Chronic Alcohol Abuse Alters Hepatic Trace Element Concentrations-Metallomic Study of Hepatic Elemental Composition by Means of ICP-OES
Abstract
1. Introduction
2. Material and Methods
2.1. Population and Sample Characterization
2.2. Sample Collection Procedure
2.3. Sample Preparation Procedure
2.4. Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) Measurements
2.5. Statistical Analysis
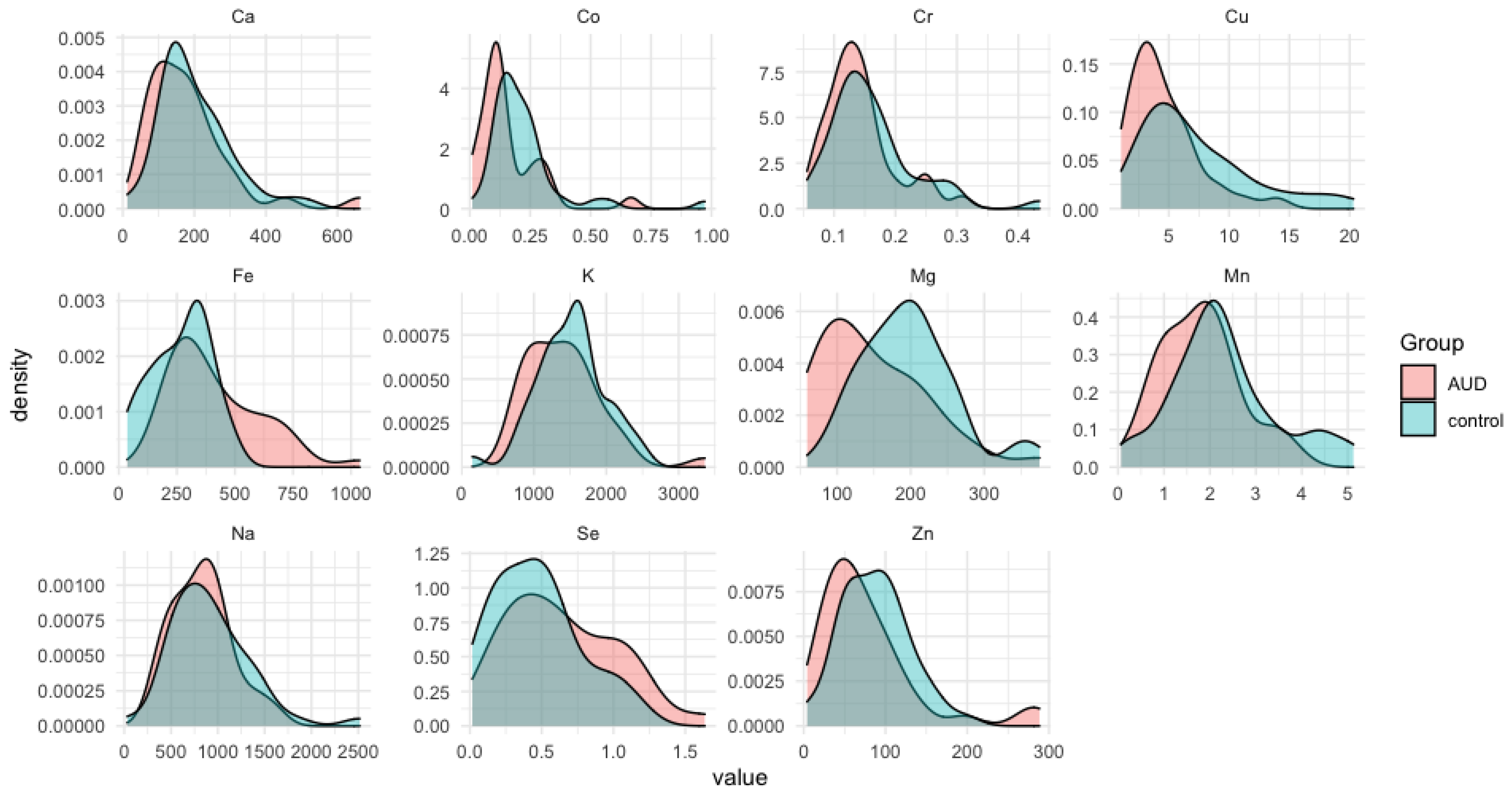
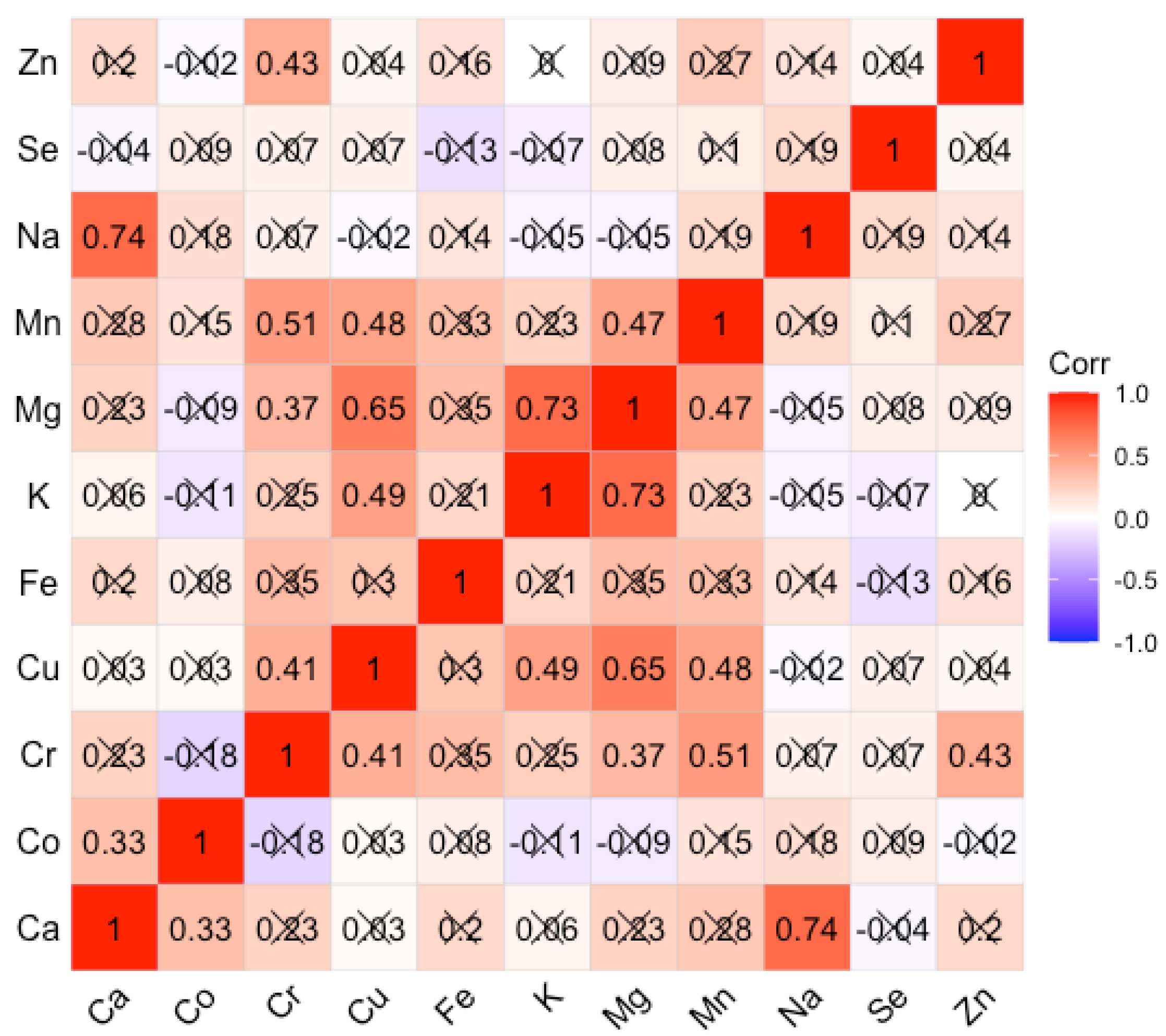
3. Results
3.1. The Average Trace Elements in the Liver Samples for the Case Group and Controls
3.2. Correlations of Average Trace Elements in the Liver Samples for the Case Group and Controls
3.3. Statistically Significant Differences between the Alcohol-Use Disoder (AUD) and Control Groupd
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Alcohol; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/alcohol (accessed on 21 September 2018).
