Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Anthropometric Measurements
2.3. Nutritional Risk
2.4. Frailty and Functional Status
2.5. Health-Related Quality of Life (HQoL)
2.6. Statistical Analysis
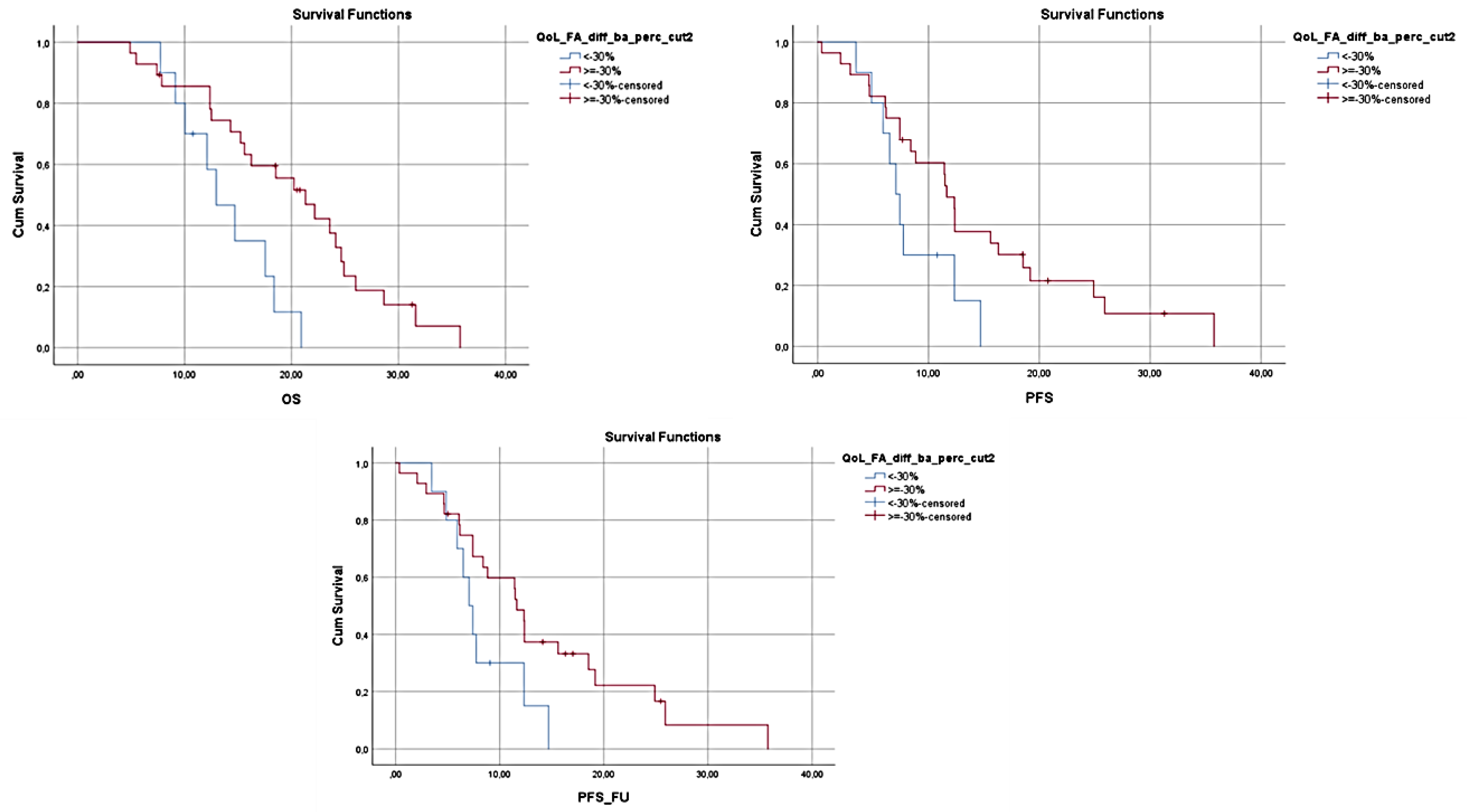
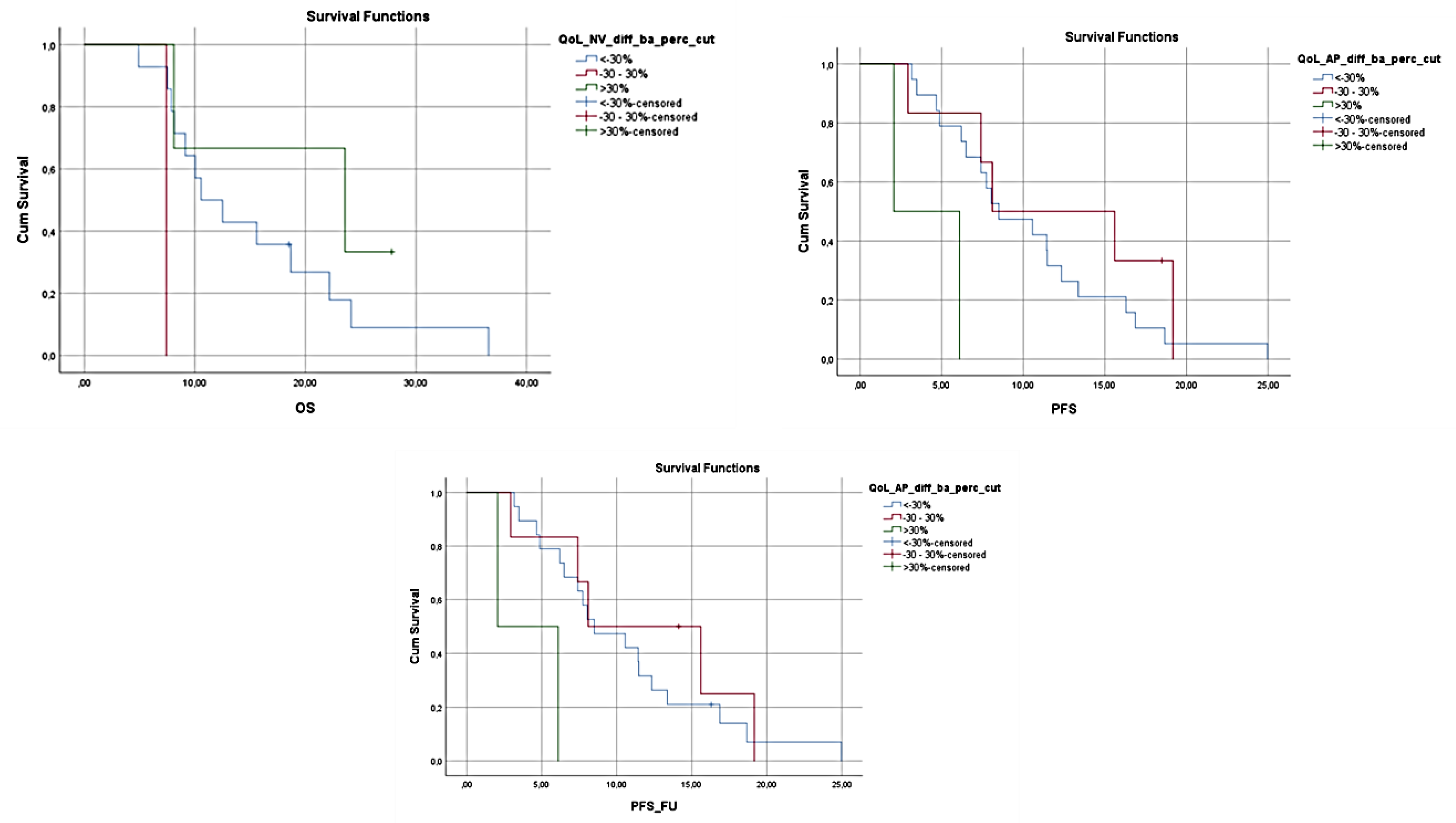
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. Stockh. Swed. 2016, 55, 1158–1160. [Google Scholar] [CrossRef]
- Gotay, C.C.; Korn, E.L.; McCabe, M.S.; Moore, T.D.; Cheson, B.D. Quality-of-life assessment in cancer treatment protocols: Research issues in protocol development. J. Natl. Cancer Inst. 1992, 84, 575–579. [Google Scholar] [CrossRef]
- Bottomley, A. The cancer patient and quality of life. Oncologist 2002, 7, 120–125. [Google Scholar] [CrossRef]
- Guan, M.; Shinde, A.M.; Hendifar, A.E. Pancreatic Cancer Cachexia: Current Concepts and Clinical Management. Frailty Sarcopenia Onset Dev. Clin. Chall. 2017. [Google Scholar] [CrossRef][Green Version]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Bishop, C.W.; Bowen, P.; Ritchey, S.J. Norms for nutritional assessment of American adults by upper arm anthropometry. Am. J. Clin. Nutr. 1981, 34, 2530–2539. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. Ser. A 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.W.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Katz, S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriatr. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Cocks, K.; King, M.T.; Velikova, G.; St-James, M.M.; Fayers, P.; Brown, J.M. Evidence-Based Guidelines for Determination of Sample Size and Interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J. Clin. Oncol. 2011, 29, 89–96. [Google Scholar] [CrossRef]
- Poulia, K.A.; Sarantis, P.; Antoniadou, D.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic Cancer and Cachexia—Metabolic Mechanisms and Novel Insights. Nutrients 2020, 12, 1543. [Google Scholar] [CrossRef]
