.J.; Pentieva, K.; Daly, D.; McPartlin, J.M.; Hughes, J.; Strain, J.J.; Scott, J.M.; McNulty, H. The effect of different cooking methods on folate retention in various foods that are amongst the major contributors to folate intake in the UK diet. Br. J. Nutr. 2002, 88, 681–688. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef]
- Rodríguez-Bernal, C.L.; Ramón, R.; Quiles, J.; Murcia, M.; Navarrete-Muñoz, E.M.; Vioque, J.; Ballester, F.; Rebagliato, M. Dietary intake in pregnant women in a Spanish Mediterranean area: As good as it is supposed to be? Public Health Nutr. 2013, 16, 1379–1389. [Google Scholar] [CrossRef]
- Cuervo, M.; Sayon-Orea, C.; Santiago, S.; Martínez, J.A. Dietary and Health Profiles of Spanish Women in Preconception, Pregnancy and Lactation. Nutrients 2014, 6, 4434–4451. [Google Scholar] [CrossRef]
- Jardí, C.; Aparicio, E.; Bedmar, C.; Aranda, N.; Abajo, S.; March, G.; Basora, J.; Arija, V.; The ECLIPSES Study Group Food. Consumption during Pregnancy and Post-Partum. ECLIPSES Study. Nutrients 2019, 11, 2447. [Google Scholar] [CrossRef]
- Stråvik, M.; Jonsson, K.; Hartvigsson, O.; Sandin, A.; Wold, A.E.; Sandberg, A.-S.; Barman, M. Food and Nutrient Intake during Pregnancy in Relation to Maternal Characteristics: Results from the NICE Birth Cohort in Northern Sweden. Nutrients 2019, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Laqueille, X.; Thibaut, F. Conséquences potentielles de la consommation de tabac, de cannabis et de cocaïne par la femme enceinte sur la grossesse, le nouveau-né et l’enfant: Revue de littérature Consequences of tobacco, cocaine and cannabis consumption during pregnancy on the pregnancy itself, on the newborn and on child development: A review. Encephale 2015, 41, S13–S20. (In French) [Google Scholar] [CrossRef] [PubMed]
- Burton, R.; Sheron, N. No level of alcohol consumption improves health. Lancet 2018, 392, 987–988. [Google Scholar] [CrossRef]
- Navas-Acien, A. Global Tobacco Use: Old and New Products. Ann. Am. Thorac. Soc. 2018, 15, S69–S75. [Google Scholar] [CrossRef]
- INEbase. Instituto Nacional de Estadística. Encuesta Nacional de Salud. 2017. Available online: https://www.ine.es/CDINEbase/consultar.do?mes=&operacion=Encuesta+nacional+de+salud&id_oper=Ir (accessed on 1 December 2021).
- Tobacco and Nicotine Cessation During Pregnancy. Obstet. Gynecol. 2020, 135, e221–e229. [CrossRef]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Skagerström, J.; Alehagen, S.; Häggström-Nordin, E.; Årestedt, K.F.; Nilsen, P. Prevalence of alcohol use before and during pregnancy and predictors of drinking during pregnancy: A cross sectional study in Sweden. BMC Public Health 2013, 13, 780. [Google Scholar] [CrossRef]
- Coathup, V.; Northstone, K.; Gray, R.; Wheeler, S.; Smith, L. Dietary Patterns and Alcohol Consumption During Pregnancy: Secondary Analysis of Avon Longitudinal Study of Parents and Children. Alcohol. Clin. Exp. Res. 2017, 41, 1120–1128. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Weisberg, P.; Spong, C. Cigarette smoking, alcohol use and adverse pregnancy outcomes: Implications for micronutrient supplementation. J. Nutr. 2003, 133, 1722S–1731S. [Google Scholar] [CrossRef]
- Halsted, C.H.; Villanueva, J.A.; Devlin, A.M.; Chandler, C.J. Metabolic Interactions of Alcohol and Folate. J. Nutr. 2002, 132, 2367S–2372S. [Google Scholar] [CrossRef]
- Sebastiani, G.; Borrás-Novell, C.; Casanova, M.A.; Tutusaus, M.P.; Martínez, S.F.; Roig, M.D.G.; García-Algar, O. The Effects of Alcohol and Drugs of Abuse on Maternal Nutritional Profile during Pregnancy. Nutrients 2018, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Mottola, M.F.; Davenport, M.H.; Ruchat, S.-M.; Davies, G.A.; Poitras, V.; Gray, C.; Garcia, A.J.; Barrowman, N.; Adamo, K.B.; Duggan, M.; et al. No. 367-2019 Canadian Guideline for Physical Activity throughout Pregnancy. J. Obstet. Gynaecol. Can. 2018, 40, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Morales-Suárez-Varela, M.; Clemente-Bosch, E.; Peraita-Costa, I.; Llopis-Morales, A.; Martínez, I.; Llopis-González, A. Maternal Physical Activity During Pregnancy and the Effect on the Mother and Newborn: A Systematic Review. J. Phys. Act. Health 2021, 18, 130–147. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240014886 (accessed on 1 December 2021).
