Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Supplementation
2.3. Cytotoxicity Evaluation
- (a)
- Viability was determined by conversion of the MTT salt to its formazan product detected at 560 nm using a Tecan Infinite M200 microplate reader (Tecan, Männedorf, Switzerland) [15]. Cell viability was expressed as percentage of viability in control cells, assigned as 100%.
- (b)
- Light microscopy examination of cell morphology and monolayer integrity was performed using an inverted confocal light microscopy model IB (Exacta Optech, Modena, Italy) using 10×, 25× and 40× as magnification [16].
2.4. HR 1H-NMR
2.5. Lipid Extraction and Fatty Acid Composition Analysis
2.6. Statistical Analysis
3. Results
3.1. Cytotoxicity
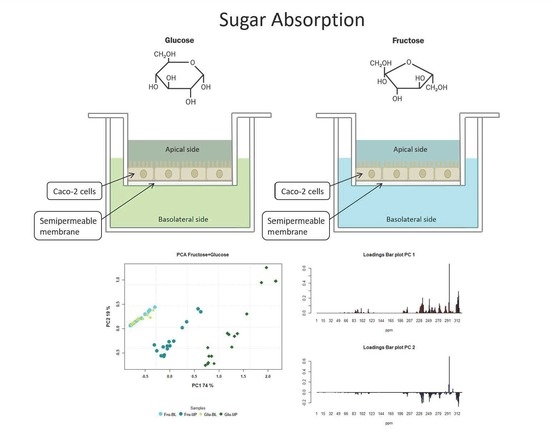
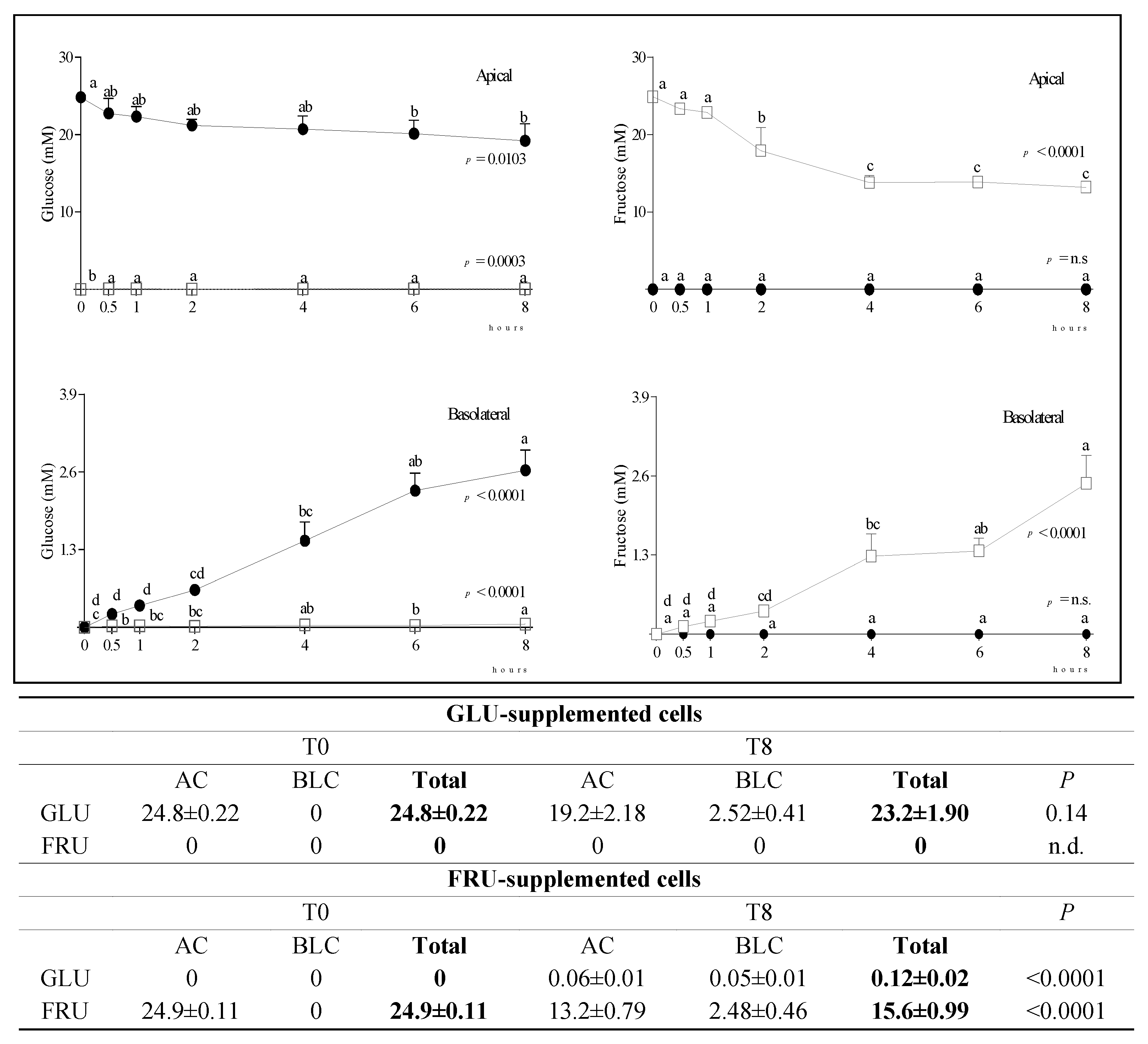
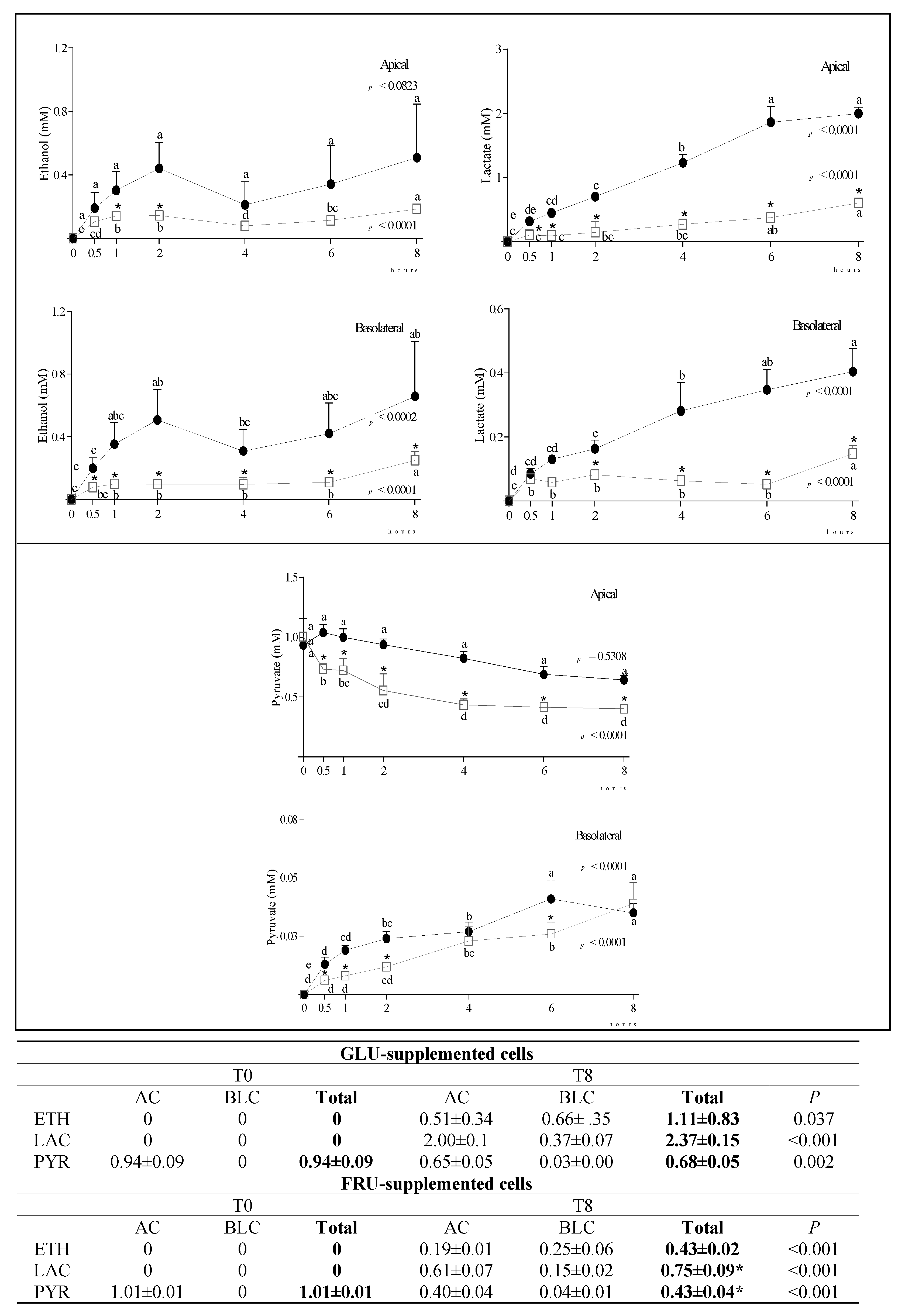
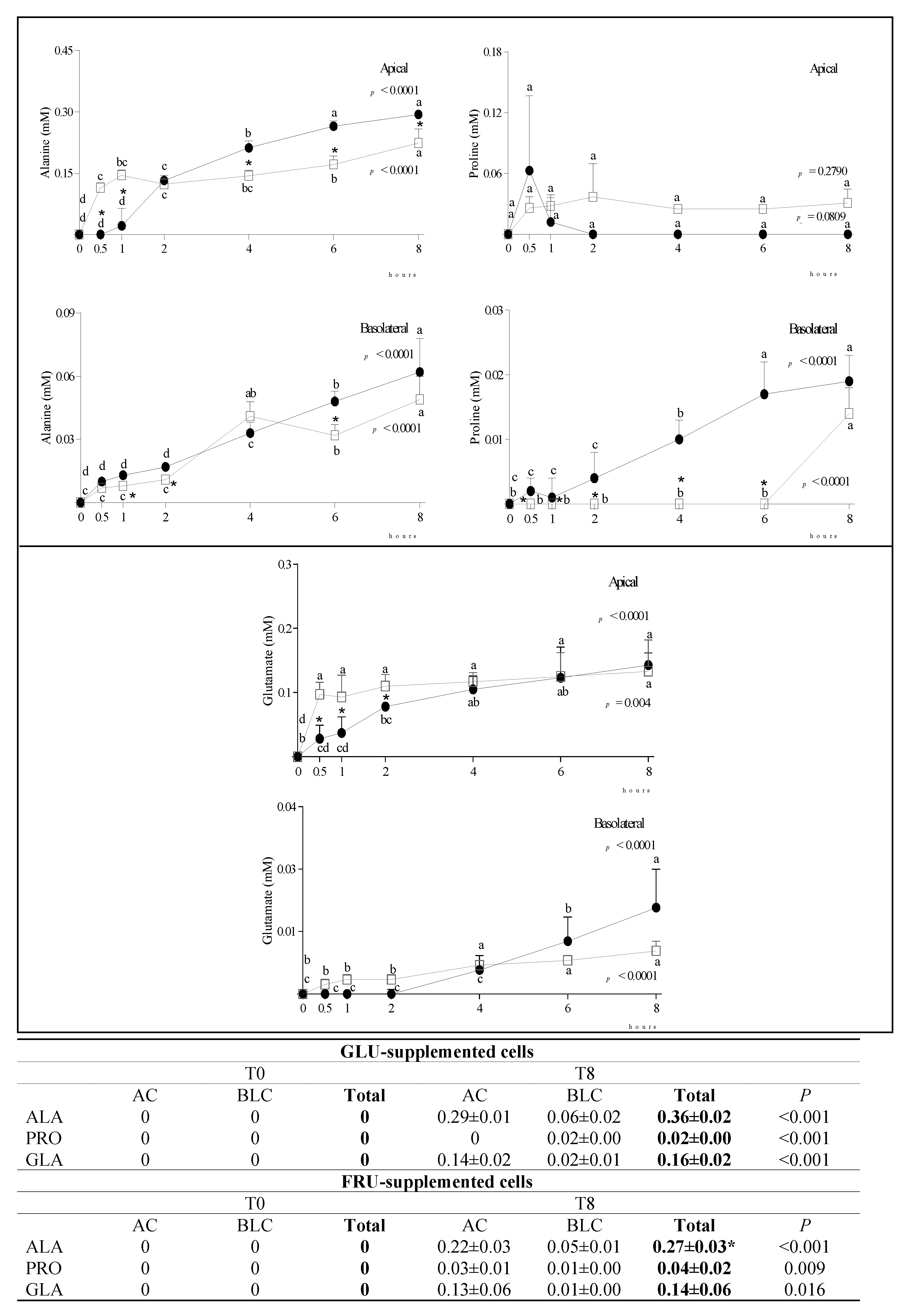
3.2. Metabolite Diffusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grembecka, M. Natural sweeteners in a human diet. Rocz. Panstw. Zakl. Hig. 2015, 66, 195–202. [Google Scholar] [PubMed]
- Page, K.; Chan, O.; Arora, J.; Belfort-Deaguiar, R.; Dzuira, J.; Roehmholdt, B.; Cline, G.; Naik, S.; Sinha, R.; Constable, R.; et al. Effects of Fructose vs Glucose on Regional Cerebral Blood Flow in Brain Regions Involved With Appetite and Reward Pathways. JAMA 2013, 309, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraris, R.P.; Choe, J.-Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Merino, B.; Fernández-Díaz, C.M.; Cózar-Castellano, I.; Perdomo, G. Intestinal Fructose and Glucose Metabolism in Health and Disease. Nutrients 2020, 12, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellett, G. The facilitated component of intestinal glucose absorption. J. Physiol. 