- de van der Schueren, M.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, C. Nutrition advice crucial, say cancer patients. Nurs. Stand. 2016, 31, 10. [Google Scholar] [CrossRef]
- Vasiloglou, M.F.; Fletcher, J.; Poulia, K.-A. Challenges and Perspectives in Nutritional Counselling and Nursing: A Narrative Review. J. Clin. Med. 2019, 8, 1489. [Google Scholar] [CrossRef] [PubMed]
- Derksen, J.W.; Kurk, S.A.; Peeters, P.H.; Dorresteijn, B.; Jourdan, M.; Van Der Velden, A.M.; Nieboer, P.; De Jong, R.S.; Honkoop, A.H.; Punt, C.J.; et al. The association between changes in muscle mass and quality of life in patients with metastatic colorectal cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Kurk, S.; Peeters, P.; Stellato, R.; Dorresteijn, B.; De Jong, P.; Jourdan, M.; Creemers, G.; Erdkamp, F.; De Jongh, F.; Kint, P.; et al. Skeletal muscle mass loss and dose-limiting toxicities in metastatic colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Arastu, A.; Patel, A.; Mohile, S.G.; Ciminelli, J.; Kaushik, R.; Wells, M.; Culakova, E.; Lei, L.; Xu, H.; Dougherty, D.W.; et al. Assessment of Financial Toxicity Among Older Adults with Advanced Cancer. JAMA Netw. Open 2020, 3, e2025810. [Google Scholar] [CrossRef] [PubMed]
- Llm, P.M.C.; Kantarjian, H.M.; Blinder, V.S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA A Cancer J. Clin. 2018, 68, 153–165. [Google Scholar] [CrossRef]
- Lentz, R.; Benson, A.B., 3rd; Kircher, S. Financial toxicity in cancer care: Prevalence, causes, consequences, and reduction strategies. J. Surg. Oncol. 2019, 120, 85–92. [Google Scholar] [CrossRef]
- Keramidou, I.; Triantafyllopoulos, L. The impact of the financial crisis and austerity policies on the service quality of public hospitals in Greece. Health Policy 2018, 122, 352–358. [Google Scholar] [CrossRef]