- Meader, N.; King, K.; Moe-Byrne, T.; Wright, K.; Graham, H.; Petticrew, M.; Power, C.; White, M.; Sowden, A.J. A systematic review on the clustering and co-occurrence of multiple risk behaviours. BMC Public Health 2016, 16, 368. [Google Scholar] [CrossRef] [PubMed]
- De Jersey, S.J.; Nicholson, J.M.; Callaway, L.K.; Daniels, L. An observational study of nutrition and physical activity behaviours, knowledge, and advice in pregnancy. BMC Pregnancy Childbirth 2013, 13, 115–118. [Google Scholar] [CrossRef]
- Bookari, K.; Yeatman, H.; Williamson, M. Informing Nutrition Care in the Antenatal Period: Pregnant Women’s Experiences and Need for Support. BioMed Res. Int. 2017, 2017, 4856527. [Google Scholar] [CrossRef]
- Mendoza, R.; Morales-Marente, E.; Palacios, M.S.; Rodríguez-Reinado, C.; Corrales-Gutiérrez, I.; García-Algar, Ó. Health advice on alcohol consumption in pregnant women in Seville (Spain). Gac. Sanit. 2020, 34, 449–458. [Google Scholar] [CrossRef]
- Babor, T.F.; Higgins-Biddle, J.C.; Saunders, J.B.; Monteiro, M.G. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care, 2nd ed.; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Werler, M.M.; Louik, C.; Mitchell, A. Achieving a public health recommendation for preventing neural tube defects with folic acid. Am. J. Public Health 1999, 89, 1637–1640. [Google Scholar] [CrossRef][Green Version]
- Williams, J.; Mai, C.T.; Mulinare, J.; Isenburg, J.; Flood, T.J.; Ethen, M.; Frohnert, B.; Kirby, R.S. Centers for Disease Control and Prevention. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification-United States, 1995–2011. MMWR Morb. Mortal. Wkly. Rep. 2015, 16, 1–5. [Google Scholar]
- Goossens, J.; Beeckman, D.; Van Hecke, A.; Delbaere, I.; Verhaeghe, S. Preconception lifestyle changes in women with planned pregnancies. Midwifery 2018, 56, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Salles, T.; Mendez, M.; Pessoa, V.; Guxens, M.; Aguilera, I.; Kogevinas, M.; Sunyer, J. Smoking during pregnancy is associated with higher dietary intake of polycyclic aromatic hydrocarbons and poor diet quality. Public Health Nutr. 2010, 13, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, H.; Song, J.-M.; Zhang, J.; Tang, Y.-L.; Xin, C.-M. A meta-analysis of risk of pregnancy loss and caffeine and coffee consumption during pregnancy. Int. J. Gynecol. Obstet. 2015, 130, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Wu, Y.; Neelakantan, N.; Chong, M.F.