- WHO. Global Status Report on Alcohol and Health. 2018. Available online: https://apps.who.int/iris/handle/10665/274603 (accessed on 27 September 2018).
- WHO. Global Health Observatory Data Repository. Available online: https://apps.who.int/gho/data/view.main.A1029SDG3v?lang=en&showonly=GISAH (accessed on 20 September 2021).
- Ji, C. Advances and New Concepts in Alcohol-Induced Organelle Stress, Unfolded Protein Responses and Organ Damage. Biomolecules 2015, 5, 1099. [Google Scholar] [CrossRef]
- Chopra, K.; Tiwari, V. Alcoholic neuropathy: Possible mechanisms and future treatment possibilities. Br. J. Clin. Pharmacol. 2012, 73, 348. [Google Scholar] [CrossRef]
- Gardner, J.D.; Mouton, A.J. Alcohol effects on cardiac function. Compr. Physiol. 2015, 5, 791. [Google Scholar] [CrossRef]
- Aghdassi, A.A.; Weiss, F.U.; Mayerle, J.; Lerch, M.M.; Simon, P. Genetic susceptibility factors for alcohol-induced chronic pancreatitis. Pancreatology 2015, 15, S23. [Google Scholar] [CrossRef]
- Rocco, A.; Compare, D.; Angrisani, D.; Sanduzzi Zamparelli, M.; Nardone, G. Alcoholic disease: Liver and beyond. World J. Gastroenterol. 2014, 20, 14652. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Hao, F.; Nelson, L.J.; Vázquez, M.T.; Canals, S.; Gómez Del Moral, M.; Martínez-Naves, E.; Nevzorova, Y.A.; Cubero, F.J. Alcoholic liver disease: Utility of animal models. World J. Gastroenterol. 2018, 24, 5063. [Google Scholar] [CrossRef]
- Grewal, P.; Viswanathen, V.A. Liver cancer and alcohol. Clin. Liver Dis. 2012, 16, 839. [Google Scholar] [CrossRef]
- Rehm, J. The risks associated with alcohol use and alcoholism. Alcohol Res. Health 2011, 34, 135. [Google Scholar]
- Grochowski, C.; Szukała, M.; Litak, J.; Budny, A.; Proch, J.; Majerek, D.; Blicharska, E.; Niedzielski, P. Correlations Between Trace Elements in Selected Locations of the Human Brain in Individuals with Alcohol Use Disorder. Molecules 2020, 25, 359. [Google Scholar] [CrossRef]
- Grochowski, C.; Blicharska, E.; Baj, J.; Mierzwińska, A.; Brzozowska, K.; Forma, A.; Maciejewski, R. Serum iron, Magnesium, Copper, and Manganese Levels in Alcoholism: A Systematic Review. Molecules 2019, 24, 1361. [Google Scholar] [CrossRef]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol Res. 2017, 38, 163. [Google Scholar]
- Saribal, D.; Hocaoglu-Emre, F.S.; Karaman, F.; Mırsal, H.; Akyolcu, M.C. Trace Element Levels and Oxidant/Antioxidant Status in Patients with Alcohol Abuse. Biol. Trace Elem. Res. 2020, 193, 7. [Google Scholar] [CrossRef]
- Versieck, J. Trace elements in human body fluids and tissues. Crit. Rev. Clin. Lab. Sci. 1985, 22, 97. [Google Scholar] [CrossRef]
- Itokawa, Y. Alcohol intake and nutrition. Nihon Arukoru Yakubutsu Igakkai Zasshi 2000, 35, 19. (In Japanese) [Google Scholar]
- Lieber, C.S. Metabolism of alcohol. Clin. Liver Dis. 2005, 9, 1–35. [Google Scholar] [CrossRef]