- Kapetanakis, E.I.; Sidiropoulou, T.; Koumarianou, A.; Kostopanagiotou, K.; Tomos, P. Delivering quality lung cancer care in crisis-wracked Greece. J. Surg. Oncol. 2017, 117, 537–538. [Google Scholar] [CrossRef]

| Number of Patients | Chemotherapy Regimen |
|---|---|
| 67 | Nab-Paclitaxel plus Gemcitabine |
| 10 | Carboplatin plus Gemcitabine |
| 16 | Gemcitabine |
| 3 | FOLFIRINOX |
| 1 | FOLFOX |
| Characteristics | n = 97 |
| Sex | |
| Men | 49 |
| Women | 48 |
| Age (years) | 68.1 ± 9.4 |
| Height (m) | 1.67 ± 0.09 |
| Body weight (kg) | 68.2 ± 13.3 |
| BMI (kg/m2) | 24.2 ± 3.5 |
| ICD-10, n (%) | |
| C25 | 76 (77.8%) |
| C22-24-25 | 21 (21.6%) |
| Clinical Frailty scale | |
| 1 (very fit) | 10.50% |
| 2 (well) | 23.30% |
| 3 (Managing well) | 47.70% |
| 4 (Vulnerable) | 16.30% |
| 5 (Mildly Frail) | 2.30% |
| 6–9 (Moderately frail—terminally ill) | 0% |
| Katz Score | |
| 0 (dependent) | 8% |
| 1 | 2.3% |
| 2 | 1.1% |
| 6 (independent) | 88.5% |
| Surgery prior to chemotherapy | |
| Yes | 17% |
| No | 83% |
| Reported involutional weight loss (kg) | |
| 3 months | 6.5 ± 7.3 |
| 6 months | 10.2 ± 7.4 |
| % of weight loss | |
| 3 months | 8.0 ± 9.5 |
| 6 months | 12.45 ± 8.99 |
| MNA-SF score n (%) | |
| Good nutritional status | 8 (8.9%) |
| Risk of malnutrition | 35 (36.8%) |
| Malnourished | 43 (44.3%) |
| Missing data | 11 (11.3%) |
| Nutrition advice prior to chemotherapy | |
| Yes | 32 (33%) |
| No | 63 (64.9%) |
| Missing | 2 |
| Dynamopenia n (%) | |
| Men | 27 (75%) |
| Women | 26 (76.5%) |
| Missing values | 27 |
| HQoL Issue | HQoL-Baseline (%) (Mean ± SD) n = 91 | HQoL-3 Months (Mean ± SD) n = 71 | HQoL-6 Months (Mean ± SD) n = 38 | HQoL-9 Months (Mean ± SD) n = 17 | p |
|---|---|---|---|---|---|
| Global HQoL | 0.59 ± 0.25 | 0.62 ± 0.25 | 0.59 ± 0.25 | 0.73 ± 0.20 | NS |
| Functional Issues | |||||
| Physical functioning | 0.71 ± 0.24 | 0.69 ± 0.25 | 0.71 ± 0.24 | 58.67 ± 0.32 | NS |
