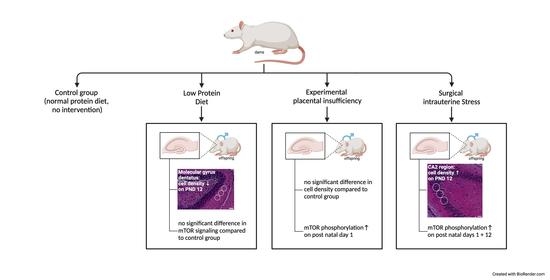
Hippocampal mTOR Dysregulation and Morphological Changes in Male Rats after Fetal Growth Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgical Procedures
2.2. Protein and RNA Isolation
2.3. Western Blot Techniques
2.4. Histology
2.5. Statistical Analysis
3. Results
3.1. Postnatal Body Weight Was Reduced in All FGR Offspring Groups, While Brain- to Body-Weight Ratios Were Elevated after Experimental Placental Insufficiency on PND 1 and after Low-Protein Diet as Well as after Intrauterine Stress on PND 12
3.2. Hippocampal Area in Relation to Brain and Body Weight Was Augmented after Low-Protein Diet and Experimental Placental Insufficiency on PND 12
3.3. Low-Protein Diet Led to Decreased Hippocampal Cell Density in the Molecular Dentate Gyrus Subregion While Intrauterine Stress Was Associated with Increased Cell Density in the Cellular CA2 Subregion
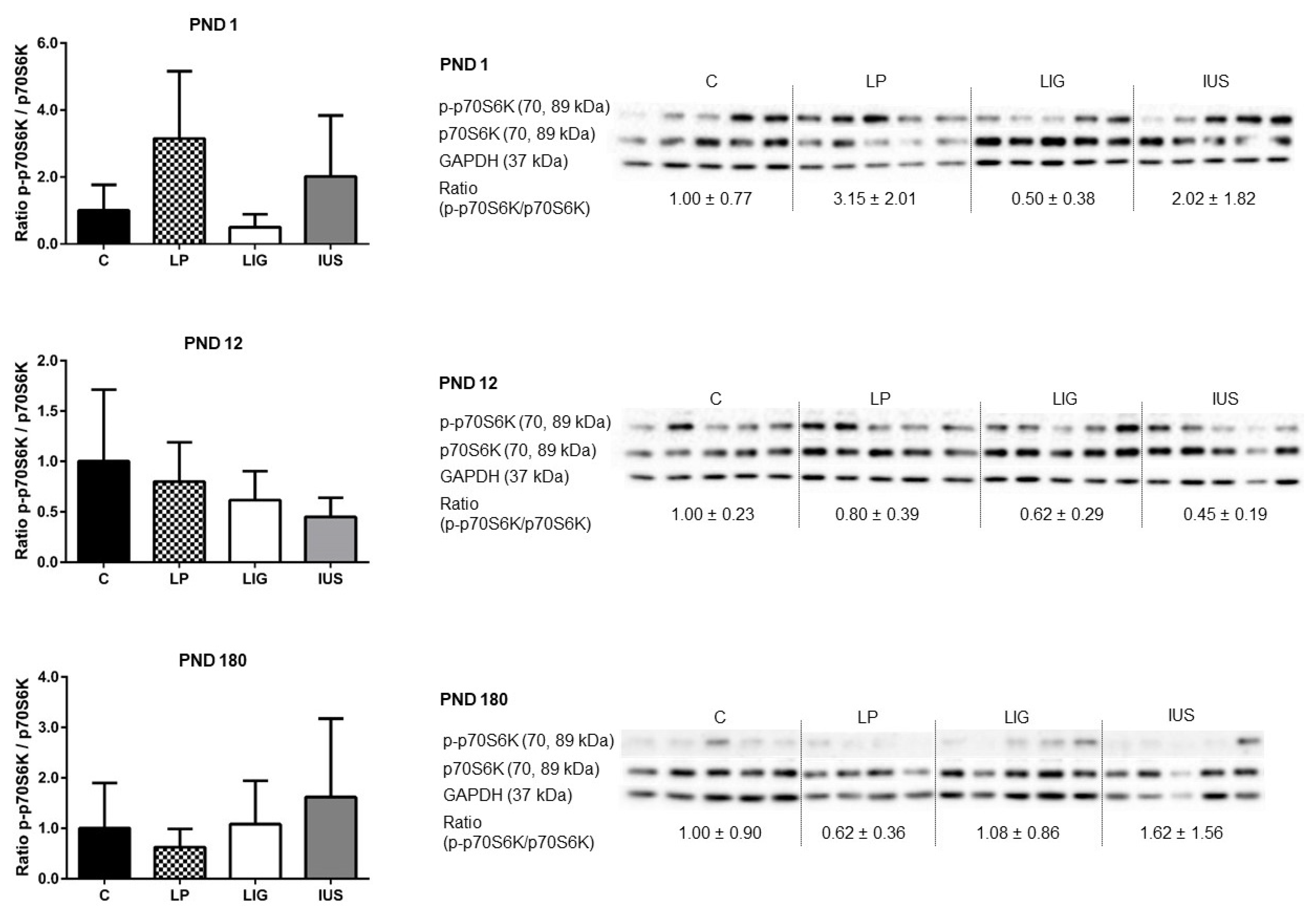
3.4. Experimental Placental Insufficiency Caused mTOR Activation on PND 1, Whereas Intrauterine Stress Caused mTOR Activation throughout Postnatal Hippocampal Development
3.5. Experimental Placental Insufficiency, Intrauterine Stress and Maternal Low-Protein Diet Did Not Have Long-Term Effects on Expression or Phosphorylation of Tau Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardosi, J. Intrauterine growth restriction: New standards for assessing adverse outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A.; Hecher, K. Fetal growth restriction due to placental disease. Semin. Perinatol. 2004, 28, 67–80. [Google Scholar] [CrossRef]
- Gagnon, R. Placental insufficiency and its consequences. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S99–S107. [Google Scholar] [CrossRef]
- Vuguin, P.M. Animal models for small for gestational age and fetal programming of adult disease. Horm. Res. 2007, 68, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef]
- Dötsch, J.; Plank, C.; Amann, K. Fetal programming of renal function. Pediatr. Nephrol. 2012, 27, 513–520. [Google Scholar] [CrossRef]
- Varvarigou, A.A. Intrauterine growth restriction as a potential risk factor for disease onset in adulthood. J. Pediatr. Endocrinol. Metab. 2010, 23, 215–224. [Google Scholar] [CrossRef]
- Geva, R.; Eshel, R.; Leitner, Y.; Fattal-Valevski, A.; Harel, S. Memory functions of children born with asymmetric intrauterine growth restriction. Brain Res. 2006, 1117, 186–194. [Google Scholar] [CrossRef]
- Von Beckerath, A.-K.; Kollmann, M.; Rotky-Fast, C.; Karpf, E.; Lang, U.; Klaritsch, P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2013, 208, 130.e1–130.e6. [Google Scholar] [CrossRef]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of intrauterine growth restriction and small for gestational age status with childhood cognitive outcomes: A systematic review and meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef]
- Sung, I.K.; Vohr, B.; Oh, W. Growth and neurodevelopmental outcome of very low birth weight infants with intrauterine growth retardation: Comparison with control subjects matched by birth weight and gestational age. J. Pediatr. 1993, 123, 618–624. [Google Scholar] [CrossRef]