- Appenzeller, B.M.; Schuman, M.; Wennig, R. Was a child poisoned by ethanol? Discrimination between ante-mortem consumption and post-mortem formation. Int. J. Legal Med. 2008, 122, 429. [Google Scholar] [CrossRef]
- Gilliland, M.G.; Bost, R.O. Alcohol in decomposed bodies: Postmortem synthesis and distribution. J. Forensic Sci. 1993, 38, 1266. [Google Scholar] [CrossRef]
- Romani, A.; Scarpa, A. Regulation of cell magnesium. Arch. Biochem. Biophys. 1992, 298, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, X.; Qi, D.; Qu, A.; Wang, G. Amelioration of Ethanol-Induced Hepatitis by Magnesium Isoglycyrrhizinate through Inhibition of Neutrophil Cell Infiltration and Oxidative Damage. Mediators Inflamm. 2017, 2017, 3526903. [Google Scholar] [CrossRef]
- Young, A.; Cefaratti, C.; Romani, A. Chronic EtOH administration alters liver Mg2+ homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G57. [Google Scholar] [CrossRef]
- Young, A.; Berti-Mattera, L.; Romani, A. Effect of repeated doses of ethanol on hepatic Mg2+ homeostasis and mobilization. Alcohol Clin. Exp. Res. 2007, 31, 1240. [Google Scholar] [CrossRef]
- Torres, L.M.; Youngner, J.; Romani, A. Role of glucose in modulating Mg2+ homeostasis in liver cells from starved rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G195. [Google Scholar] [CrossRef][Green Version]
- Shah, S.C.; Zhu, X.; Dai, Q.; Peek, R.M.; Shrubsole, M.J. Magnesium intake is associated with a reduced risk of incident liver cancer, based on an analysis of the NIH-American Association of Retired Persons (NIH-AARP) Diet and Health Study prospective cohort. Am. J. Clin. Nutr. 2021, 113, 630. [Google Scholar] [CrossRef]
- Wu, J.; Meng, Q.H. Current understanding of the metabolism of micronutrients in chronic alcoholic liver disease. World J. Gastroenterol. 2020, 26, 4567. [Google Scholar] [CrossRef]
- Liu, M.; Yang, H.; Mao, Y. Magnesium and liver disease. Ann. Transl. Med. 2019, 7, 578. [Google Scholar] [CrossRef]
- Osna, N.A.; Donohue, T.M., Jr.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 2017, 38, 147. [Google Scholar]
- Chen, L.H.; Xi, S.; Cohen, D.A. Liver antioxidant defenses in mice fed ethanol and the AIN-76A diet. Alcohol 1995, 12, 453. [Google Scholar] [CrossRef]
- Zhao, M.; Matter, K.; Laissue, J.A.; Zimmermann, A. Copper/zinc and manganese superoxide dismutases in alcoholic liver disease: Immunohistochemical quantitation. Histol. Histopathol. 1996, 11, 899. [Google Scholar]
- Dong, X.; Liu, H.; Chen, F.; Li, D.; Zhao, Y. MiR-214 promotes the alcohol-induced oxidative stress via down-regulation of glutathione reductase and cytochrome P450 in liver cells. Alcohol. Clin. Exp. Res. 2014, 38, 68. [Google Scholar] [CrossRef]
- Shibazaki, S.; Uchiyama, S.; Tsuda, K.; Taniuchi, N. Copper deficiency caused by excessive alcohol consumption. BMJ Case Rep. 2017, 2017, bcr2017220921. [Google Scholar] [CrossRef]