2001, 531, 585–595. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Moore, J.B.; Fielding, B.A. Sugar and metabolic health: Is there still a debate? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, R.P. Dietary and developmental regulation of intestinal sugar transport. Biochem. J. 2001, 360, 265–276. [Google Scholar] [CrossRef]
- Schreck, K.; Melzig, M.F. Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells. Molecules 2018, 23, 2544. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.; Cotter, P.; Lopez-Exposito, I.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and ex Vivo Models. 2015. Available online: https://library.oapen.org/bitstream/handle/20.500.12657/28028/1001968.pdf?sequence=1 (accessed on 1 December 2021).
- Fanjul, C.; Barrenetxe, J.; Iñigo, C.; Sakar, Y.; Ducroc, R.; Barber, A.; Lostao, M.P. Leptin regulates sugar and amino acids transport in the human intestinal cell line Caco-2. Acta Physiol. 2012, 205, 82–91. [Google Scholar] [CrossRef]
- O’Brien, P.; Corpe, C.P. Acute Effects of Sugars and Artificial Sweeteners on Small Intestinal Sugar Transport: A Study Using CaCo-2 Cells As an In Vitro Model of the Human Enterocyte. PLoS ONE 2016, 11, e0167785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraris, R.P.; Yasharpour, S.; Lloyd, K.C.; Mirzayan, R.; Diamond, J.M. Luminal glucose concentrations in the gut under normal conditions. Am. J. Physiol.-Gastrointest. Liver Physiol. 1990, 259, G822–G837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Q.; Ma, L.Q.; Cui, X. Effects of Food Constituents on Absorption and Bioaccessibility of Dietary Synthetic Phenolic Antioxidant by Caco-2 Cells. J. Agric. Food Chem. 2020, 68, 4670–4677. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef] [Green Version]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Di Nunzio, M.; van Deursen, D.; Verhoeven, A.J.; Bordoni, A. n-3 and n-6 Polyunsaturated fatty acids suppress sterol regulatory element binding protein activity and increase flow of non-esterified cholesterol in HepG2 cells. Br. J. Nutr. 2010, 103, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Bub, A.; Malpuech-Brugère, C.; Orfila, C.; Amat, J.; Arianna, A.; Blot, A.; Di Nunzio, M.; Holmes, M.; Kertész, Z.; Marshall, L.; et al. A Dietary Intervention of Bioactive Enriched Foods Aimed at Adults at Risk of Metabolic Syndrome: Protocol and Results from PATHWAY-27 Pilot Study. Nutrients 2019, 11, 1814. [Google Scholar] [CrossRef] [Green Version]
- Gouyon, F.; Onesto, C.; Dalet, V.; Pages, G.; Leturque, A.; Brot-Laroche, E. Fructose modulates GLUT5 mRNA stability in differentiated Caco-2 cells: Role of cAMP-signalling pathway and PABP (polyadenylated-binding protein)-interacting protein (Paip) 2. Biochem. J. 2003, 375, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Steffansen, B.; Pedersen, M.D.L.; Laghmoch, A.M.; Nielsen, C.U. SGLT1-Mediated Transport in Caco-2 Cells Is Highly Dependent on Cell Bank Origin. J. Pharm. Sci. 2017, 106, 2664–2670. [Google Scholar] [CrossRef] [Green Version]
- Alzaid, F.; Cheung, H.-M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The Small Intestine Converts Dietary Fructose into Glucose and Organic Acids. Cell Metab. 2018, 27, 351–361.e353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, C.; Douard, V.; Yu, S.; Tharabenjasin, P.; Gao, N.; Ferraris, R.P. Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R499–R509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öckerman, P.A.; Lundborg, H. Conversion of fructose to glucose by human jejunum absence of galactose-to-glucose conversion. Biochim. Biophys. Acta (BBA)-Enzymol. Biol. Oxid. 1965, 105, 34–42. [Google Scholar] [CrossRef]
- Felmlee, M.A.; Jones, R.S.; Rodriguez-Cruz, V.; Follman, K.E.; Morris, M.E. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol. Rev. 2020, 72, 466. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.K.; Jones, A.W. Endogenous Ethanol ‘Auto-Brewery Syndrome’ as a Drunk-Driving Defence Challenge. Med. Sci. Law 2000, 40, 206–215. [Google Scholar] [CrossRef]
- Antoshechkin, A.G. On intracellular formation of ethanol and its possible role in energy metabolism. Alcohol Alcohol. 2001, 36, 608. [Google Scholar] [CrossRef]
- Sakai, R.; Ooba, Y.; Watanabe, A.; Nakamura, H.; Kawamata, Y.; Shimada, T.; Takumi, A.; van Goudoever, J.B.; Narita, T. Glutamate metabolism in a human intestinal epithelial cell layer model. Amino Acids 2020, 52, 1505–1519. [Google Scholar] [CrossRef]
- Steenson, S.; Umpleby, A.M.; Lovegrove, J.A.; Jackson, K.G.; Fielding, B.A. Role of the Enterocyte in Fructose-Induced Hypertriglyceridaemia. Nutrients 2017, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Al-Jawadi, A.; Patel, C.R.; Shiarella, R.J.; Romelus, E.; Auvinen, M.; Guardia, J.; Pearce, S.C.; Kishida, K.; Yu, S.; Gao, N.; et al. Cell-Type–Specific, Ketohexokinase-Dependent Induction by Fructose of Lipogenic Gene Expression in Mouse Small Intestine. J. Nutr. 2020, 150, 1722–1730. [Google Scholar] [CrossRef]