- Paz, I.; Gale, R.; Laor, A.; Danon, Y.L.; Stevenson, D.K.; Seidman, D.S. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstet. Gynecol. 1995, 85, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Leitner, Y.; Fattal-Valevski, A.; Geva, R.; Bassan, H.; Posner, E.; Kutai, M.; Many, A.; Jaffa, A.J.; Harel, S. Six-year follow-up of children with intrauterine growth retardation: Long-term, prospective study. J. Child Neurol. 2000, 15, 781–786. [Google Scholar] [CrossRef]
- Strauss, R.S. Adult functional outcome of those born small for gestational age: Twenty-six-year follow-up of the 1970 British Birth Cohort. J. Am. Med. Assoc. 2000, 283, 625–632. [Google Scholar] [CrossRef]
- Zubrick, S.R.; Kurinczuk, J.J.; McDermott, B.M.C.; McKelvey, R.S.; Silburn, S.R.; Davies, L.C. Fetal growth and subsequent mental health problems in children aged 4 to 13 years. Dev. Med. Child Neurol. 2000, 42, 14–20. [Google Scholar] [CrossRef]
- Frisk, V.; Amsel, R.; Whyte, H.E.A. The importance of head growth patterns in predicting the cognitive abilities and literacy skills of small-for-gestational-age children. Dev. Neuropsychol. 2002, 22, 565–593. [Google Scholar] [CrossRef]
- Leitner, Y.; Fattal-Valevski, A.; Geva, R.; Eshel, R.; Toledano-Alhadef, H.; Rotstein, M.; Bassan, H.; Radianu, B.; Bitchonsky, O.; Jaffa, A.J.; et al. Neurodevelopmental outcome of children with intrauterine growth retardation: A longitudinal, 10-Year prospective study. J. Child Neurol. 2007, 22, 580–587. [Google Scholar] [CrossRef]
- Mosing, M.A.; Lundholm, C.; Cnattingius, S.; Gatz, M.; Pedersen, N.L. Associations between birth characteristics and age-related cognitive impairment and dementia: A registry-based cohort study. PLoS Med. 2018, 15, e1002609. [Google Scholar] [CrossRef]
- Jarvis, S.; Glinianaia, S.V.; Arnaud, C.; Fauconnier, J.; Johnson, A.; McManus, V.; Topp, M.; Uvebrant, P.; Cans, C.; Krägeloh-Mann, I. Registers S collaboration of ECP. Case gender and severity in cerebral palsy varies with intrauterine growth. Arch. Dis. Child. 2005, 90, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Caprau, D.; Schober, M.E.; Bass, K.; O’Grady, S.; Ke, X.; Block, B.; Callaway, C.W.; Hale, M.; Yu, X.; McKnight, R.A.; et al. Altered expression and chromatin structure of the hippocampal IGF1r gene is associated with impaired hippocampal function in the adult IUGR male rat. J. Dev. Orig. Health Dis. 2012, 3, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Dötsch, J.; Plank, C.; Amann, K.; Ingelfinger, J. The implications of fetal programming of glomerular number and renal function. J. Mol. Med. 2009, 87, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Nüsken, E.; Wohlfarth, M.; Lippach, G.; Rauh, M.; Schneider, H.; Dötsch, J.; Nüsken, K.-D. Reduced perinatal leptin availability may contribute to adverse metabolic programming in a rat model of uteroplacental insufficiency. Endocrinology 2016, 157, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Nüsken, K.-D.; Dötsch, J.; Rauh, M.; Rascher, W.; Schneider, H. Uteroplacental Insufficiency after Bilateral Uterine Artery Ligation in the Rat: Impact on Postnatal Glucose and Lipid Metabolism and Evidence for Metabolic Programming of the Offspring by Sham Operation. Endocrinology 2008, 149, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.S.; Walker, J.A.; Gage, F.H. Hippocampal Connections and Spatial Discrimination. Brain Res. 1978, 139, 295–308. [Google Scholar] [CrossRef]