- Sassine, M.; Mergler, D.; Bowler, R.; Hudnell, H. Manganese accentuates adverse mental health effects associated with alcohol use disorders. Biol. Psychiat. 2002, 51, 909. [Google Scholar] [CrossRef]
- Gerhardt, R.E.; Crecelius, E.A.; Hudson, J.B. Trace element content of moonshine. Arch. Environ. Health 1980, 35, 332. [Google Scholar] [CrossRef]
- Morin, Y.L.; Foley, A.R.; Martineau, G.; Roussel, J. Quebec beer-drinkers’ cardiomyopathy: Forty-eight cases. Can. Med. Assoc. J. 1967, 97, 881. [Google Scholar]
- Fletcher, L.M.; Halliday, J.W.; Powell, L.W. Interrelationships of alcohol and iron in liver disease with particular reference to the iron-binding proteins, ferritin and transferrin. J. Gastroenterol. Hepatol. 1999, 14, 202. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Dominitz, J.A.; Weiss, N.S.; Heagerty, P.J.; Kowdley, K.V. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology 2004, 126, 1293. [Google Scholar] [CrossRef]
- Lou, D.Q.; Nicolas, G.; Lesbordes, J.C.; Viatte, L.; Grimber, G.; Szajnert, M.F.; Kahn, A.; Vaulont, S. Functional differences between hepcidin 1 and 2 in transgenic mice. Blood 2004, 103, 2816. [Google Scholar] [CrossRef]
- Gunshin, H.; Fujiwara, Y.; Custodio, A.O.; Direnzo, C.; Robine, S.; Andrews, N.C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Investig. 2005, 115, 1258. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, H.; Suzuki, M.; Hosoki, Y.; Sakurai, S.; Fujimoto, Y.; Kohgo, Y. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin. Exp. Res. 2002, 26, 26S. [Google Scholar] [CrossRef]
- Flieger, J.; Dolar-Szczasny, J.; Rejdak, R.; Majerek, D.; Tatarczak-Michalewska, M.; Proch, J.; Blicharska, E.; Flieger, W.; Baj, J.; Niedzielski, P. The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 9413. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Święch, A.; Flieger, J.; Tatarczak-Michalewska, M.; Niedzielski, P.; Proch, J.; Majerek, D.; Kawka, J.; Mackiewicz, J. Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2019, 24, 4127. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Flieger, J.; Kowalska, B.; Majerek, D.; Tatarczak-Michalewska, M.; Zakrocka, I.; Załuska, W.; Rejdak, R. Hemodialysis Effect on the Composition of the Eye Fluid of Cataract Patients. J. Clin. Med. 2021, 10, 5485. [Google Scholar] [CrossRef]
- Rayssiguier, Y.; Boirie, Y.Y.; Durlach, J. Magnésium. In Apports Nutritionnels. Conseillés Pour la Population Française, 3rd ed.; Martin, A., Ed.; Editions Tec & Doc.: Paris, France, 2001. [Google Scholar]
- Leevy, C.M.; Moroianu, S.A. Nutritional aspects of alcoholic liver disease. Clin. Liver Dis. 2005, 9, 67. [Google Scholar] [CrossRef]
- Pitts, T.O.; Van Thiel, D.H. Disorders of the serum electrolytes, acid-base balance, and renal function in alcoholism. Recent Dev. Alcohol 1986, 4, 311. [Google Scholar] [CrossRef]
- Fitsanakis, V.A.; Zhang, N.; Avison, M.J.; Erikson, K.M.; Gore, J.C.; Aschner, M. Changes in dietary iron exacerbate regional brain manganese accumulation as determined by magnetic resonance imaging. Toxicol. Sci. 2011, 120, 146. [Google Scholar] [CrossRef]
