Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Creatine’s Ergogenic Role in Mitochondrial Dysfunction
3.1. Acute, Traumatic Mitochondrial Dysfunction
3.2. Chronic, Atraumatic Mitochondrial Dysfunction
4. Noncommunicable Chronic Diseases (NCD)
5. Cardiovascular Disease and Ischemic Heart Failure
6. Traumatic and Ischemic Central Nervous System Injuries
7. Neurodegenerative Disorders
8. Psychological Disorders
9. Chronic Fatigue Syndrome, Post Viral Fatigue Syndrome, and Long COVID
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef]
- Harris, R. Creatine in health, medicine and sport: An introduction to a meeting held at Downing College, University of Cambridge, July 2010. Amino Acids 2011, 40, 1267. [Google Scholar] [CrossRef]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef]
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef]
- Huertas, J.R.; Casuso, R.A.; Agustín, P.H.; Cogliati, S. Stay Fit, Stay Young: Mitochondria in Movement: The Role of Exercise in the New Mitochondrial Paradigm. Oxidative Med. Cell. Longev. 2019, 2019, e7058350. [Google Scholar] [CrossRef]
- Negro, M.; Avanzato, I.; D’Antona, G. Chapter 2.7—Creatine in Skeletal Muscle Physiology. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 59–68. [Google Scholar]
- Nelson, A.G.; Arnall, D.A.; Kokkonen, J.; Day, R.; Evans, J. Muscle glycogen supercompensation is enhanced by prior creatine supplementation. Med. Sci. Sports Exerc. 2001, 33, 1096–1100. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Parise, G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 1999, 22, 1228–1233. [Google Scholar] [CrossRef]
- McKenna, M.J.; Morton, J.; Selig, S.E.; Snow, R.J. Creatine supplementation increases muscle total creatine but not maximal intermittent exercise performance. J. Appl. Physiol. 1999, 87, 2244–2252. [Google Scholar] [CrossRef]
- Greenhaff, P.L.; Bodin, K.; Soderlund, K.; Hultman, E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am. J. Physiol. 1994, 266, E725–E730. [Google Scholar] [CrossRef]
- Greenwood, M.; Kreider, R.B.; Earnest, C.P.; Rasmussen, C.; Almada, A. Differences in creatine retention among three nutritional formulations of oral creatine supplements. J. Exerc. Physiol. Online 2003, 6, 37–43. [Google Scholar]
- Choi, J.K.; Kustermann, E.; Dedeoglu, A.; Jenkins, B.G. Magnetic resonance spectroscopy of regional brain metabolite markers in FALS mice and the effects of dietary creatine supplementation. Eur. J. Neurosci. 2009, 30, 2143–2150. [Google Scholar] [CrossRef]
- Lyoo, I.K.; Kong, S.W.; Sung, S.M.; Hirashima, F.; Parow, A.; Hennen, J.; Cohen, B.M.; Renshaw, P.F. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003, 123, 87–100. [Google Scholar] [CrossRef]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef]
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef]
- Cornish, S.M.; Chilibeck, P.D.; Burke, D.G. The effect of creatine monohydrate supplementation on sprint skating in ice-hockey players. J. Sports Med. Phys. Fitness 2006, 46, 90–98. [Google Scholar]
- Dawson, B.; Vladich, T.; Blanksby, B.A. Effects of 4 weeks of creatine supplementation in junior swimmers on freestyle sprint and swim bench performance. J. Strength Cond. Res. 2002, 16, 485–490. [Google Scholar]
- Grindstaff, P.D.; Kreider, R.; Bishop, R.; Wilson, M.; Wood, L.; Alexander, C.; Almada, A. Effects of creatine supplementation on repetitive sprint performance and body composition in competitive swimmers. Int. J. Sport Nutr. 1997, 7, 330–346. [Google Scholar] [CrossRef]
- Juhasz, I.; Gyore, I.; Csende, Z.; Racz, L.; Tihanyi, J. Creatine supplementation improves the anaerobic performance of elite junior fin swimmers. Acta Physiol. Hung. 2009, 96, 325–336. [Google Scholar] [CrossRef]
- Silva, A.J.; Machado Reis, V.; Guidetti, L.; Bessone Alves, F.; Mota, P.; Freitas, J.; Baldari, C. Effect of creatine on swimming velocity, body composition and hydrodynamic variables. J. Sports Med. Phys. Fitness 2007, 47, 58–64. [Google Scholar]
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef]
- Stone, M.H.; Sanborn, K.; Smith, L.L.; O’Bryant, H.S.; Hoke, T.; Utter, A.C.; Johnson, R.L.; Boros, R.; Hruby, J.; Pierce, K.C.; et al. Effects of in-season (5 weeks) creatine and pyruvate supplementation on anaerobic performance and body composition in American football players. Int. J. Sport Nutr. 1999, 9, 146–165. [Google Scholar] [CrossRef]
- Bemben, M.G.; Bemben, D.A.; Loftiss, D.D.; Knehans, A.W. Creatine supplementation during resistance training in college football athletes. Med. Sci. Sports Exerc. 2001, 33, 1667–1673. [Google Scholar] [CrossRef]
- Hoffman, J.; Ratamess, N.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Magnus, C.; Anderson, M. Effect of in-season creatine supplementation on body composition and performance in rugby union football players. Appl. Physiol. Nutr. Metab. 2007, 32, 1052–1057. [Google Scholar] [CrossRef]
- Claudino, J.G.; Mezencio, B.; Amaral, S.; Zanetti, V.; Benatti, F.; Roschel, H.; Gualano, B.; Amadio, A.C.; Serrao, J.C. Creatine monohydrate supplementation on lower-limb muscle power in Brazilian elite soccer players. J. Int. Soc. Sports Nutr. 2014, 11, 32. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Rasmussen, C.; Lancaster, S.; Starks, M.; Smith, P.; Melton, C.; Greenwood, M.; Almada, A.; Kreider, R. Impact of differing protein sources and a creatine containing nutritional formula after 12 weeks of resistance training. Nutrition 2007, 23, 647–656. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Campbell, W.I.; Harvey, T.M.; Marcello, B.M.; Roberts, M.D.; Parker, A.G.; Byars, A.G.; Greenwood, L.D.; Almada, A.L.; et al. The effects of creatine monohydrate supplementation with and without D-pinitol on resistance training adaptations. J. Strength Cond. Res. 2009, 23, 2673–2682. [Google Scholar] [CrossRef]
- Galvan, E.; Walker, D.K.; Simbo, S.Y.; Dalton, R.; Levers, K.; O’Connor, A.; Goodenough, C.; Barringer, N.D.; Greenwood, M.; Rasmussen, C.; et al. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 12. [Google Scholar] [CrossRef]
- Volek, J.S.; Kraemer, W.J.; Bush, J.A.; Boetes, M.; Incledon, T.; Clark, K.L.; Lynch, J.M. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J. Am. Diet. Assoc. 1997, 97, 765–770. [Google Scholar] [CrossRef]
- Volek, J.S.; Mazzetti, S.A.; Farquhar, W.B.; Barnes, B.R.; Gomez, A.L.; Kraemer, W.J. Physiological responses to short-term exercise in the heat after creatine loading. Med. Sci. Sports Exerc. 2001, 33, 1101–1108. [Google Scholar] [CrossRef]
- Volek, J.S.; Ratamess, N.A.; Rubin, M.R.; Gomez, A.L.; French, D.N.; McGuigan, M.M.; Scheett, T.P.; Sharman, M.J.; Hakkinen, K.; Kraemer, W.J. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur. J. Appl. Physiol. 2004, 91, 628–637. [Google Scholar] [CrossRef]
- Buford, T.W.; Kreider, R.B.; Stout, J.R.; Greenwood, M.; Campbell, B.; Spano, M.; Ziegenfuss, T.; Lopez, H.; Landis, J.; Antonio, J. International Society of Sports Nutrition position stand: Creatine supplementation and exercise. J. Int. Soc. Sports Nutr. 2007, 4, 6. [Google Scholar] [CrossRef]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN exercise & sport nutrition review: Research & recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef]