-F.; Pan, A.; van Dam, R. Maternal caffeine intake during pregnancy and risk of pregnancy loss: A categorical and dose–response meta-analysis of prospective studies. Public Health Nutr. 2015, 19, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Miyake, Y.; Tanaka, K.; Sasaki, S.; Hirota, Y. Maternal total caffeine intake, mainly from Japanese and Chinese tea, during pregnancy was associated with risk of preterm birth: The Osaka Maternal and Child Health Study. Nutr. Res. 2015, 35, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef]
- Olney, J.W.; Wozniak, D.F.; Farber, N.; Jevtovic-Todorovic, V.; Bittigau, P.; Ikonomidou, C. The enigma of fetal alcohol neurotoxicity. Ann. Med. 2002, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J. Nutritional issues in perinatal alcohol exposure. Neurobehav. Toxicol. Teratol. 1984, 6, 261–269. [Google Scholar]
- Rasch, V. Cigarette, alcohol, and caffeine consumption: Risk factors for spontaneous abortion. Acta Obstet. Gynecol. Scand. 2003, 82, 182–188. [Google Scholar] [CrossRef]
- Cao, H.; Wei, X.; Guo, X.; Song, C.; Luo, Y.; Cui, Y.; Hu, X.; Zhang, Y. Screening high-risk clusters for developing birth defects in mothers in Shanxi Province, China: Application of latent class cluster analysis. BMC Pregnancy Childbirth 2015, 15, 343. [Google Scholar] [CrossRef]
- Poels, M.; van Stel, H.F.; Franx, A.; Koster, M.P. Actively preparing for pregnancy is associated with healthier lifestyle of women during the preconception period. Midwifery 2017, 50, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Patel, D.; Brima, N.; Stephenson, J. Alcohol, smoking, folic acid and multivitamin use among women attending maternity care in London: A cross-sectional study. Sex. Reprod. Health 2019, 22, 100461. [Google Scholar] [CrossRef] [PubMed]

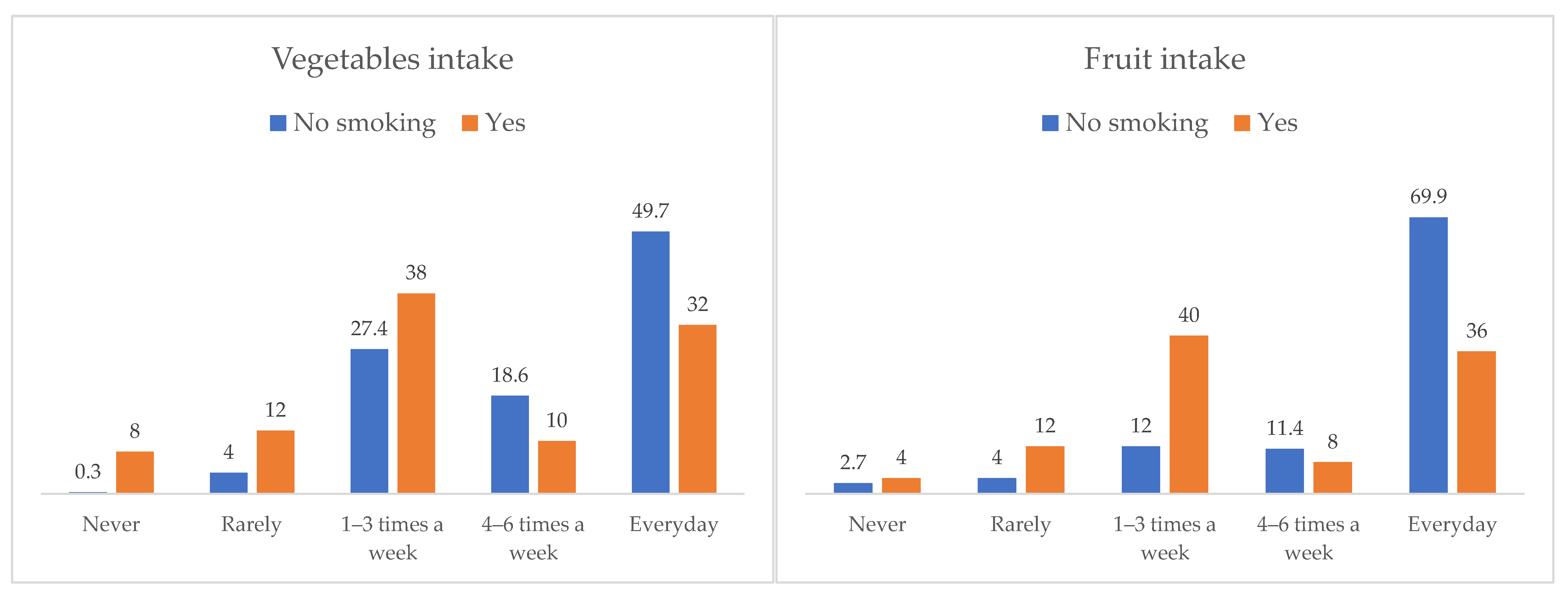
| Variable | Categories | Percentage |
|---|---|---|
| Age | Up to 25 years old | 12.4 |
| 26–30 years old | 23.4 | |
| 31–35 years old | 38.0 | |
| More than 35 years old | 26.3 | |
| Educational level | Low level of studies | 16.9 |
| Medium level of studies | 45.5 | |
| University studies | 37.6 | |
| Employment status | Full-time employment | 39.3 |
| Part-time employment | 12.7 | |
| Unemployed | 28.0 | |
| Other employment status | 20.0 | |
| In a relationship | Yes | 98.1 |
| No | 1.9 | |