- Yin, Z.; Jiang, H.; Lee, E.S.; Ni, M.; Erikson, K.M.; Milatovic, D.; Bowman, A.B.; Aschner, M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J. Neurochem. 2010, 112, 1190. [Google Scholar] [CrossRef]
- Skalnaya, M.G.; Skalny, A.V. Essential Trace Elements in Human Health: A Physician’s View; Publishing House of Tomsk State University: Tomsk, Russia, 2018; ISBN 978-5-94621-683-8. [Google Scholar]
- Baj, J.; Flieger, W.; Teresiński, G.; Buszewicz, G.; Sitarz, E.; Forma, A.; Karakuła, K.; Maciejewski, R. Magnesium, Calcium, Potassium, Sodium, Phosphorus, Selenium, Zinc, and Chromium Levels in Alcohol Use Disorder: A Review. J. Clin. Med. 2020, 9, 1901. [Google Scholar] [CrossRef]
- Grochowski, C.; Blicharska, E.; Bogucki, J.; Proch, J.; Mierzwińska, A.; Baj, J.; Litak, J.; Podkowiński, A.; Flieger, J.; Teresiński, G.; et al. Increased Aluminum Content in Certain Brain Structures is Correlated with Higher Silicon Concentration in Alcoholic Use Disorder. Molecules 2019, 24, 1721. [Google Scholar] [CrossRef]


| Group | Controls (n = 45) | Cases (n = 39) | p |
|---|---|---|---|
| Mean age ± SD | 50.33 ± 19.10 | 47.89 ± 13.32 | 0.413 |
| Gender (n/%) | female: 14 (31.11%) | female: 10 (25.64%) | 0.579 |
| male: 31 (68.89%) | male: 29 (74.36%) | ||
| Mean weight [kg] | 81.955 ± 20.944 | 81.358 ± 25.438 | 0.642 |
| BMI [kg m−2] (mean ± SD) | 28.456 ± 7.32 | 26.512 ± 7.41 | 0.650 |
| Element | Λ [nm] | LOD [mg L−1] | R |
|---|---|---|---|
| Mg | 279.553 | 0.01 | 0.9996 |
| K | 766.491 | 0.2 | 0.9996 |
| Na | 589.592 | 0.1 | 0.9997 |
| Ca | 422.673 | 0.01 | 0.9998 |
| Cr | 267.716 | 0.2 | 1.0000 |
| Zn | 213.857 | 0.1 | 0.9999 |
| Co | 238.892 | 0.3 | 0.9999 |
| Mn | 257.610 | 0.03 | 1.0000 |
| Cu | 327.395 | 0.15 | 1.0000 |
| Se | 196.026 | 2.0 | 0.9999 |
| Fe | 238.204 | 0.2 | 1.0000 |
| Element | The AUD Group | The Control Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean [µg/g] | Median [µg/g] | Min [µg/g] | Max [µg/g] | SD | SE | N | Mean [µg/g] | Median [µg/g] | Min [µg/g] | Max [µg/g] | SD | SE | |
| Ca | 35 | 181.415 | 166.990 | 59.105 | 662.016 | 121.220 | 20.490 | 45 | 199.943 | 160.422 | 12.601 | 513.214 | 98.153 | 14.631 |
| Co | 31 | 0.155 | 0.117 | 0.011 | 0.667 | 0.131 | 0.024 | 43 | 0.221 | 0.185 | 0.019 | 0.972 | 0.158 | 0.024 |
| Cr | 35 | 0.142 | 0.133 | 0.066 | 0.308 | 0.056 | 0.009 | 44 | 0.162 | 0.146 | 0.056 | 0.434 | 0.072 | 0.011 |
| Cu | 36 | 4.561 | 3.532 | 1.041 | 13.965 | 2.852 | 0.473 | 45 | 7.274 | 5.731 | 1.541 | 20.310 | 4.627 | 0.690 |
| Fe | 39 | 415.811 | 365.519 | 170.468 | 1037.217 | 197.492 | 31.624 | 31 | 267.058 | 315.300 | 36.368 | 480.465 | 125.073 | 22.464 |
| K | 36 | 1413.296 | 1361.149 | 749.301 | 3359.705 | 537.728 | 89.621 | 45 | 1507.900 | 1567.416 | 149.818 | 2506.878 | 470.254 | 70.101 |