- Schmidt-Kastner, R.; Freund, T.F. Selective vulnerability oft he hippocampus in brain ischemia. Neuroscience 1991, 40, 599–636. [Google Scholar] [CrossRef]
- Kuchna, I. Quantitative studies of human newborns’ hippocampal pyramidal cells after perinatal hypoxia. Folia Neuropathol. 1994, 32, 9–16. [Google Scholar]
- Isaacs, E.B.; Lucas, A.; Chong, W.K.; Wood, S.J.; Johnson, C.L.; Marshall, C.; Vargha-Khadem, F.; Gadian, D.G. Hippocampal volume and everyday memory in children of very low birth weight. Pediatr. Res. 2000, 47, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.C.; Rose, A.; Rao, M.; Gallego, J.; Gressens, P.; Mani, S. Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience 2008, 152, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Mallard, C.; Loeliger, M.; Copolov, D.; Rees, S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience 2000, 100, 327–333. [Google Scholar] [CrossRef]
- Fung, C.; Ke, X.; Brown, A.S.; Yu, X.; McKnight, R.A.; Lane, R.H. Uteroplacental insufficiency alters rat hippocampal cellular phenotype in conjunction with ErbB receptor expression. Pediatr. Res. 2012, 72, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Lei, Q.; James, S.J.; Kelleher, S.L.; Melnyk, S.; Jernigan, S.; Yu, X.; Wang, L.; Callaway, C.W.; Gill, G.; et al. Uteroplacental insufficiency affects epigenetic determinants of chromatin structure in brains of neonatal and juvenile IUGR rats. Physiol. Genom. 2006, 25, 16–28. [Google Scholar] [CrossRef]
- Ke, X.; Schober, M.E.; McKnight, R.A.; O’Grady, S.; Caprau, D.; Yu, X.; Callaway, C.W.; Lane, R.H. Intrauterine growth retardation affects expression and epigenetic characteristics of the rat hippocampal glucocorticoid receptor gene. Physiol. Genom. 2010, 42, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Xing, B.; Yu, B.; Yu, X.; Majnik, A.; Cohen, S.; Lane, R.; Joss-Moore, L. IUGR disrupts the PPARγ-Setd8-H4K20me1 and Wnt signaling pathways in the juvenile rat hippocampus. Int. J. Dev. Neurosci. 2014, 38, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; Pavan, I.C.B.; Amaral, C.L.; Meneguello, L.; Luchessi, A.D.; Simabuco, F.M. The S6K protein family in health and disease. Life Sci. 2015, 131, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.S.; Holt, C.E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 2001, 32, 1013–1026. [Google Scholar] [CrossRef]
- Jaworski, J. Control of Dendritic Arborization by the Phosphoinositide-3’-Kinase-Akt-Mammalian Target of Rapamycin Pathway. J. Neurosci. 2005, 25, 11300–11312. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Anderson, R.; Dwivedy, A.; Weinl, C.; Van Horck, F.; Leung, K.; Cogill, E.; Holt, C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 2006, 49, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Di Nardo, A.; Han, J.M.; Baharanyi, H.; Kramvis, I.; Huynh, T.; Dabora, S.; Codeluppi, S.; Pandolfi, P.; Pasquale, E.B.; et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat. Neurosci. 2010, 12, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Nawa, H. mTOR signaling and its roles in normal and abnormal brain development. Front. Mol. Neurosci. 2014, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Mizuno, M.; Yonezawa, K.; Nawa, H.; Takei, N. Activation of mammalian target of rapamycin signaling in spatial learning. Neurosci. Res. 2010, 68, 88–93. [Google Scholar] [CrossRef] [PubMed]