| Number of pregnancies (including the current one) | One | 40.4 |
| Two | 31.0 | |
| More than two | 28.6 | |
| Was the pregnancy planned? | Yes | 74.6 |
| No | 25.4 |
| Never | Rarely | 1–3 Days a Week | 4–6 Days a Week | Every Day | ||
|---|---|---|---|---|---|---|
| Vegetables | 1.2 | 4.9 | 28.6 | 17.6 | 47.7 | |
| Fruits | 2.8 | 4.9 | 15.3 | 11.0 | 66.0 | |
| Nuts | 16.5 | 36.2 | 32.9 | 7.5 | 6.8 | |
| Rye or wholemeal bread | 58.9 | 8.0 | 6.1 | 4.0 | 23.0 | |
| Legumes | 1.2 | 5.6 | 71.8 | 18.1 | 3.3 | |
| Never | Rarely | Once a month | Weekly | Daily | ||
| Coffee | 68.5 | 3.8 | 1.9 | 3.5 | 22.3 | |
| Tea | 88.3 | 3.5 | 1.4 | 4.2 | 2.6 | |
| Caffeinated soft drinks | 46.2 | 8.2 | 3.5 | 26.8 | 15.3 | |
| No | Since before pregnancy | Since 1st trimester | Since 2nd trimester | |||
| Folic acid | 5.2 | 34.3 | 57.5 | 3.1 | ||
| None | Half an hour | One hour | 2–3 h | 4–6 h | 7 or more hours | |
| Weekly physical activity | 32.5 | 2.4 | 7.5 | 18.6 | 23.5 | 15.5 |
| Age | |||||
|---|---|---|---|---|---|
| Up to 25 | 26–30 | 31–35 | More than 35 | ||
| Smoking | No | 77.4 | 80.8 | 93.2 | 92.9 |
| Yes | 22.6 | 19.2 | 6.8 | 7.1 | |
| Alcohol | No | 83 | 73.7 | 72.2 | 72.3 |
| Yes | 17 | 26.3 | 27.8 | 27.7 | |
| Vegetables | Never | 3.8 | 2 | 0 | 0.9 |
| Rarely | 15.1 | 5.1 | 1.9 | 4.5 | |
| 1–3 days a week | 20.8 | 40.4 | 28.4 | 22.3 | |
| 4–6 days a week | 18.9 | 15.2 | 19.8 | 16.1 | |
| Every day | 41.5 | 37.4 | 50 | 56.3 | |
| Fruits | Never | 3.8 | 5.1 | 2.5 | 0.9 |
| Rarely | 11.3 | 5.1 | 4.3 | 2.7 | |
| 1–3 days a week | 26.4 | 20.2 | 12.3 | 9.8 | |
| 4–6 days a week | 9.4 | 11.1 | 16 | 4.5 | |
| Every day | 49.1 | 58.6 | 64.8 | 82.1 | |
| Nuts | Never | 26.4 | 22.2 | 12.4 | 12.5 |
| Rarely | 45.3 | 38.4 | 32.3 | 35.7 | |
| 1–3 days a week | 13.2 | 26.3 | 41.6 | 35.7 | |
| 4–6 days a week | 15.1 | 9.1 | 5 | 6.3 | |
| Every day | 0 | 4 | 8.7 | 9.8 | |
| Rye or wholemeal bread | Never | 84.9 | 64.6 | 54.9 | 47.3 |
| Rarely | 3.8 | 5.1 | 8 | 7.1 | |
| 1–3 days a week | 3.8 | 3 | 8 | 7.1 | |
| 4–6 days a week | 3.8 | 6.1 | 3.1 | 3.6 | |
| Every day | 3.8 | 21.2 | 23.5 | 33 | |
| Legumes | Never | 3.8 | 1 | 0 | 1.8 |
| Rarely | 3.8 | 8.1 | 4.3 | 6.3 | |
| 1–3 days a week | 64.2 | 66.7 | 74.7 | 75.9 | |
| 4–6 days a week | 22.6 | 23.2 | 17.9 | 11.6 | |
| Every day | 5.7 | 1 | 3.1 | 4.5 | |
| Coffee | Never | 75.5 | 74.7 | 63 | 67.9 |
| Rarely | 9.4 | 4 | 2.5 | 2.7 | |