- Branch, J.D. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Lanhers, C.; Pereira, B.; Naughton, G.; Trousselard, M.; Lesage, F.X.; Dutheil, F. Creatine Supplementation and Lower Limb Strength Performance: A Systematic Review and Meta-Analyses. Sports Med. 2015, 45, 1285–1294. [Google Scholar] [CrossRef]
- Wiroth, J.B.; Bermon, S.; Andrei, S.; Dalloz, E.; Hebuterne, X.; Dolisi, C. Effects of oral creatine supplementation on maximal pedalling performance in older adults. Eur. J. Appl. Physiol. 2001, 84, 533–539. [Google Scholar] [CrossRef]
- McMorris, T.; Mielcarz, G.; Harris, R.C.; Swain, J.P.; Howard, A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2007, 14, 517–528. [Google Scholar] [CrossRef]
- Rawson, E.S.; Clarkson, P.M. Acute creatine supplementation in older men. Int. J. Sports Med. 2000, 21, 71–75. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Potential benefits of creatine monohydrate supplementation in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 497–502. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Januario, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Read, C.Y.; Calnan, R.J. Mitochondrial disease: Beyond etiology unknown. J. Pediatr. Nurs. 2000, 15, 232–241. [Google Scholar] [CrossRef]
- Cohen, B.H.; Gold, D.R. Mitochondrial cytopathy in adults: What we know so far. Clev. Clin. J. Med. 2001, 68, 625–642. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The Neurometabolic Cascade of Concussion. J. Athl. Train 2001, 36, 228–235. [Google Scholar] [CrossRef]
- Dean, A.; Philip, J.; Arikan, G.; Opitz, B.; Sterr, A. Potential for use of creatine supplementation following mild traumatic brain injury. Concussion 2017, 2, CNC34. [Google Scholar] [CrossRef]
- Gaetz, M. The neurophysiology of brain injury. Clin. Neurophysiol. 2004, 115, 4–18. [Google Scholar] [CrossRef]
- Brooke, N.S.; Ouwerkerk, R.; Adams, C.B.; Radda, G.K.; Ledingham, J.G.; Rajagopalan, B. Phosphorus-31 magnetic resonance spectra reveal prolonged intracellular acidosis in the brain following subarachnoid hemorrhage. Proc. Natl. Acad. Sci. USA 1994, 91, 1903–1907. [Google Scholar] [CrossRef]
- Abe, K.; Aoki, M.; Kawagoe, J.; Yoshida, T.; Hattori, A.; Kogure, K.; Itoyama, Y. Ischemic Delayed Neuronal Death. Stroke 1995, 26, 1478–1489. [Google Scholar] [CrossRef]
- Ankarcrona, M.; Dypbukt, J.M.; Bonfoco, E.; Zhivotovsky, B.; Orrenius, S.; Lipton, S.A.; Nicotera, P. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995, 15, 961–973. [Google Scholar] [CrossRef]
- Fiskum, G.; Murphy, A.N.; Beal, M.F. Mitochondria in Neurodegeneration: Acute Ischemia and Chronic Neurodegenerative Diseases. J. Cereb. Blood Flow Metab. 1999, 19, 351–369. [Google Scholar] [CrossRef]
- Schinder, A.F.; Olson, E.C.; Spitzer, N.C.; Montal, M. Mitochondrial Dysfunction Is a Primary Event in Glutamate Neurotoxicity. J. Neurosci. 1996, 16, 6125–6133. [Google Scholar] [CrossRef]
- Béard, E.; Braissant, O. Synthesis and transport of creatine in the CNS: Importance for cerebral functions. J. Neurochem. 2010, 115, 297–313. [Google Scholar] [CrossRef]
- Rabinowitz, A.R.; Li, X.; Levin, H.S. Sport and nonsport etiologies of mild traumatic brain injury: Similarities and differences. Annu. Rev. Psychol. 2014, 65, 301–331. [Google Scholar] [CrossRef]
- Signoretti, S.; Lazzarino, G.; Tavazzi, B.; Vagnozzi, R. The pathophysiology of concussion. PM R 2011, 3, S359–S368. [Google Scholar] [CrossRef]
- Andres, R.H.; Ducray, A.D.; Schlattner, U.; Wallimann, T.; Widmer, H.R. Functions and effects of creatine in the central nervous system. Brain Res. Bull. 2008, 76, 329–343. [Google Scholar] [CrossRef]
- Gualano, B.; Roschel, H.; Lancha, A.H.; Brightbill, C.E.; Rawson, E.S. In sickness and in health: The widespread application of creatine supplementation. Amino Acids 2012, 43, 519–529. [Google Scholar] [CrossRef]
- Rae, C.D.; Bröer, S. Creatine as a booster for human brain function. How might it work? Neurochem. Int. 2015, 89, 249–259. [Google Scholar] [CrossRef]
- Perasso, L.; Spallarossa, P.; Gandolfo, C.; Ruggeri, P.; Balestrino, M. Therapeutic Use of Creatine in Brain or Heart Ischemia: Available Data and Future Perspectives. Med. Res. Rev. 2013, 33, 336–363. [Google Scholar] [CrossRef]
- O’Gorman, E.; Beutner, G.; Dolder, M.; Koretsky, A.P.; Brdiczka, D.; Wallimann, T. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997, 414, 253–257. [Google Scholar] [CrossRef]
- Meyer, L.E.; Machado, L.B.; Santiago, A.P.; Da-Silva, W.S.; De Felice, F.G.; Holub, O.; Oliveira, M.F.; Galina, A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: Antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J. Biol. Chem. 2006, 281, 37361–37371. [Google Scholar] [CrossRef]
- Sakellaris, G.; Kotsiou, M.; Tamiolaki, M.; Kalostos, G.; Tsapaki, E.; Spanaki, M.; Spilioti, M.; Charissis, G.; Evangeliou, A. Prevention of complications related to traumatic brain injury in children and adolescents with creatine administration: An open label randomized pilot study. J. Trauma 2006, 61, 322–329. [Google Scholar] [CrossRef]
- Sakellaris, G.; Nasis, G.; Kotsiou, M.; Tamiolaki, M.; Charissis, G.; Evangeliou, A. Prevention of traumatic headache, dizziness and fatigue with creatine administration. A pilot study. Acta Paediatr. 2008, 97, 31–34. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ferreira, G.; Settineri, R.; Ellithorpe, R.R.; Breeding, P.; Ash, M.E. Mitochondrial Dysfunction and Chronic Disease: Treatment with Membrane Lipid Replacement and Other Natural Supplements. In Mitochondrial Biology and Experimental Therapeutics; Oliveira, P.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 499–522. [Google Scholar]
- Newell, C.; Leduc-Pessah, H.; Khan, A.; Shearer, J. Mitochondrial Dysfunction in Chronic Disease. In The Routledge Handbook on Biochemistry of Exercise; Routledge: Abingdon, UK, 2020. [Google Scholar]
- Victor, M.V.; Rocha, M.; Herance, R.; Hernandez-Mijares, A. Oxidative Stress and Mitochondrial Dysfunction in Type 2 Diabetes. Curr. Pharm. Des. 2011, 17, 3947–3958. [Google Scholar] [CrossRef]
- Picard, M.; Turnbull, D.M. Linking the Metabolic State and Mitochondrial DNA in Chronic Disease, Health, and Aging. Diabetes 2013, 62, 672–678. [Google Scholar] [CrossRef]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial Dysfunction in Atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef]
- Cloonan, S.M.; Kim, K.; Esteves, P.; Trian, T.; Barnes, P.J. Mitochondrial dysfunction in lung ageing and disease. Eur. Respir. Rev. 2020, 29, 157. [Google Scholar] [CrossRef]
- Wei, P.Z.; Szeto, C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.-H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef]
- Fang, T.; Wang, M.; Xiao, H.; Wei, X. Mitochondrial dysfunction and chronic lung disease. Cell Biol. Toxicol. 2019, 35, 493–502. [Google Scholar] [CrossRef] [PubMed]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, V.; Menzies, K.J.; Auwerx, J. Repairing Mitochondrial Dysfunction in Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 353–389. [Google Scholar] [CrossRef]
- Abrigo, J.; Simon, F.; Cabrera, D.; Vilos, C.; Cabello-Verrugio, C. Mitochondrial Dysfunction in Skeletal Muscle Pathologies. Curr. Protein Pept. Sci. 2019, 20, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S.; Pabelick, C.M.; Sieck, G.C. Mitochondrial Dysfunction in Airway Disease. Chest 2017, 152, 618–626. [Google Scholar] [CrossRef]