- Schwenk, R.W.; Holloway, G.P.; Luiken, J.J.; Bonen, A.; Glatz, J.F. Fatty acid transport across the cell membrane: Regulation by fatty acid transporters. Prostaglandins Leukot Essent Fat. Acids 2010, 82, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Rescigno, A.; Piras, A.; Atzeri, A.; Scano, P.; Porcedda, S.; Zucca, P.; Assunta Dessì, M. Chemical composition and effect on intestinal Caco-2 cell viability and lipid profile of fixed oil from Cynomorium coccineum L. Food Chem. Toxicol. 2012, 50, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Reiser, S.; Hallfrisch, J. Stimulation of Neutral Amino Acid Transport by Fructose in Epithelial Cells Isolated from Rat Intestine. J. Nutr. 1977, 107, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, L.; Li, H.; Bai, X.; Sun, F.; Xiao, X.; Fu, S. Metabolic Complementation between Glucose and Amino Acid Drives Hepatic De Novo Lipogenesis and Steatosis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Sun, S.Z.; Empie, M.W. Fructose metabolism in humans—What isotopic tracer studies tell us. Nutr. Metab. 2012, 9, 89. [Google Scholar] [CrossRef] [Green Version]
| GLU-Supplemented | FRU-Supplemented | ANOVA | ||
|---|---|---|---|---|
| FAME | T0 | T8 | ||
| 14:0 | 3.2 ± 0.3 b | 4.0 ± 0.3 a | 4.7 ± 0.3 a | p = 0.001 |
| 16:0 | 40.7 ± 3.5 b | 47.5 ± 6.3 ab | 55.0 ± 8.5 a | p = 0.0504 |
| 16:1 n-7 | 7.8 ± 0.9 b | 9.9 ± 0.7 a | 11.4 ± 1.1 a | p = 0.0038 |
| 18:0 | 39.3 ± 4.6 a | 45.0 ± 7.4 a | 52.0 ± 10.1 a | p = 0.1457 |
| 18:1 n-9 | 44.8 ± 5.0 b | 55.6 ± 3.6 a | 61.3 ± 3.5 a | p = 0.0037 |
| 18:1 n-7 | 11.6 ± 1.6 b | 14.1 ± 0.8 ab | 16.0 ± 1.4 a | p = 0.0109 |
| 18:2 n-6 | 1.6 ± 0.3 b | 1.9 ± 0.1 ab | 2.1 ± 0.1 a | p = 0.0446 |