| Role functioning | 0.62 ± 0.35 | 0.64 ± 0.36 | 0.58 ± 0.37 | 0.66 ± 0.37 | NS |
| Emotional functioning | 0.71 ± 0.27 | 0.71 ± 0.28 | 0.60 ± 0.33 | 0.64 ± 0.21 | NS |
| Cognitive functioning | 0.84 ± 0.24 | 0.82 ± 0.23 | 0.77 ± 0.25 | 0.77 ± 0.31 | NS |
| Social Functioning | 0.82 ± 0.28 | 0.75 ± 0.29 | 0.65 ± 0.35 | 0.56 ± 0.41 | NS |
| Symptom scales | |||||
| Fatigue | 0.42 ± 0.31 | 0.40 ± 0.30 | 0.44 ± 0.29 | 0.42 ± 0.34 | NS |
| Nausea and vomiting | 0.12 ± 0.24 | 0.10 ± 0.23 | 0.10 ± 0.21 | 0.07 ± 0.13 | NS |
| Pain | 0.28 ± 0.28 | 0.27 ± 0.28 | 0.37 ± 0.28 | 0.30 ± 0.33 | NS |
| Dyspnea | 0.22 ± 0.3 | 0.25 ± 0.28 | 0.29 ± 0.31 | 0.23 ± 0.20 | NS |
| Insomnia | 0.37 ± 0.36 | 0.31 ± 0.34 | 0.41 ± 0.36 | 0.33 ± 0.33 | NS |
| Appetite | 0.29 ± 036 | 0.19 ± 0.30 | 0.28 ± 0.38 | 0.31 ± 0.42 | NS |
| Constipation | 0.24 ± 0.31 | 0.20 ± 0.29 | 0.31 ± 0.36 | 0.12 ± 0.26 | NS |
| Diarrhea | 0.14 ± 0.25 | 0.18 ± 0.29 | 0.17 ± 0.23 | 0.22 ± 0.33 | NS |
| Financial | 0.23 ± 0.30 | 0.32 ± 0.31 * | 0.42 ± 0.34 * | 0.45 ± 0.35 * | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulia, K.-A.; Antoniadou, D.; Sarantis, P.; Karamouzis, M.V. Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study. Nutrients 2022, 14, 442. https://doi.org/10.3390/nu14030442
Poulia K-A, Antoniadou D, Sarantis P, Karamouzis MV. Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study. Nutrients. 2022; 14(3):442. https://doi.org/10.3390/nu14030442
Chicago/Turabian StylePoulia, Kalliopi-Anna, Dimitra Antoniadou, Panagiotis Sarantis, and Michalis V. Karamouzis. 2022. "Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study" Nutrients 14, no. 3: 442. https://doi.org/10.3390/nu14030442
APA StylePoulia, K.-A., Antoniadou, D., Sarantis, P., & Karamouzis, M. V. (2022). Pancreatic Cancer Prognosis, Malnutrition Risk, and Quality of Life: A Cross-Sectional Study. Nutrients, 14(3), 442. https://doi.org/10.3390/nu14030442