- Diaz-Vegas, A.; Sanchez-Aguilera, P.; Krycer, J.R.; Morales, P.E.; Monsalves-Alvarez, M.; Cifuentes, M.; Rothermel, B.A.; Lavandero, S. Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases? Endocr. Rev. 2020, 41, 491–517. [Google Scholar] [CrossRef]
- Novak, E.A.; Mollen, K.P. Mitochondrial dysfunction in inflammatory bowel disease. Front. Cell Dev. Biol. 2015, 3, 62. [Google Scholar] [CrossRef]
- Ballinger, S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005, 38, 1278–1295. [Google Scholar] [CrossRef]
- Rosca, M.G.; Hoppel, C.L. Mitochondrial dysfunction in heart failure. Heart Fail. Rev. 2013, 18, 607–622. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Cao, Q.; Wang, Z.; Zhao, M.; Xu, L.; Zhuang, Q. Mitochondrial dysfunction in fibrotic diseases. Cell Death Discov. 2020, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.; Beal, M.F. Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial Dysfunction in Retinal Diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Liu, F. Mitochondrial stress: A bridge between mitochondrial dysfunction and metabolic diseases? Cell. Signal. 2011, 23, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Myhill, S.; Booth, N.E.; McLaren-Howard, J. Chronic fatigue syndrome and mitochondrial dysfunction. Int. J. Clin. Exp. Med. 2009, 2, 1–16. [Google Scholar]
- Haas, R.H. Mitochondrial Dysfunction in Aging and Diseases of Aging. Biology 2019, 8, 48. [Google Scholar] [CrossRef]
- Kemp, G.J. Mitochondrial dysfunction in chronic ischemia and peripheral vascular disease. Mitochondrion 2004, 4, 629–640. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zapelini, P.H.; Rezin, G.T.; Cardoso, M.R.; Ritter, C.; Klamt, F.; Moreira, J.C.F.; Streck, E.L.; Dal-Pizzol, F. Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 2008, 8, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.G.; Kun, S.; Sélley, E.; Kertész, M.; Szélig, L.; Csontos, C.; Böddi, K.; Bogár, L.; Miseta, A.; Wittmann, I. Role of Tyrosine Isomers in Acute and Chronic Diseases Leading to Oxidative Stress—A Review. Curr. Med. Chem. 2016, 23, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, G.G.; Pacheco Moisés, F.P.; Mireles-Ramírez, M.; Flores-Alvarado, L.J.; González-Usigli, H.; Sánchez-González, V.J.; Sánchez-López, A.L.; Sánchez-Romero, L.; Díaz-Barba, E.I.; Santoscoy-Gutiérrez, J.F.; et al. Chapter One-Oxidative Stress: Love and Hate History in Central Nervous System. In Advances in Protein Chemistry and Structural Biology; Stress and Inflammation in Disorders; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 108, pp. 1–31. [Google Scholar]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L.; Levine, S. Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, e3085756. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J. Energy demands in competitive soccer. J. Sports Sci. 1994, 12, S5–S12. [Google Scholar] [CrossRef]
- Adeva, M.M.; Souto, G.; Blanco, N.; Donapetry, C. Ammonium metabolism in humans. Metabolism 2012, 61, 1495–1511. [Google Scholar] [CrossRef]
- Mutch, B.J.; Banister, E.W. Ammonia metabolism in exercise and fatigue: A review. Med. Sci. Sports Exerc. 1983, 15, 41–50. [Google Scholar] [CrossRef]
- Srinivasan, S.; Guha, M.; Kashina, A.; Avadhani, N.G. Mitochondrial Dysfunction and Mitochondrial Dynamics-The Cancer Connection. Biochim. Biophys. Acta 2017, 1858, 602–614. [Google Scholar] [CrossRef]
- Stork, C.; Renshaw, P.F. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol. Psychiatry 2005, 10, 900–919. [Google Scholar] [CrossRef]
- Devic, S. Warburg Effect-a Consequence or the Cause of Carcinogenesis? J. Cancer 2016, 7, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.M.; Heaton, R.; Rankin, S.; Murphy, A.; Bentley, J.; Sexton, D.; Hargreaves, I.P. Evidence of Oxidative Stress and Secondary Mitochondrial Dysfunction in Metabolic and Non-Metabolic Disorders. J. Clin. Med. 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Mlynárik, V. Introduction to nuclear magnetic resonance. Anal. Biochem. 2017, 529, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Prost, R.W. Magnetic resonance spectroscopy. Med. Phys. 2008, 35, 4530–4544. [Google Scholar] [CrossRef] [PubMed]
- Henning, A. Proton and multinuclear magnetic resonance spectroscopy in the human brain at ultra-high field strength: A review. Neuroimage 2018, 168, 181–198. [Google Scholar] [CrossRef]
- Porter, D.A.; Smith, M.A. Magnetic resonance spectroscopy in vivo. J. Biomed. Eng. 1988, 10, 562–568. [Google Scholar] [CrossRef]
- Bertolino, A.; Frye, M.; Callicott, J.H.; Mattay, V.S.; Rakow, R.; Shelton-Repella, J.; Post, R.; Weinberger, D.R. Neuronal pathology in the hippocampal area of patients with bipolar disorder: A study with proton magnetic resonance spectroscopic imaging. Biol. Psychiatry 2003, 53, 906–913. [Google Scholar] [CrossRef]
- Dager, S.R.; Friedman, S.D.; Parow, A.; Demopulos, C.; Stoll, A.L.; Lyoo, I.K.; Dunner, D.L.; Renshaw, P.F. Brain Metabolic Alterations in Medication-Free Patients with BipolarDisorder. Arch. Gen. Psychiatry 2004, 61, 450–458. [Google Scholar] [CrossRef]
- Kato, T.; Takahashi, S.; Shioiri, T.; Inubushi, T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J. Affect. Disord. 1992, 26, 223–230. [Google Scholar] [CrossRef]
- Kato, T.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Takahashi, Y.; Inubushi, T.; Takahashi, S. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol. Med. 1995, 25, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Riesberg, L.A.; Weed, S.A.; McDonald, T.L.; Eckerson, J.M.; Drescher, K.M. Beyond muscles: The untapped potential of creatine. Int. Immunopharmacol. 2016, 37, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Schulze, A. Health implications of creatine: Can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience 2002, 112, 243–260. [Google Scholar] [CrossRef]
- Guimarães-Ferreira, L.; Pinheiro, C.H.J.; Gerlinger-Romero, F.; Vitzel, K.F.; Nachbar, R.T.; Curi, R.; Nunes, M.T. Short-term creatine supplementation decreases reactive oxygen species content with no changes in expression and activity of antioxidant enzymes in skeletal muscle. Eur. J. Appl. Physiol. 2012, 112, 3905–3911. [Google Scholar] [CrossRef]
- Hunter, D.J.; Reddy, K.S. Noncommunicable Diseases. N. Engl. J. Med. 2013, 369, 1336–1343. [Google Scholar] [CrossRef]
- Peña-Oyarzun, D.; Bravo-Sagua, R.; Diaz-Vega, A.; Aleman, L.; Chiong, M.; Garcia, L.; Bambs, C.; Troncoso, R.; Cifuentes, M.; Morselli, E.; et al. Autophagy and oxidative stress in non-communicable diseases: A matter of the inflammatory state? Free Radic. Biol. Med. 2018, 124, 61–78. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Lopez, A.D. Measuring the Global Burden of Disease. N. Engl. J. Med. 2013, 369, 448–457. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases: Progress Monitor 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Yu, E.P.K.; Reinhold, J.; Yu, H.; Starks, L.; Uryga, A.K.; Foote, K.; Finigan, A.; Figg, N.; Pung, Y.-F.; Logan, A.; et al. Mitochondrial Respiration Is Reduced in Atherosclerosis, Promoting Necrotic Core Formation and Reducing Relative Fibrous Cap Thickness. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2322–2332. [Google Scholar] [CrossRef]