- An, W.L.; Cowburn, R.F.; Li, L.; Braak, H.; Alafuzoff, I.; Iqbal, K.; Iqbal, I.G.; Winblad, B.; Pei, J.J. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. Am. J. Pathol. 2003, 163, 591–607. [Google Scholar] [CrossRef]
- Li, X.; Alafuzoff, I.; Soininen, H.; Winblad, B.; Pei, J. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer’s disease brain. FEBS J. 2005, 272, 4211–4220. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Bereczki, E.; Zhang, H.; Wang, S.; Li, C.; Ji, X.; Branca, R.M.; Lehtiö, J.; Guan, Z.; Filipcik, P.; et al. Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis: Implication for Alzheimer disease. J. Biol. Chem. 2013, 288, 15556–15570. [Google Scholar] [CrossRef] [PubMed]
- Lahti, J.; Räikkönen, K.; Kajantie, E.; Heinonen, K.; Pesonen, A.K.; Järvenpää, A.L.; Strandberg, T. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J. Child Psychol. Psychiatry Allied Discip. 2006, 47, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, S.P.; Caprau, D.; Ke, X.; Contreras, Y.; Haley, S.; Ermini, F.; Penn, A.; Moyer-Mileur, L.; McKnight, R.; Lane, R. Intrauterine growth restriction alters hippocampal expression and chromatin structure of Cyp19a1 variants. Syst. Biol. Reprod. Med. 2010, 56, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Numpang, B.; Ke, X.; Yu, X.; Callaway, C.; McKnight, R.; Joss-Moore, L.; Lane, R. Fetal growth restriction alters hippocampal 17-beta estradiol and estrogen receptor alpha levels in the newborn male rat. Syst. Biol. Reprod. Med. 2013, 59, 184–190. [Google Scholar] [CrossRef][Green Version]
- Nüsken, E.; Fink, G.; Lechner, F.; Voggel, J.; Wohlfarth, M.; Sprenger, L.; Mehdiani, N.; Weber, L.T.; Liebau, M.C.; Brachvogel, B.; et al. Altered molecular signatures during kidney development after intrauterine growth restriction of different origins. J. Mol. Med. 2020, 98, 395–407. [Google Scholar] [CrossRef]
- Roza, S.J.; Steegers, E.A.P.; Verburg, B.O.; Jaddoe, V.W.V.; Moll, H.A.; Hofman, A.; Verhulst, F.C.; Tiemeier, H. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am. J. Epidemiol. 2008, 168, 1145–1152. [Google Scholar] [CrossRef]
- Van den Broek, A.J.M.; Kok, J.H.; Houtzager, B.A.; Scherjon, S.A. Behavioural problems at the age of eleven years in preterm-born children with or without fetal brain sparing: A prospective cohort study. Early Hum. Dev. 2010, 86, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Cruz-Martinez, R.; Sanz-Cortes, M.; Arranz, A.; Illa, M.; Botet, F.; Costas-Moragas, C.; Gratacos, E. Neurobehavioral outcomes in preterm, growth-restricted infants with and without prenatal advanced signs of brain-sparing. Ultrasound Obstet. Gynecol. 2011, 38, 288–294. [Google Scholar] [CrossRef]