| Mg | 36 | 149.136 | 133.003 | 59.156 | 375.039 | 73.063 | 12.177 | 45 | 196.713 | 196.362 | 75.629 | 372.551 | 64.797 | 9.659 |
| Mn | 36 | 1.797 | 1.791 | 0.193 | 3.846 | 0.859 | 0.143 | 45 | 2.318 | 2.118 | 0.066 | 5.122 | 1.175 | 0.171 |
| Na | 36 | 827.503 | 840.872 | 288.063 | 1630.992 | 321.576 | 53.596 | 45 | 916.410 | 805.901 | 31.939 | 2515.700 | 431.902 | 64.384 |
| Se | 29 | 0.651 | 0.610 | 0.117 | 1.636 | 0.385 | 0.071 | 36 | 0.469 | 0.460 | 0.017 | 1.181 | 0.305 | 0.051 |
| Zn | 36 | 78.048 | 56.925 | 8.822 | 288.344 | 63.526 | 10.5878 | 44 | 87.267 | 88.918 | 4.089 | 198.083 | 41.251 | 6.219 |
| Sum.Rank AUD | Sum.Rank Control | U | Z | p | N AUD | N Control | |
|---|---|---|---|---|---|---|---|
| Ca | 1279.0 | 1961.0 | 649.0 | −1.34325 | 0.179191 | 35 | 45 |
| Co | 901.5 | 1873.5 | 405.5 | −2.85947 | 0.004244 | 31 | 43 |
| Cr | 1263.0 | 1897.0 | 633.0 | −1.35209 | 0.176347 | 35 | 44 |
| Cu | 1160.0 | 2161.0 | 494.0 | −3.00340 | 0.002670 | 36 | 45 |
| Fe | 1631.0 | 854.0 | 358.0 | 2.91451 | 0.003563 | 39 | 31 |
| K | 1345.0 | 1976.0 | 679.0 | −1.24508 | 0.213103 | 36 | 45 |
| Mg | 1134.0 | 2187.0 | 468.0 | −3.25052 | 0.001152 | 36 | 45 |
| Mn | 1252.0 | 2069.0 | 586.0 | −2.12899 | 0.033256 | 36 | 45 |
| Na | 1393.0 | 1928.0 | 727.0 | −0.78887 | 0.430190 | 36 | 45 |
| Se | 1098.0 | 1047.0 | 381.0 | 1.86075 | 0.062781 | 29 | 36 |
| Zn | 1260.0 | 1980.0 | 594.0 | −1.91485 | 0.055512 | 36 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baj, J.; Teresiński, G.; Forma, A.; Flieger, M.; Proch, J.; Niedzielski, P.; Grochowski, C.; Blicharska, E.; Buszewicz, G.; Bogucki, J.; et al. Chronic Alcohol Abuse Alters Hepatic Trace Element Concentrations-Metallomic Study of Hepatic Elemental Composition by Means of ICP-OES. Nutrients 2022, 14, 546. https://doi.org/10.3390/nu14030546
Baj J, Teresiński G, Forma A, Flieger M, Proch J, Niedzielski P, Grochowski C, Blicharska E, Buszewicz G, Bogucki J, et al. Chronic Alcohol Abuse Alters Hepatic Trace Element Concentrations-Metallomic Study of Hepatic Elemental Composition by Means of ICP-OES. Nutrients. 2022; 14(3):546. https://doi.org/10.3390/nu14030546
Chicago/Turabian StyleBaj, Jacek, Grzegorz Teresiński, Alicja Forma, Michał Flieger, Jędrzej Proch, Przemysław Niedzielski, Cezary Grochowski, Eliza Blicharska, Grzegorz Buszewicz, Jacek Bogucki, and et al. 2022. "Chronic Alcohol Abuse Alters Hepatic Trace Element Concentrations-Metallomic Study of Hepatic Elemental Composition by Means of ICP-OES" Nutrients 14, no. 3: 546. https://doi.org/10.3390/nu14030546
APA StyleBaj, J., Teresiński, G., Forma, A., Flieger, M., Proch, J., Niedzielski, P., Grochowski, C., Blicharska, E., Buszewicz, G., Bogucki, J., Majerek, D., Karakuła, K., Czeczelewski, M., & Flieger, J. (2022). Chronic Alcohol Abuse Alters Hepatic Trace Element Concentrations-Metallomic Study of Hepatic Elemental Composition by Means of ICP-OES. Nutrients, 14(3), 546. https://doi.org/10.3390/nu14030546