| Once a month | 1.9 | 4 | 0.6 | 1.8 | |
| Weekly | 7.5 | 4 | 2.5 | 2.7 | |
| Daily | 5.7 | 13.1 | 31.5 | 25 | |
| Tea | Never | 86.8 | 86.9 | 89.5 | 88.4 |
| Rarely | 7.5 | 2 | 3.7 | 2.7 | |
| Once a month | 1.9 | 2 | 0.6 | 1.8 | |
| Weekly | 3.8 | 6.1 | 4.3 | 2.7 | |
| Daily | 0 | 3 | 1.9 | 4.5 | |
| Soft drinks | Never | 28.3 | 46.5 | 47.5 | 52.7 |
| Rarely | 5.7 | 4 | 9.9 | 10.7 | |
| Once a month | 5.7 | 2 | 2.5 | 5.4 | |
| Weekly | 32.1 | 27.3 | 29 | 20.5 | |
| Daily | 28.3 | 20.2 | 11.1 | 10.7 | |
| Folic acid | No | 5.7 | 5.1 | 4.3 | 6.3 |
| Before pregnancy | 7.5 | 29.3 | 37 | 47.3 | |
| 1st trimester | 81.1 | 62.6 | 55.6 | 44.6 | |
| 2nd trimester | 5.7 | 3 | 3.1 | 1.8 | |
| Physical activity | None | 36.5 | 36.4 | 32.7 | 26.8 |
| Half an hour | 3.8 | 4 | 1.2 | 1.8 | |
| One hour | 7.7 | 7.1 | 6.2 | 9.8 | |
| 2–3 h | 13.5 | 16.2 | 19.8 | 21.4 | |
| 4–6 h | 26.9 | 23.2 | 22.2 | 24.1 | |
| 7 or more hours | 11.5 | 13.1 | 17.9 | 16.1 | |
| Educational level | ||||
|---|---|---|---|---|
| Low | Medium | University | ||
| Smoking | No | 66.7 | 88.7 | 97.5 |
| Yes | 33.3 | 11.3 | 2.5 | |
| Alcohol | No | 66.7 | 74.2 | 76.9 |
| Yes | 33.3 | 25.8 | 23.1 | |
| Vegetables | Never | 4.2 | 1 | 0 |
| Rarely | 12.5 | 4.6 | 1.9 | |
| 1–3 days a week | 37.5 | 35.6 | 16.3 | |
| 4–6 days a week | 12.5 | 19.6 | 17.5 | |
| Every day | 33.3 | 39.2 | 64.4 | |
| Fruits | Never | 2.8 | 5.2 | 0 |
| Rarely | 9.7 | 6.2 | 1.3 | |
| 1–3 days a week | 23.6 | 18.6 | 7.5 | |
| 4–6 days a week | 8.3 | 11.3 | 11.9 | |
| Every day | 55.6 | 58.8 | 79.4 | |
| Nuts | Never | 26.4 | 20.6 | 6.9 |
| Rarely | 33.3 | 41.2 | 31.4 | |
| 1–3 days a week | 26.4 | 25.8 | 44.7 | |
| 4–6 days a week | 6.9 | 8.8 | 6.3 | |
| Every day | 6.9 | 3.6 | 10.7 | |
| Rye or wholemeal bread | Never | 72.2 | 66 | 44.4 |
| Rarely | 6.9 | 6.7 | 10 | |
| 1–3 days a week | 4.2 | 4.6 | 8.8 | |
| 4–6 days a week | 5.6 | 4.1 | 3.1 | |
| Every day | 11.1 | 18.6 | 33.8 | |
| Legumes | Never | 0 | 2.1 | 0.6 |
| Rarely | 4.2 | 5.2 | 6.9 | |
| 1–3 days a week | 68.1 | 68.6 | 77.5 | |
| 4–6 days a week | 22.2 | 21.6 | 11.9 | |
| Every day | 5.6 | 2.6 | 3.1 | |
| Coffee | Never | 61.1 | 73.7 | 65.6 |
| Rarely | 4.2 | 5.2 | 1.9 | |
| Once a month | 5.6 | 1.5 | 0.6 | |
| Weekly | 5.6 | 4.1 | 1.9 | |
| Daily | 23.6 | 15.5 | 30 | |
| Tea | Never | 90.3 | 88.7 | 86.9 |
| Rarely | 5.6 | 3.1 | 3.1 | |
| Once a month | 1.4 | 1.5 | 1.3 | |
| Weekly | 2.8 | 4.6 | 4.4 | |