- Shimizu, S.; Ishibashi, M.; Kumagai, S.; Wajima, T.; Hiroi, T.; Kurihara, T.; Ishii, M.; Kiuchi, Y. Decreased cardiac mitochondrial tetrahydrobiopterin in a rat model of pressure overload. Int. J. Mol. Med. 2013, 31, 589–596. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.; Mi, C.; Liu, J.; Gao, F.; Long, J. Compromised mitochondrial remodeling in compensatory hypertrophied myocardium of spontaneously hypertensive rat. Cardiovasc. Pathol. 2014, 23, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Tschöpe, C.; Sterner-Kock, A.; Westermann, D.; Heringer-Walther, S.; Riad, A.; Nikolic, A.; Wang, Y.; Ebermann, L.; Siems, W.-E.; et al. Accelerated Mitochondrial Adenosine Diphosphate/Adenosine Triphosphate Transport Improves Hypertension-Induced Heart Disease. Circulation 2007, 115, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Rolo, A.P.; Duarte, F.V.; Simões, A.M.; Palmeira, C.M. Differential alterations in mitochondrial function induced by a choline-deficient diet: Understanding fatty liver disease progression. Mitochondrion 2008, 8, 367–376. [Google Scholar] [CrossRef]
- Galloway, C.A.; Lee, H.; Brookes, P.S.; Yoon, Y. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G632–G641. [Google Scholar] [CrossRef]
- Tubbs, E.; Chanon, S.; Robert, M.; Bendridi, N.; Bidaux, G.; Chauvin, M.-A.; Ji-Cao, J.; Durand, C.; Gauvrit-Ramette, D.; Vidal, H.; et al. Disruption of Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Contributes to Muscle Insulin Resistance in Mice and Humans. Diabetes 2018, 67, 636–650. [Google Scholar] [CrossRef]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.T.; Hartley, R.C.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.-T.; Price, J.W.; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 119, 573–581. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Parra, V.; Troncoso, R.; Pennanen, C.; Contreras-Ferrat, A.; Vasquez-Trincado, C.; Morales, P.E.; Lopez-Crisosto, C.; Sotomayor-Flores, C.; Chiong, M.; et al. Alteration in mitochondrial Ca2+ uptake disrupts insulin signaling in hypertrophic cardiomyocytes. Cell Commun. Signal. 2014, 12, 68. [Google Scholar] [CrossRef]
- Tubbs, E.; Theurey, P.; Vial, G.; Bendridi, N.; Bravard, A.; Chauvin, M.-A.; Ji-Cao, J.; Zoulim, F.; Bartosch, B.; Ovize, M.; et al. Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Is Required for Insulin Signaling and Is Implicated in Hepatic Insulin Resistance. Diabetes 2014, 63, 3279–3294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wakabayashi, N.; Wakabayashi, J.; Tamura, Y.; Song, W.-J.; Sereda, S.; Clerc, P.; Polster, B.M.; Aja, S.M.; Pletnikov, M.V.; et al. The dynamin-related GTPase Opa1 is required for glucose-stimulated ATP production in pancreatic beta cells. Mol. Biol. Cell 2011, 22, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, F.; Schultz, J.; Waterstradt, R.; Baltrusch, S. Drp1 guarding of the mitochondrial network is important for glucose-stimulated insulin secretion in pancreatic beta cells. Biochem. Biophys. Res. Commun. 2016, 474, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Anello, M.; Lupi, R.; Spampinato, D.; Piro, S.; Masini, M.; Boggi, U.; Del Prato, S.; Rabuazzo, A.M.; Purrello, F.; Marchetti, P. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 2005, 48, 282–289. [Google Scholar] [CrossRef]
- Scheuermann-Freestone, M.; Madsen, P.L.; Manners, D.; Blamire, A.M.; Buckingham, R.E.; Styles, P.; Radda, G.K.; Neubauer, S.; Clarke, K. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003, 107, 3040–3046. [Google Scholar] [CrossRef]
- Rider, O.J.; Francis, J.M.; Ali, M.K.; Holloway, C.; Pegg, T.; Robson, M.D.; Tyler, D.; Byrne, J.; Clarke, K.; Neubauer, S. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation 2012, 125, 1511–1519. [Google Scholar] [CrossRef]
- Lamb, H.J.; Beyerbacht, H.P.; Van der Laarse, A.; Stoel, B.C.; Doornbos, J.; Van der Wall, E.E.; De Roos, A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation 1999, 99, 2261–2267. [Google Scholar] [CrossRef]
- Guescini, M.; Tiano, L.; Genova, M.L.; Polidori, E.; Silvestri, S.; Orlando, P.; Fimognari, C.; Calcabrini, C.; Stocchi, V.; Sestili, P. The Combination of Physical Exercise with Muscle-Directed Antioxidants to Counteract Sarcopenia: A Biomedical Rationale for Pleiotropic Treatment with Creatine and Coenzyme Q10. Oxidative Med. Cell. Longev. 2017, 2017, e7083049. [Google Scholar] [CrossRef]
- Alves, C.R.R.; Filho, C.A.A.M.; Benatti, F.B.; Brucki, S.; Pereira, R.M.R.; Pinto, A.L.d.S.; Lima, F.R.; Roschel, H.; Gualano, B. Creatine Supplementation Associated or Not with Strength Training upon Emotional and Cognitive Measures in Older Women: A Randomized Double-Blind Study. PLoS ONE 2013, 8, e76301. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Variables Influencing the Effectiveness of Creatine Supplementation as a Therapeutic Intervention for Sarcopenia. Front. Nutr. 2019, 6, 124. [Google Scholar] [CrossRef]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; De Sa Pinto, A.L.; Lima, F.R.; Pereira, R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.M.; Tritto, A.C.; Da Silva, L.R.; De Oliveira, P.B.; Benatti, F.B.; Roschel, H.; Nieß, B.; Gualano, B.; Pereira, R.M.R. Effects of long-term low-dose dietary creatine supplementation in older women. Exp. Gerontol. 2015, 70, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.L.; Botelho, P.B.; Carneiro, J.A.; Mota, J.F. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J. Cach. Sarc. Muscle 2016, 7, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Artioli, G.G.; Poortmans, J.R.; Lancha Junior, A.H. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010, 38, 31–44. [Google Scholar] [CrossRef]
- Candow, D.G.; Vogt, E.; Johannsmeyer, S.; Forbes, S.C.; Farthing, J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 2015, 40, 689–694. [Google Scholar] [CrossRef]
- De Sousa, M.V.; Da Silva Soares, D.B.; Caraça, E.R.; Cardoso, R. Dietary protein and exercise for preservation of lean mass and perspectives on type 2 diabetes prevention. Exp. Biol. Med. 2019, 244, 992–1004. [Google Scholar] [CrossRef]
- Barney, B.; Beck, G.R. Nutrition Interventions in Heart Failure. In Manual of Heart Failure Management; Bisognano, J.D., Earley, M.B., Baker, M.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 207–217. [Google Scholar]
- Solis, M.Y.; Artioli, G.G.; Gualano, B. Potential of Creatine in Glucose Management and Diabetes. Nutrients 2021, 13, 570. [Google Scholar] [CrossRef]
- Gualano, B.; De Salles Painneli, V.; Roschel, H.; Artioli, G.G.; Neves, M.; De Sá Pinto, A.L.; Da Silva, M.E.R.; Cunha, M.R.; Otaduy, M.C.G.; Leite, C.D.C.; et al. Creatine in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Med. Sci. Sports Exerc. 2011, 43, 770–778. [Google Scholar] [CrossRef]
- Earnest, C.P.; Almada, A.L.; Mitchell, T.L. High-performance capillary electrophoresis-pure creatine monohydrate reduces blood lipids in men and women. Clin. Sci. 1996, 91, 113–118. [Google Scholar] [CrossRef]
- Deminice, R.; De Castro, G.S.F.; Francisco, L.V.; Da Silva, L.E.C.M.; Cardoso, J.F.R.; Frajacomo, F.T.T.; Teodoro, B.G.; Dos Reis Silveira, L.; Jordao, A.A. Creatine supplementation prevents fatty liver in rats fed choline-deficient diet: A burden of one-carbon and fatty acid metabolism. J. Nutr. Biochem. 2015, 26, 391–397. [Google Scholar] [CrossRef]