| 18:3 n-3 | 2.8 ± 0.2 a | 2.7 ± 1.2 a | 3.8 ± 0.4 a | p = 0.1584 |
| 20:4 n-6 | 3.7 ± 0.6 b | 4.3 ± 0.2 ab | 4.8 ± 0.3 a | p = 0.0366 |
| 20:5 n-3 | 0.7 ± 0.8 a | 1.2 ± 0.2 a | 1.3 ± 0.2 a | p = 0.3442 |
| 22:6 n-3 | 2.7 ± 0.5 a | 3.7 ± 0.7 a | 3.8 ± 0.6 a | p = 0.1298 |
| Total | 158.9 ± 11.3 b | 189.7 ± 15.5 a | 216.0 ± 10.8 a | p = 0.0017 |
| GLU-Supplemented | FRU-Supplemented | |||
|---|---|---|---|---|
| FAME | AC | BLC | AC | BLC |
| 14:0 | 0.5 ± 0.6 | 0.23 ± 0.06 | 0.50 ± 0.13 | 0.32 ± 0.05 |
| 16:0 | 11.8 ± 2.8 | 5.80 ± 0.91 | 13.58 ± 6.19 | 7.40 ± 0.25 * |
| 18:0 | 11.1 ± 2.5 | 5.31 ± 0.81 | 13.95 ± 5.45 | 7.13 ± 0.19 * |
| 18:1 n-9 | 4.3 ± 3.9 | 0.0 ± 0.0 | 3.86 ± 3.08 | 2.39 ± 3.28 |
| Total | 27.76 ± 6.12 | 11.34 ± 1.76 | 31.89 ± 9.27 | 17.24 ± 3.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiarello, E.; Di Nunzio, M.; Picone, G.; Antonelli, G.; Capozzi, F.; Bordoni, A. Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu. Nutrients 2022, 14, 517. https://doi.org/10.3390/nu14030517
Chiarello E, Di Nunzio M, Picone G, Antonelli G, Capozzi F, Bordoni A. Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu. Nutrients. 2022; 14(3):517. https://doi.org/10.3390/nu14030517
Chicago/Turabian StyleChiarello, Elena, Mattia Di Nunzio, Gianfranco Picone, Giorgia Antonelli, Francesco Capozzi, and Alessandra Bordoni. 2022. "Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu" Nutrients 14, no. 3: 517. https://doi.org/10.3390/nu14030517
APA StyleChiarello, E., Di Nunzio, M., Picone, G., Antonelli, G., Capozzi, F., & Bordoni, A. (2022). Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu. Nutrients, 14(3), 517. https://doi.org/10.3390/nu14030517