- Lee, I.; Kesner, R.P. Different contributions of dorsal hippocampal subregios to emory acquisation and retrieval in contextual fear-conditioning. Hippocampus 2004, 14, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Berardino, B.G.; Ballarini, F.; Chertoff, M.; Igaz, L.M.; Cánepa, E.T. Nutritional stress timing differentially programs cognitive abilities in young adult male mice. Nutr. Neurosci. 2020, 1–13. [Google Scholar] [CrossRef]
- Ferroni, N.M.; Berardino, B.G.; Belluscio, L.M.; Fernández, M.S.; Fesser, E.A.; Sonzogni, S.V.; Cánepa, E.T. Perinatal protein malnutrition induces the emergence of enduring effects and age-related impairment behaviors, increasing the death risk in a mouse model. Nutr. Neurosci. 2020, 1–14. [Google Scholar] [CrossRef]
- Wang, L.; Xu, R.J. The effects of perinatal protein malnutrition on spatial learning and memory behaviour and brain-derived neurotrophic factor concentration in the brain tissue in young rats. Asia Pac. J. Clin. Nutr. 2007, 16, 467–472. [Google Scholar] [PubMed]
- Murmu, M.S.; Salomon, S.; Biala, Y.; Weinstock, M.; Braun, K.; Bock, J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur. J. Neurosci. 2006, 24, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Behan, Á.T.; van den Hove, D.L.A.; Mueller, L.; Jetten, M.J.A.; Steinbusch, H.W.M.; Cotter, D.R.; Prickaerts, J. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur. Neuropsychopharmacol. 2011, 21, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.; Wainstock, T.; Braun, K.; Segal, M. Stress In Utero: Prenatal Programming of Brain Plasticity and Cognition. Biol. Psychiatry 2015, 78, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.R.; Bale, T.L. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 2007, 91, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Jiang, D.; Ryu, Y.K.; Lim, S.; Kwak, M.; Gray, C.D.; Xu, M.; Choi, J.H.; Junn, S.; Kim, J.; et al. Erratum: Correction: Early postnatal exposure to isoflurane causes cognitive deficits and disrupts development of newborn hippocampal neurons via activation of the mTOR pathway. PLoS Biol. 2018, 16, e1002625. [Google Scholar] [CrossRef]
- Xu, J.; Mathena, R.P.; Xu, M.; Wang, Y.; Chang, C.; Fang, Y.; Zhang, P.; Mintz, C.D. Early developmental exposure to general anesthetic agents in primary neuron culture disrupts synapse formation via actions on the mTOR pathway. Int. J. Mol. Sci. 2018, 19, 2183. [Google Scholar] [CrossRef] [PubMed]
- Herbert, T.P.; Tee, A.R.; Proud, C.G. The Extracellular Signal-regulated Kinase Pathway Regulates the Phosphorylation of 4E-BP1 at Multiple Sites. J. Biol. Chem. 2002, 277, 11591–11596. [Google Scholar] [CrossRef]
- Lehman, J.A.; Calvo, V.; Gomez-Cambronero, J. Mechanism of ribosomal p70s6 kinase activation by granulocyte macrophage colony-stimulating factor in neutrophils. Cooperation of a MEK-related, Thr421/Ser424 kinase and a rapamycin-sensitive, mTOR-related Thr389 kinase. J. Biol. Chem. 2003, 278, 28130–28138. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Jiang, B.; Zhang, Y. 4E-BP1, a multifactor regulated multifunctional protein. Cell Cycle 2016, 15, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; De Pinto, V.; Messina, A.; Branca, C.; Oddo, S. Genetic Reduction of Mammalian Target of Rapamycin Ameliorates Alzheimer’s Disease-Like Cognitive and Pathological Deficits by Restoring Hippocampal Gene Expression Signature. J. Neurosci. 2014, 34, 7988–7998. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, S.; Min, F.; Ma, J.; Shi, X.; Bereczki, E.; Wu, J. mTOR-mediated hyperphosphorylation of tau in the hippocampus is involved in cognitive deficits in streptozotocin-induced diabetic mice. Metab. Brain Dis. 2014, 29, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. Sex-dependent changes induced by perinatal stress in cortical and hippocampal morphology and behaviour in rats: An update. Stress 2011, 14, 604–613. [Google Scholar] [CrossRef]
- Lodygensky, G.A.; Seghier, M.L.; Warfield, S.K.; Tolsa, C.B.; Sizonenko, S.; Lazeyras, F.; Hüppi, P.S. Intrauterine growth restriction affects the preterm infant’s hippocampus. Pediatr. Res. 2008, 63, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Aanes, S.; Bjuland, K.J.; Skranes, J.; Lohaugen, G.C.C. Memory function and hippocampal volumes in preterm born very-low-birth-weight (VLBW) young adults. Neuroimage 2015, 105, 76–83. [Google Scholar] [CrossRef]
- Fleiss, B.; Wong, F.; Brownfoot, F.; Shearer, I.K.; Baud, O.; Walker, D.W.; Gressens, P.; Tolcos, M. Knowledge Gaps and Emerging Research Areas in Intrauterine Growth Restriction-Associated Brain Injury. Front. Endocrinol. 2019, 10, 188. [Google Scholar] [CrossRef] [PubMed]






| Antibody | Manufacturer | Molecular Weight | Dilution * |
|---|---|---|---|
| 4E-BP1 Rabbit mAb | Cell Signaling Technology (#9644) | 15–20 kDa | 1:1000 |
| Phospho-4E-BP1 (Thr37/46) Rabbit mAb | Cell Signaling Technology (#2855) | 15–20 kDa | 1:2000 |
| GAPDH Rabbit mAb | Cell Signaling Technology (#2118) | 20 kDa | 1:3000 |
| mTOR Rabbit mAb | Cell Signaling Technology (#2983) | 289 kDa | 1:1000 |
| Phospho-mTOR (Ser2448) Rabbit mAb | Cell Signaling Technology (#5536) | 289 kDa | 1:500 |
| p70S6K Rabbit mAb | Cell Signaling Technology (#2708) | 70/89 kDa | 1:500 |
| Phospho-p70S6K (Thr389) Rabbit mAb | Cell Signaling Technology (#9234) | 70/89 kDa | 1:500 |
| Tau Rabbit mAb | Abcam (ab32057) | 50–79 kDa | 1:2000 |
| Phospho-Tau (Ser396) Rabbit mAb | Abcam (ab109390) | 50–79 kDa | 1:10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schömig, C.; Oberholz, L.; Fink, G.; Voggel, J.; Wohlfarth, M.; Dötsch, J.; Nüsken, K.-D.; Nüsken, E. Hippocampal mTOR Dysregulation and Morphological Changes in Male Rats after Fetal Growth Restriction. Nutrients 2022, 14, 451. https://doi.org/10.3390/nu14030451
Schömig C, Oberholz L, Fink G, Voggel J, Wohlfarth M, Dötsch J, Nüsken K-D, Nüsken E. Hippocampal mTOR Dysregulation and Morphological Changes in Male Rats after Fetal Growth Restriction. Nutrients. 2022; 14(3):451. https://doi.org/10.3390/nu14030451
Chicago/Turabian StyleSchömig, Charlotte, Laura Oberholz, Gregor Fink, Jenny Voggel, Maria Wohlfarth, Jörg Dötsch, Kai-Dietrich Nüsken, and Eva Nüsken. 2022. "Hippocampal mTOR Dysregulation and Morphological Changes in Male Rats after Fetal Growth Restriction" Nutrients 14, no. 3: 451. https://doi.org/10.3390/nu14030451
APA StyleSchömig, C., Oberholz, L., Fink, G., Voggel, J., Wohlfarth, M., Dötsch, J., Nüsken, K.-D., & Nüsken, E. (2022). Hippocampal mTOR Dysregulation and Morphological Changes in Male Rats after Fetal Growth Restriction. Nutrients, 14(3), 451. https://doi.org/10.3390/nu14030451