- Gupta, A.; Akki, A.; Wang, Y.; Leppo, M.K.; Chacko, V.P.; Foster, D.B.; Caceres, V.; Shi, S.; Kirk, J.A.; Su, J.; et al. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J. Clin. Investig. 2012, 122, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Wallis, J.; Lygate, C.A.; Fischer, A.; ten Hove, M.; Schneider, J.E.; Sebag-Montefiore, L.; Dawson, D.; Hulbert, K.; Zhang, W.; Zhang, M.H.; et al. Supranormal Myocardial Creatine and Phosphocreatine Concentrations Lead to Cardiac Hypertrophy and Heart Failure. Circulation 2005, 112, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Nascimben, L.; Ingwall, J.S.; Pauletto, P.; Friedrich, J.; Gwathmey, J.K.; Saks, V.; Pessina, A.C.; Allen, P.d. Creatine Kinase System in Failing and Nonfailing Human Myocardium. Circulation 1996, 94, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Spindler, M.; Higgins, M.A.; Jin, N.; Gill, R.M.; Bloem, L.J.; Ryan, T.P.; Ingwall, J.S. The fall in creatine levels and creatine kinase isozyme changes in the failing heart are reversible: Complex post-transcriptional regulation of the components of the CK system. J. Mol. Cell. Cardiol. 2005, 39, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lygate, C.A.; Fischer, A.; Sebag-Montefiore, L.; Wallis, J.; ten Hove, M.; Neubauer, S. The creatine kinase energy transport system in the failing mouse heart. J. Mol. Cell. Cardiol. 2007, 42, 1129–1136. [Google Scholar] [CrossRef]
- Liao, R.; Nascimben, L.; Friedrich, J.; Gwathmey, J.K.; Ingwall, J.S. Decreased Energy Reserve in an Animal Model of Dilated Cardiomyopathy. Circ. Res. 1996, 78, 893–902. [Google Scholar] [CrossRef]
- Zervou, S.; Whittington, H.J.; Russell, A.J.; Lygate, C.A. Augmentation of Creatine in the Heart. Mini Rev. Med. Chem. 2016, 16, 19–28. [Google Scholar] [CrossRef]
- Neubauer, S.; Krahe, T.; Schindler, R.; Horn, M.; Hillenbrand, H.; Entzeroth, C.; Mader, H.; Kromer, E.P.; Riegger, G.A.; Lackner, K. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 1992, 86, 1810–1818. [Google Scholar] [CrossRef]
- Neubauer, S.; Horn, M.; Pabst, T.; Gödde, M.; Lübke, D.; Jilling, B.; Hahn, D.; Ertl, G. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur. Heart J. 1995, 16, 115–118. [Google Scholar] [CrossRef]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Peters, W.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; et al. Myocardial Phosphocreatine-to-ATP Ratio Is a Predictor of Mortality in Patients with Dilated Cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.; Remkes, H.; Dienesch, C.; Hu, K.; Ertl, G.; Neubauer, S. Chronic high-dose creatine feeding does not attenuate left ventricular remodeling in rat hearts post-myocardial infarction. Cardiovasc. Res. 1999, 43, 117–124. [Google Scholar] [CrossRef][Green Version]
- McClung, J.; Hand, G.; Davis, J.; Carson, J. Effect of creatine supplementation on cardiac muscle of exercise-stressed rats. Eur. J. Appl. Physiol. 2003, 89, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.; Jiang, N.; Ma, G.; Qu, J.; Zhang, G.; Cao, D.; Wen, L.; Liu, S.; Ji, L.L.; Zhang, Y. Regulation of mitochondrial uncoupling respiration during exercise in rat heart: Role of reactive oxygen species (ROS) and uncoupling protein 2. Free Radic. Biol. Med. 2008, 44, 1373–1381. [Google Scholar] [CrossRef]
- Cao, F.; Zervou, S.; Lygate, C.A. The creatine kinase system as a therapeutic target for myocardial ischaemia–reperfusion injury. Biochem. Soc. Trans. 2018, 46, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Hultman, J.; Ronquist, G.; Forsberg, J.O.; Hansson, H.E. Myocardial energy restoration of ischemic damage by administration of phosphoenolpyruvate during reperfusion. A study in a paracorporeal rat heart model. Eur. Surg. Res. 1983, 15, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Osbakken, M.; Ito, K.; Zhang, D.; Ponomarenko, I.; Ivanics, T.; Jahngen, E.G.; Cohn, M. Creatine and cyclocreatine effects on ischemic myocardium: 31P nuclear magnetic resonance evaluation of intact heart. Cardiology 1992, 80, 184–195. [Google Scholar] [CrossRef]
- Sharov, V.G.; Saks, V.A.; Kupriyanov, V.V.; Lakomkin, V.L.; Kapelko, V.I.; Steinschneider, A.Y.; Javadov, S.A. Protection of ischemic myocardium by exogenous phosphocreatine. I. Morphologic and phosphorus 31-nuclear magnetic resonance studies. J. Thorac. Cardiovasc. Surg. 1987, 94, 749–761. [Google Scholar] [CrossRef]
- Balestrino, M.; Sarocchi, M.; Adriano, E.; Spallarossa, P. Potential of creatine or phosphocreatine supplementation in cerebrovascular disease and in ischemic heart disease. Amino Acids 2016, 48, 1955–1967. [Google Scholar] [CrossRef]
- ten Hove, M.; Lygate, C.A.; Fischer, A.; Schneider, J.E.; Sang, A.E.; Hulbert, K.; Sebag-Montefiore, L.; Watkins, H.; Clarke, K.; Isbrandt, D.; et al. Reduced Inotropic Reserve and Increased Susceptibility to Cardiac Ischemia/Reperfusion Injury in Phosphocreatine-Deficient Guanidinoacetate-N-Methyltransferase–Knockout Mice. Circulation 2005, 111, 2477–2485. [Google Scholar] [CrossRef]
- Elgebaly, S.A.; Wei, Z.; Tyles, E.; Elkerm, A.F.; Houser, S.L.; Gillies, C.; Kaddurah-Daouk, R. Enhancement of the recovery of rat hearts after prolonged cold storage by cyclocreatine phosphate. Transplantation 1994, 57, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Cisowski, M.; Bochenek, A.; Kucewicz, E.; Wnuk-Wojnar, A.M.; Morawski, W.; Skalski, J.; Grzybek, H. The use of exogenous creatine phosphate for myocardial protection in patients undergoing coronary artery bypass surgery. J. Cardiovasc. Surg. 1996, 37, 75–80. [Google Scholar]
- Ruda, M.Y.; Samarenko, M.B.; Afonskaya, N.I.; Saks, V.A. Reduction of ventricular arrhythmias by phosphocreatine (Neoton) in patients with acute myocardial infarction. Am. Heart J. 1988, 116, 393–397. [Google Scholar] [CrossRef]
- Elgebaly, S.A.; Poston, R.; Todd, R.; Helmy, T.; Almaghraby, A.M.; Elbayoumi, T.; Kreutzer, D.L. Cyclocreatine protects against ischemic injury and enhances cardiac recovery during early reperfusion. Expert Rev. Cardiovasc. Ther. 2019, 17, 683–697. [Google Scholar] [CrossRef]
- Roberts, J.J.; Walker, J.B. Feeding a creatine analogue delays ATP depletion and onset of rigor in ischemic heart. Am. J. Physiol. Heart Circ. Physiol. 1982, 243, H911–H916. [Google Scholar] [CrossRef]
- Chida, K.; Otani, H.; Kohzuki, M.; Saito, H.; Kagaya, Y.; Takai, Y.; Takahashi, S.; Yamada, S.; Zuguchi, M. The Relationship between Plasma BNP Level and the Myocardial Phosphocreatine/Adenosine Triphosphate Ratio Determined by Phosphorus-31 Magnetic Resonance Spectroscopy in Patients with Dilated Cardiomyopathy. Cardiology 2006, 106, 132–136. [Google Scholar] [CrossRef]
- Russo, E.; Nguyen, H.; Lippert, T.; Tuazon, J.; Borlongan, C.V.; Napoli, E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018, 4, 84–94. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Zaaroor, M. Mitochondrial targeting for development of novel drug strategies in brain injury. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 131–145. [Google Scholar] [CrossRef]
- Niizuma, K.; Endo, H.; Chan, P.H. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 2009, 109, 133–138. [Google Scholar] [CrossRef]