| Daily | 0 | 2.1 | 4.4 | |
| Soft drinks | Never | 30.6 | 45.4 | 54.4 |
| Rarely | 6.9 | 8.8 | 8.1 | |
| Once a month | 4.2 | 3.6 | 3.1 | |
| Weekly | 22.2 | 27.8 | 27.5 | |
| Daily | 36.1 | 14.4 | 6.9 | |
| Folic acid | No | 6.9 | 5.7 | 3.8 |
| Before pregnancy | 9.7 | 32 | 48.1 | |
| 1st trimester | 77.8 | 58.8 | 46.9 | |
| 2nd trimester | 5.6 | 3.6 | 1.3 | |
| Physical activity | None | 41.7 | 37.8 | 21.9 |
| Half an hour | 4.2 | 2.6 | 1.3 | |
| One hour | 8.3 | 6.7 | 8.1 | |
| 2–3 h | 22.2 | 14 | 22.5 | |
| 4–6 h | 15.3 | 21.8 | 29.4 | |
| 7 or more hours | 8.3 | 17.1 | 16.9 | |
| Coffee | ||||||
|---|---|---|---|---|---|---|
| Never | Rarely | Once a Month | Weekly | Daily | ||
| Tobacco | No | 70.7 | 3.5 | 1.3 | 3.7 | 20.7 |
| Yes | 52.0 | 6.0 | 6.0 | 2.0 | 34.0 | |
| Alcohol | No | 74.6 | 2.9 | 1.6 | 3.2 | 17.8 |
| Yes | 51.4 | 6.3 | 2.7 | 4.5 | 35.1 | |
| Folic Acid | |||||
|---|---|---|---|---|---|
| No | Since before Pregnancy | Since 1st Trimester | Since 2nd Trimester | ||
| Tobacco | No | 5.1 | 36.7 | 55.6 | 2.7 |
| Yes | 6.0 | 16.0 | 72.0 | 6.0 | |
| Alcohol | No | 4.8 | 38.7 | 55.2 | 1.3 |
| Yes | 6.3 | 21.6 | 64.0 | 8.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrales-Gutierrez, I.; Baena-Antequera, F.; Gomez-Baya, D.; Leon-Larios, F.; Mendoza, R. Relationship between Eating Habits, Physical Activity and Tobacco and Alcohol Use in Pregnant Women: Sociodemographic Inequalities. Nutrients 2022, 14, 557. https://doi.org/10.3390/nu14030557
Corrales-Gutierrez I, Baena-Antequera F, Gomez-Baya D, Leon-Larios F, Mendoza R. Relationship between Eating Habits, Physical Activity and Tobacco and Alcohol Use in Pregnant Women: Sociodemographic Inequalities. Nutrients. 2022; 14(3):557. https://doi.org/10.3390/nu14030557
Chicago/Turabian StyleCorrales-Gutierrez, Isabel, Francisca Baena-Antequera, Diego Gomez-Baya, Fatima Leon-Larios, and Ramon Mendoza. 2022. "Relationship between Eating Habits, Physical Activity and Tobacco and Alcohol Use in Pregnant Women: Sociodemographic Inequalities" Nutrients 14, no. 3: 557. https://doi.org/10.3390/nu14030557
APA StyleCorrales-Gutierrez, I., Baena-Antequera, F., Gomez-Baya, D., Leon-Larios, F., & Mendoza, R. (2022). Relationship between Eating Habits, Physical Activity and Tobacco and Alcohol Use in Pregnant Women: Sociodemographic Inequalities. Nutrients, 14(3), 557. https://doi.org/10.3390/nu14030557