- Niizuma, K.; Yoshioka, H.; Chen, H.; Kim, G.S.; Jung, J.E.; Katsu, M.; Okami, N.; Chan, P.H. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 92–99. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ning, N.; Zhou, Q.; Khoshnam, S.E.; Farzaneh, M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic. Biol. Med. 2020, 146, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Zarriello, S.; Rajani, M.; Tuazon, J.; Napoli, E.; Borlongan, C.V. Understanding the Role of Dysfunctional and Healthy Mitochondria in Stroke Pathology and Its Treatment. Int. J. Mol. Sci. 2018, 19, 2127. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Hüttemann, M. Molecular Mechanisms of Ischemia–Reperfusion Injury in Brain: Pivotal Role of the Mitochondrial Membrane Potential in Reactive Oxygen Species Generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Ischemic stroke and mitochondria: Mechanisms and targets. Protoplasma 2020, 257, 335–343. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim. Biophys. Acta 2009, 1787, 1416–1424. [Google Scholar] [CrossRef]
- Blennow, K.; Hardy, J.; Zetterberg, H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012, 76, 886–899. [Google Scholar] [CrossRef]
- Turner, C.E.; Byblow, W.D.; Gant, N. Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J. Neurosci. 2015, 35, 1773–1780. [Google Scholar] [CrossRef]
- Zhu, S.; Li, M.; Figueroa, B.E.; Liu, A.; Stavrovskaya, I.G.; Pasinelli, P.; Beal, M.F.; Brown, R.H.; Kristal, B.S.; Ferrante, R.J.; et al. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J. Neurosci 2004, 24, 5909–5912. [Google Scholar] [CrossRef]
- Hausmann, O.N.; Fouad, K.; Wallimann, T.; Schwab, M.E. Protective effects of oral creatine supplementation on spinal cord injury in rats. Spin. Cord 2002, 40, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Geiger, J.D.; Mattson, M.P.; Scheff, S.W. Dietary supplement creatine protects against traumatic brain injury. Ann. Neurol. 2000, 48, 723–729. [Google Scholar] [CrossRef]
- Prass, K.; Royl, G.; Lindauer, U.; Freyer, D.; Megow, D.; Dirnagl, U.; Stöckler-Ipsiroglu, G.; Wallimann, T.; Priller, J. Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. J. Cereb. Blood Flow Metab. 2007, 27, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Freire Royes, L.F.; Cassol, G. The Effects of Creatine Supplementation and Physical Exercise on Traumatic Brain Injury. Mini Rev. Med. Chem. 2016, 16, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Novelli, A.; Reilly, J.A.; Lysko, P.G.; Henneberry, R.C. Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988, 451, 205–212. [Google Scholar] [CrossRef]
- Tsuji, K.; Nakamura, Y.; Ogata, T.; Shibata, T.; Kataoka, K. Rapid decrease in ATP content without recovery phase during glutamate-induced cell death in cultured spinal neurons. Brain Res. 1994, 662, 289–292. [Google Scholar] [CrossRef]
- Carter, A.J.; Müller, R.E.; Pschorn, U.; Stransky, W. Preincubation with Creatine Enhances Levels of Creatine Phosphate and Prevents Anoxic Damage in Rat Hippocampal Slices. J. Neurochem. 1995, 64, 2691–2699. [Google Scholar] [CrossRef]
- Brustovetsky, N.; Brustovetsky, T.; Dubinsky, J.M. On the mechanisms of neuroprotection by creatine and phosphocreatine. J. Neurochem. 2001, 76, 425–434. [Google Scholar] [CrossRef]
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef]
- Grimm, A.; Friedland, K.; Eckert, A. Mitochondrial dysfunction: The missing link between aging and sporadic Alzheimer’s disease. Biogerontology 2016, 17, 281–296. [Google Scholar] [CrossRef]
- Leuner, K.; Hauptmann, S.; Abdel-Kader, R.; Scherping, I.; Keil, U.; Strosznajder, J.B.; Eckert, A.; Müller, W.E. Mitochondrial dysfunction: The first domino in brain aging and Alzheimer’s disease? Antiox. Redox Signal. 2007, 9, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Grimm, A.; Eckert, A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Geary, D.C. Mitochondrial Functioning and the Relations among Health, Cognition, and Aging: Where Cell Biology Meets Cognitive Science. Int. J. Mol. Sci. 2021, 22, 3562. [Google Scholar] [CrossRef] [PubMed]
- González-Reyes, R.E.; Aliev, G.; Ávila-Rodrigues, M.; Barreto, G.E. Alterations in Glucose Metabolism on Cognition: A Possible Link Between Diabetes and Dementia. Curr. Pharm. Des. 2016, 22, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.C.-C.; Huang, P.-T.; Lin, Y.-F. Alzheimer’s Disease and Diabetes: Insulin Signaling as the Bridge Linking Two Pathologies. Mol. Neurobiol. 2020, 57, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yan, Z.; Zhou, T.; Wang, G. SIRT1 Regulates Cognitive Performance and Ability of Learning and Memory in Diabetic and Nondiabetic Models. J. Diabetes. Res. 2017, 2017, 7121827. [Google Scholar] [CrossRef]
- Jo, D.; Kim, B.C.; Cho, K.A.; Song, J. The Cerebral Effect of Ammonia in Brain Aging: Blood-Brain Barrier Breakdown, Mitochondrial Dysfunction, and Neuroinflammation. J. Clin. Med. 2021, 10, 2773. [Google Scholar] [CrossRef]
- Bustamante, J.; Czerniczyniec, A.; Lores-Arnaiz, S. Brain nitric oxide synthases and mitochondrial function. Front. Biosci. 2007, 12, 1034–1040. [Google Scholar] [CrossRef]
- Felipo, V.; Butterworth, R.F. Mitochondrial dysfunction in acute hyperammonemia. Neurochem. Int. 2002, 40, 487–491. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Alzheimer’s Disease is Type 3 Diabetes—Evidence Reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Links Between Obesity-Induced Brain Insulin Resistance, Brain Mitochondrial Dysfunction, and Dementia. Front. Endocrinol. 2018, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Eckert, A.; Kurz, C.; Eckert, G.P.; Leuner, K. Mitochondrial dysfunction: Common final pathway in brain aging and Alzheimer’s disease—Therapeutic aspects. Mol. Neurobiol. 2010, 41, 159–171. [Google Scholar] [CrossRef]
- Kaliszewska, A.; Allison, J.; Martini, M.; Arias, N. The Interaction of Diet and Mitochondrial Dysfunction in Aging and Cognition. Int. J. Mol. Sci. 2021, 22, 3574. [Google Scholar] [CrossRef] [PubMed]
- Francis, H.M.; Stevenson, R.J. Potential for diet to prevent and remediate cognitive deficits in neurological disorders. Nutr. Rev. 2018, 76, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Head, E. Oxidative damage and cognitive dysfunction: Antioxidant treatments to promote healthy brain aging. Neurochem. Res. 2009, 34, 670–678. [Google Scholar] [CrossRef]
- Poddar, J.; Pradhan, M.; Ganguly, G.; Chakrabarti, S. Biochemical deficits and cognitive decline in brain aging: Intervention by dietary supplements. J. Chem. Neuroanat. 2019, 95, 70–80. [Google Scholar] [CrossRef]
- Hammett, S.T.; Wall, M.B.; Edwards, T.C.; Smith, A.T. Dietary supplementation of creatine monohydrate reduces the human fMRI BOLD signal. Neurosci. Lett. 2010, 479, 201–205. [Google Scholar] [CrossRef]
- Watanabe, A.; Kato, N.; Kato, T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci. Res. 2002, 42, 279–285. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.C.; Howard, A.N.; Langridge, G.; Hall, B.; Corbett, J.; Dicks, M.; Hodgson, C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol. Behav. 2007, 90, 21–28. [Google Scholar] [CrossRef]
- Dworak, M.; Kim, T.; McCarley, R.W.; Basheer, R. Creatine supplementation reduces sleep need and homeostatic sleep pressure in rats. J. Sleep Res. 2017, 26, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Starkov, A.; Blass, J.P.; Ratan, R.R.; Beal, M.F. Cause and consequence: Mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim. Biophys. Acta 2010, 1802, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; De Bari, L.; De Filippis, B.; Henrion-Caude, A.; Vacca, R.A. Mitochondrial dysfunction as a central actor in intellectual disability-related diseases: An overview of Down syndrome, autism, Fragile X and Rett syndrome. Neurosci. Biobehav. Rev. 2014, 46, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Adhihetty, P.J.; Beal, M.F. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromol. Med. 2008, 10, 275–290. [Google Scholar] [CrossRef]
- Kley, R.A.; Tarnopolsky, M.A.; Vorgerd, M. Creatine for treating muscle disorders. Cochrane Database Syst. Rev. 2013, 2013, CD004760. [Google Scholar] [CrossRef]
- Shao, A.; Lin, D.; Wang, L.; Tu, S.; Lenahan, C.; Zhang, J. Oxidative Stress at the Crossroads of Aging, Stroke and Depression. Aging Dis. 2020, 11, 1537–1566. [Google Scholar] [CrossRef]
- Martin, E.I.; Ressler, K.J.; Binder, E.; Nemeroff, C.B. The Neurobiology of Anxiety Disorders: Brain Imaging, Genetics, and Psychoneuroendocrinology. Psychiatr. Clin. 2009, 32, 549–575. [Google Scholar] [CrossRef]
- Yildiz-Yesiloglu, A.; Ankerst, D.P. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: A meta-analysis. Psychiatry Res. Neuroimag. 2006, 147, 1–25. [Google Scholar] [CrossRef]
- Scaglia, F. The role of mitochondrial dysfunction in psychiatric disease. Dev. Disabil. Res. Rev. 2010, 16, 136–143. [Google Scholar] [CrossRef]
- Agren, H.; Niklasson, F. Creatinine and creatine in CSF: Indices of brain energy metabolism in depression. Short note. J. Neural. Transm. 1988, 74, 55–59. [Google Scholar] [CrossRef]
- Mirza, Y.; O’Neill, J.; Smith, E.A.; Russell, A.; Smith, J.M.; Banerjee, S.P.; Bhandari, R.; Boyd, C.; Rose, M.; Ivey, J.; et al. Increased medial thalamic creatine-phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J. Child Neurol. 2006, 21, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.; Hall, K.H.; Tate, W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 “long-haulers”? Chronic Dis. Transl. Med. 2021, 7, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Watzl, J.; Banakar, S.; O’Neill, J.; Mintz, J.; Davanzo, P.; Fischer, J.; Chirichigno, J.W.; Ventura, J.; Elman, S.; et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology 2007, 32, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.G.; Sung, Y.-H.; Hellem, T.L.; Fiedler, K.K.; Shi, X.; Jeong, E.-K.; Renshaw, P.F. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011, 135, 354–361. [Google Scholar] [CrossRef]
- Roitman, S.; Green, T.; Osher, Y.; Karni, N.; Levine, J. Creatine monohydrate in resistant depression: A preliminary study. Bipolar Disord. 2007, 9, 754–758. [Google Scholar] [CrossRef]
- Toniolo, R.A.; Silva, M.; Fernandes, F.d.B.F.; Amaral, J.A.d.M.S.; Dias, R.d.S.; Lafer, B. A randomized, double-blind, placebo-controlled, proof-of-concept trial of creatine monohydrate as adjunctive treatment for bipolar depression. J. Neural. Transm. 2018, 125, 247–257. [Google Scholar] [CrossRef]
- Kious, B.M.; Kondo, D.G.; Renshaw, P.F. Creatine for the Treatment of Depression. Biomolecules 2019, 9, 406. [Google Scholar] [CrossRef]
- Pazini, F.L.; Cunha, M.P.; Rodrigues, A.L.S. The possible beneficial effects of creatine for the management of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 193–206. [Google Scholar] [CrossRef]
- Kondo, D.G.; Forrest, L.N.; Shi, X.; Sung, Y.-H.; Hellem, T.L.; Huber, R.S.; Renshaw, P.F. Creatine target engagement with brain bioenergetics: A dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids 2016, 48, 1941–1954. [Google Scholar] [CrossRef]
- D’Anci, K.E.; Allen, P.J.; Kanarek, R.B. A potential role for creatine in drug abuse? Mol. Neurobiol. 2011, 44, 136–141. [Google Scholar] [CrossRef]
- Amital, D.; Vishne, T.; Roitman, S.; Kotler, M.; Levine, J. Open Study of Creatine Monohydrate in Treatment-Resistant Posttraumatic Stress Disorder. J. Clin. Psychiatry 2006, 67, 836–837. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J. Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic value? Neurosci. Biobehav. Rev. 2012, 36, 1442–1462. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, T.C.; Majeroni, B.A.; Pretorius, R.; Malik, K. Fatigue: An overview. Am. Fam. Physician 2008, 78, 1173–1179. [Google Scholar]
- Jamal, G.A.; Hansen, S. Post-Viral Fatigue Syndrome: Evidence for Underlying Organic Disturbance in the Muscle Fibre. Eur. Neurol. 1989, 29, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.H.T.; Newham, D.J.; Peters, T.J. Muscle biochemistry and pathophysiology in postviral fatigue syndrome. Br. Med. Bull. 1991, 47, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.J.M.; Barrett, M.C.; Taylor, D.J.; Kemp, G.J.; Lodi, R. Heterogeneity in chronic fatigue syndrome: Evidence from magnetic resonance spectroscopy of muscle. Neuromuscul. Disord. 1998, 8, 204–209. [Google Scholar] [CrossRef]
- Behan, W.M.H.; More, I.A.R.; Behan, P.O. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol. 1991, 83, 61–65. [Google Scholar] [CrossRef]
- Zhang, C.; Baumer, A.; Mackay, I.R.; Linnane, A.W.; Nagley, P. Unusual pattern of mitochondrial DNA deletions in skeletal muscle of an adult human with chronic fatigue syndrome. Hum. Mol. Genet. 1995, 4, 751–754. [Google Scholar] [CrossRef]
- Filler, K.; Lyon, D.; Bennett, J.; McCain, N.; Elswick, R.; Lukkahatai, N.; Saligan, L.N. Association of mitochondrial dysfunction and fatigue: A review of the literature. BBA Clin. 2014, 1, 12–23. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in Myalgic Encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Malatji, B.G.; Meyer, H.; Mason, S.; Engelke, U.F.H.; Wevers, R.A.; Van Reenen, M.; Reinecke, C.J. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, M. Viral infection, a suggestive hypothesis for aetiology of chronic fatigue syndrome. J. Med. Hypotheses Ideas 2008, 2, 10–11. [Google Scholar]
- Smith, A.P. Post-viral Fatigue: Implications for Long Covid. Asian J. Res. Infect. Dis. 2021, 17–23. [Google Scholar] [CrossRef]
- Carfi, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Doykov, I.; Hällqvist, J.; Gilmour, K.C.; Grandjean, L.; Mills, K.; Heywood, W.E. ‘The long tail of Covid-19’-The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Research 2021, 9, 1349. [Google Scholar] [CrossRef]
- Poenaru, S.; Abdallah, S.J.; Corrales-Medina, V.; Cowan, J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: A narrative review. Ther. Adv. Infect. 2021, 8, 20499361211009385. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.; Drid, P.; Hoffman, J.R.; Sekulic, D.; Zenic, N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients 2016, 8, 72. [Google Scholar] [CrossRef]
- Ostojic, S.M. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients 2021, 13, 503. [Google Scholar] [CrossRef]
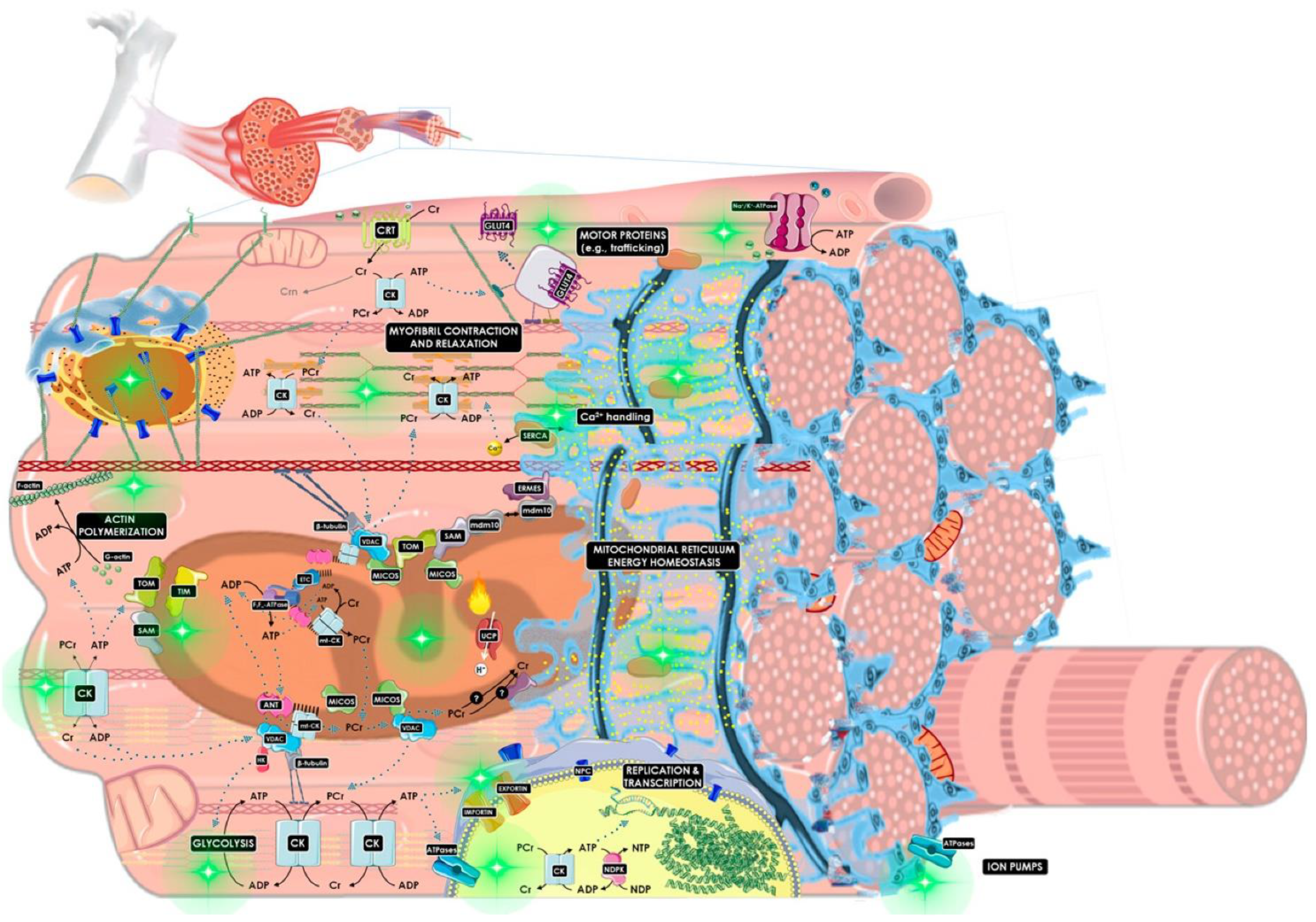

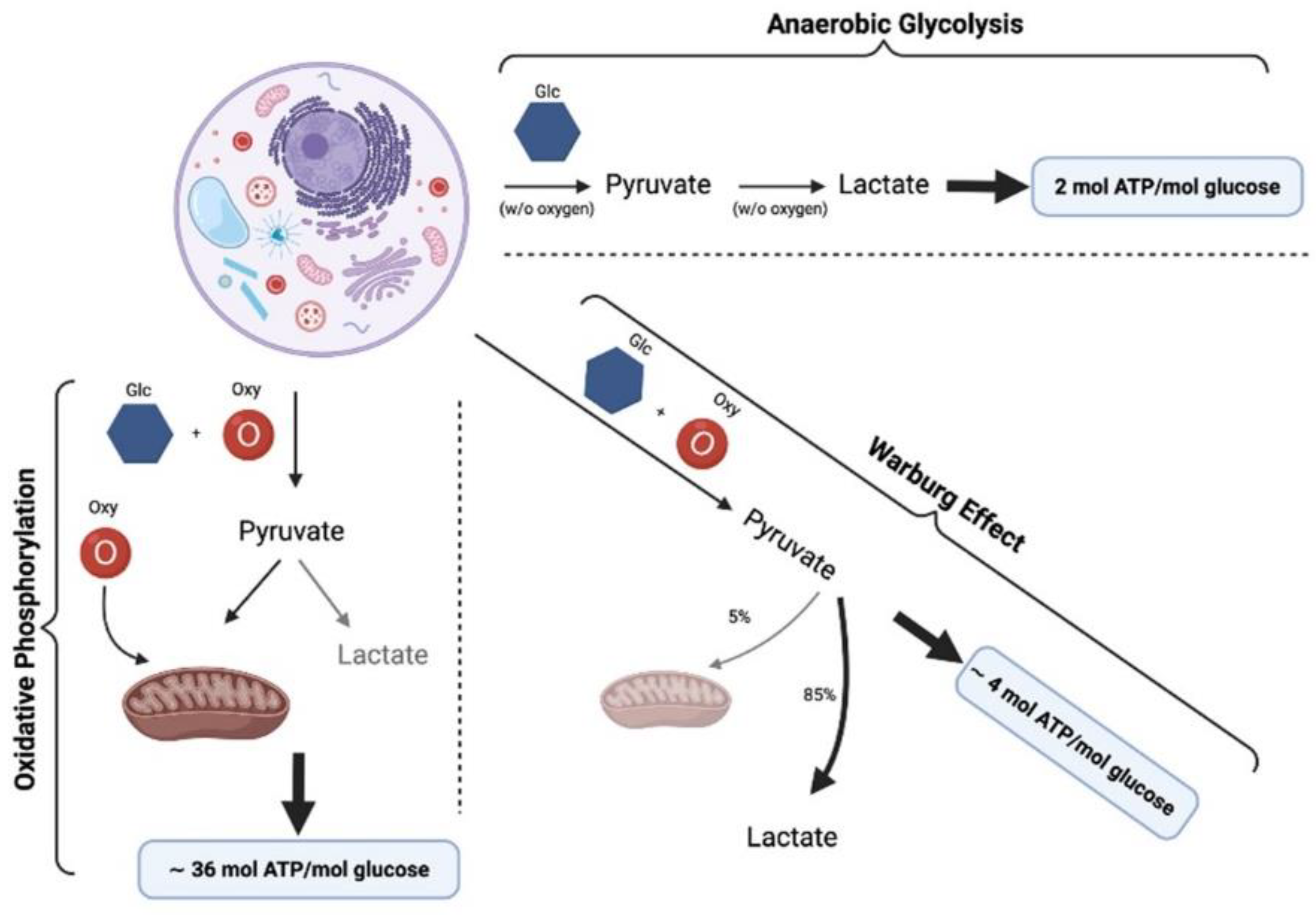
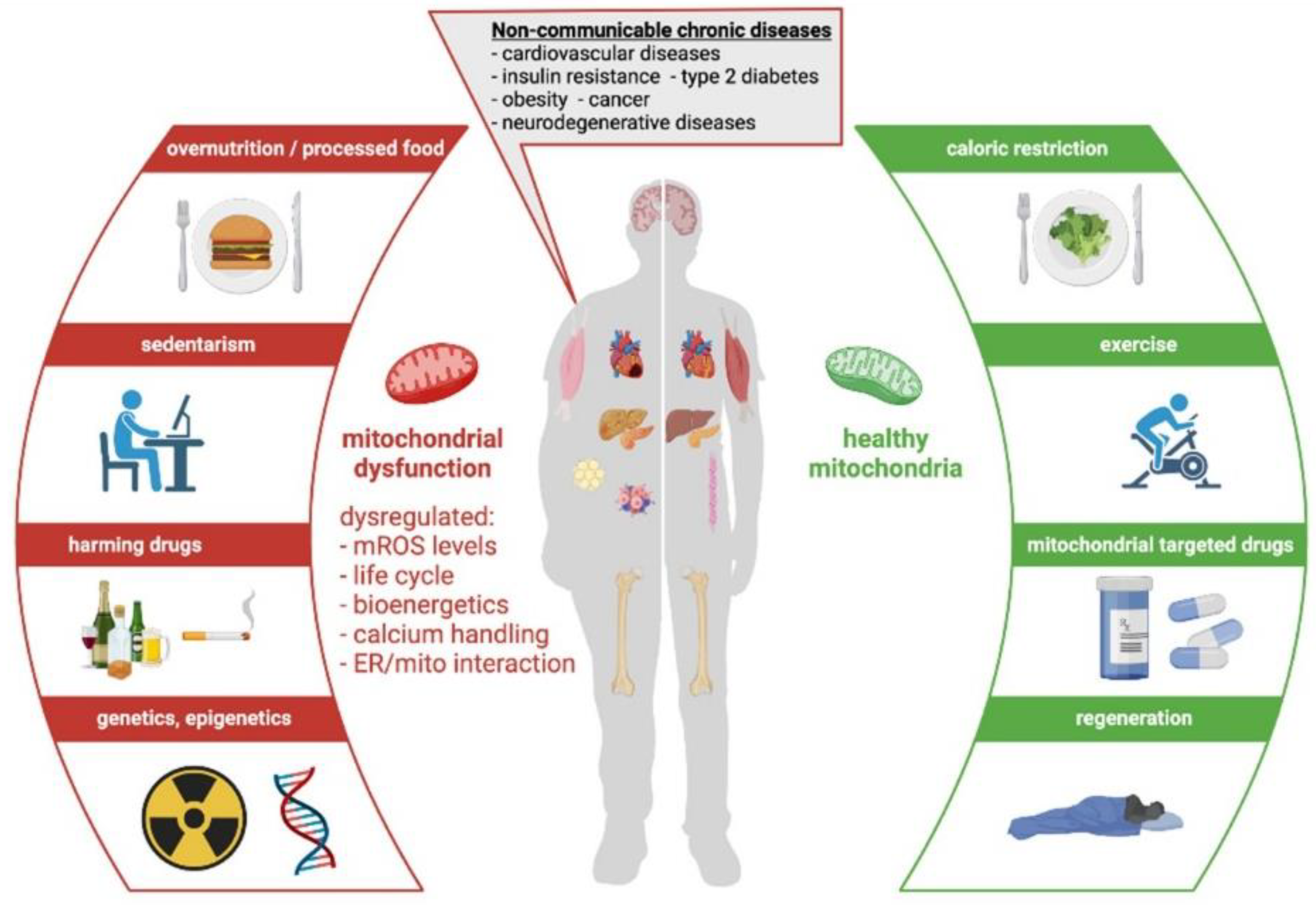
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Sakellaris et al. [71] | Traumatic brain injury | Human | 0.4 g/kg per day for 6 months | Yes | 39 | Improved self-care, cognition, behavior functions and communication | Direct effect on disease |
| Sakellaris et al. [72] | Traumatic brain injury | Human | 0.4 g/kg per day for 6 months | Yes | 39 | Reduced fatigue, headache and dizziness | Direct effect on disease |
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Guimarães-Ferreira et al. [128] | - | Animal/vitro | 5 g/kg per day for 6 days | no | 39 | Decrease in ROS in muscle tissue | Anima model |
| Kato et al. [124] | Bipolar disorder | Humans | None | No | 25 (disease) vs. 21 (control) | Abnormal energy phosphate metabolism in bipolar disorder | No intervention, only descriptive, observational findings |
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Rider et al. [151] | Obesity | Human | None | None | 64 | Deranged cardiac energetics and diastolic dysfunction in obesity group | Observational, disease related changes in metabolism |
| Scheuermann-Freestone et al. [150] | Diabetes Type 2 | Human | None | None | 36 | Impaired myocardial and skeletal muscle metabolism (reduced PCR/ATP ratio) | Observational disease related changes in metabolism |
| Lamb et al. [152] | Hypertension | Human | None | None | 24 | Altered high-energy phosphate metabolism in hypertension. Cardiac dysfunction correlates with metabolic alterations | Observational, disease related changes in metabolism |
| Gualano et al. [164] | Diabetes Type 2 | Human | 5 g creatine for 12 weeks + physical activity program | Yes | 25 | Improved glycemic control in supplementation group (by GLUT-4 recruitment) | Direct effect on disease related metabolic effects |
| Earnest et al. [165] | Hyper-cholester-inaemia | Human | 4 × 5 g creatine for 5 days and afterwards 2 times per day for 51 days (orally) | Yes | 34 | Minor reduction of total cholesterol during supplementation. Reduction of triacylglycerol’s and very-low-density-lipoprotein c 4 weeks after finishing supplementation | Direct effect of supplementation on metabolism. |
| Deminice et al. [166] | Fatty liver | Animal | Control vs. 0.25% choline diet vs. 0.25% choline + 2% creatine diet | None | 24 | Prevention of fat liver accumulation and hepatic events in creatine-fed group | Animal model |
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Elgebaly et al. [187] | - | Animal/vitro | 500 mg/kg BW | no | 6 | Better aortic flow, coronary flow, cardiac output, stroke volume, and stroke work | Animal model |
| Cisowski et al. [188] | Cardiac surgery | Humans | 6 g 3 days pre-surgery, intra-surgical and two days post- surgery i.v. | yes | 40 | Reduced arrhythmic events, reduced need of ionotropic medication | Direct effect on surgical procedure |
| Ruda et al. [189] | Ischemic myocardial infarct | human | 2 g bolus + 4 g/h over 2 h | Yes | 60 | Reduced arrhythmic events | Direct effect on short term outcome |
| Chida et al. [192] | Dilated Cardio-myopathy | Human | None | None | 13 | Plasma BNP level was correlated negatively with the myocardial phosphocreatine/adenosine triphosphate | Observational finding |
| Roberts et al. [191]. | None | Animal | Oral creatine-feeding | None | Not clear | Higher cellular ATP during ischemia in creatine-fed rat hearts | Animal model |
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Zhu et al. [206] | None/induced ischemia | Animal | 2% creatine-supplemented diet for 4 weeks | None | 6 per group | Reduction in ischemia induced infarct size | Animal model |
| Turner et al. [205] | None/induced hypoxia | Human | 7-ds oral creatine-supplementation | Yes | 15 | Less decrease in cognitive performance, attentional capacity, corticomotor excitability for creatine-group | Human brain metabolism |
| Hausmann et al. [207] | None/induced spinal cord injury | Animal | 4 weeks oral creatine-supplementation | none | 20 | Better locomotor scores after 1 week for creatine-group. Less scar tissue for creatine-group after 2 weeks | Animal model |
| Sullivan et al. [208] | None/induced traumatic brain injury | Animal | Mice: 0.1 mL/10 g/BW creatine monohydrate injection for 1, 3 or 5 days | none | 40 mice/24 rats | Reduction of brain tissue damage size by 36% mice and 50% rats | Animal model |
| Rats: 1% creatine diet for 4 weeks. | |||||||
| Prass et al. [209] | None/induced experimental stroke | Animal | Creatine-rich diet (0%, 0.5%, 1%, 2% for 3 weeks | None | Unclear | Reduction of infarct size by 40% in 2% creatine-fed group | Animal model |
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Hammett et al. [234] | None | Human | 20 g/d creatine for 5 days + 5 g/d for 2-days | Yes | 22 | Reduction of stress related blood oxygen level dependent in fMRI in creatine-group | Human metabolic response |
| Watanabe et al. [235] | None | Human | 8 g/d for 5-days | Yes | 24 | Reduction of mental fatigue and increased brain oxygen consumption in creatine-group | Human metabolic response |
| McMorris et al. [236] | None | Human | 4 × 5 g/d | yes | 20 | Better in central complex executive tasks with creatine while sleep deprivation | Human metabolic response |
| McMorris et al. [45] | None | Human | 4 × 5 g/d | Yes | 15 | random number generation, forward number and spatial recall, and long-term memory | Human metabolism |
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Kondo et al. [250] | Adolescent major depressive disorder | Human | 4 g/d creatine for 8 weeks | None | 15 | Reduction in children-depression symptom scores. Significant increase in brain phosphocreatine level. | Direct effect on disease (no RCT) |
| Roitman et al. [251] | Treatment resistant depression | Human | 3–5 g/d creatine for 4 weeks | None | 8 unipolar depressed patients and two bipolar patients | Development of hypomania/mania in bipolar patients. Improved Hamilton Depression Rating Scale, Hamilton Anxiety Scale, and Clinical Global Impression for 7 of 8 unipolar depressed patients | Direct effect on disease (no RCT) |
| Toniolo et al. [252] | Depressive episode of Bipolar Type 1 and Type 2 | Human | 6 g/d creatine for 6 weeks | Yes | 35 | No significant difference in Montgomery-Åsberg Depression Rating Scale by intervention but higher remission rate in creatine supplemented group | Direct effect on disease |
| Kondo et al. [255] | Adolescent with SSRI resistant major depressive disorder | Human | 0 g vs. 2 g vs. 4 g vs. 10 g creatine supplementation for 8 weeks | Yes | 34 | Clinical depression scores correlated inversely with brain phosphocreatine (PCR) levels. PCR level improved with higher dose. | Potential direct effect on disease |
| Study | Disease | Subject | Treatment | Randomized | Subjects | Efficacy | Effect Role |
|---|---|---|---|---|---|---|---|
| Ostojic et al. [264] | Chronic Fatigue syndrome | Human | 2 g, 4 g oral Guanidinoacetic Acid for 3 months vs. placebo | Yes | 21 | Higher muscle creatine-phosphate level and better oxidative capacity. However, no significant improvement of fatigue symptoms | Direct effect on disease related metabolism |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marshall, R.P.; Droste, J.-N.; Giessing, J.; Kreider, R.B. Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review. Nutrients 2022, 14, 529. https://doi.org/10.3390/nu14030529
Marshall RP, Droste J-N, Giessing J, Kreider RB. Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review. Nutrients. 2022; 14(3):529. https://doi.org/10.3390/nu14030529
Chicago/Turabian StyleMarshall, Robert Percy, Jan-Niklas Droste, Jürgen Giessing, and Richard B. Kreider. 2022. "Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review" Nutrients 14, no. 3: 529. https://doi.org/10.3390/nu14030529
APA StyleMarshall, R. P., Droste, J.-N., Giessing, J., & Kreider, R. B. (2022). Role of Creatine Supplementation in Conditions Involving Mitochondrial Dysfunction: A Narrative Review. Nutrients, 14(3), 529. https://doi.org/10.3390/nu14030529







