Strategies to Mitigate Chemotherapy and Radiation Toxicities That Affect Eating
Abstract
1. Introduction
| Chemotherapy Regimen | Effect on Normal Tissue | Tumor Versus Normal Tissue Consideration(s) |
|---|---|---|
| Dose | Side effects against a normal tissue (for example, production of platelets by bone marrow) are dose-limiting | An optimal biologic dose (OBD) instead of the maximally tolerated dose (MTD) may facilitate normal tissue healing. An area under the curve (AUC) strategy with oral dosing or continuous infusion can decrease toxicity of some drugs (e.g., cyclophosphamide or ifosfamide [22,23]) |
| Mechanisms of action against dividing cells | Marrow, mouth, esophagus, intestines, and skin are easily damaged by chemotherapy | Chemotherapy guidelines should allow adequate tissue recovery before administration of the next cycle allowing for improvement in blood counts, mucositis, diarrhea, and skin) |
| Biodistribution | Oral mucosa and skin blood flow are temperature dependent | Oral cryotherapy can reduce mucositis [24,25,26,27] Cold packs may decrease hand–foot erythroderma |
| Drug metabolism | Elimination and inactivation of chemotherapy drugs vary between tissues and persons | Dose adjustment if excessive toxicity is seen Facilitate detoxification by normal cells (e.g., improve glutathione [28,29,30]) |
| Protective drugs | Can reduce damage to normal tissues to mitigate or avoid significant short-term or long-term side effects | Mesna to protect the bladder from acrolein metabolite after cyclophosphamide or ifosfamide Dexrazoxane to protect heart from doxorubicin Dexamethasone to prevent taxane reactions Leucovorin to rescue from methotrexate |
| Drug combinations | Combinations of chemotherapy drugs can be more toxic to organs and tissue than a single agent | Chemotherapy combinations with non-overlapping toxicities and alternating regimens to achieve less toxicity are often used |
| Damage Association | Variable | Tumor Versus Normal Tissue Consideration(s) |
|---|---|---|
| Fraction dose size | Amount of radiation energy per dose | Larger fractions are biologically more effective against tumors than normal cells |
| Schedule | One-time, daily for 1 week, or daily (e.g., M–F) for 3 to 5 weeks | Time between radiation doses allows both tumor and normal tissue repair |
| Tumor radiosensitivity | Some cancers (e.g., Wilms tumor, lymphomas) are very radiosensitive. Other cancers (e.g., carcinomas, brain tumors, sarcomas, metastases) can be more difficult to kill with radiation. | Smaller total dose is needed to treat some tumors with curative intent. If a tumor is relatively radioresistant, a combination of chemotherapy and radiation may work better against tumor cells [32,33,34] |
| Radiation particle | Photons and electrons have less energy than protons and alpha particles. More energy results in hard to repair double strand DNA breaks in cancer cells | Choice of the type of radiation often depends on normal tissue nearby as well as the dose needed to treat |
| Precision of radiation treatment plan | Stereotactic body radiotherapy (SBRT) sterotactic radiosurgery (SRS) and proton radiotherapy plans are very precise. These require not only expensive radiation machines, but a highly specialized radiation physicist and oncologist time and effort for each individualized treatment plan | Palliative radiation plans are less precise and use lower doses for rapid treatment planning to reduce pain with acceptable (low) damage to nearby tissue. Image guidance provides more precise radiation treatment plans (more to tumor and less to normal tissue) Very precise SBRT, SRS, or proton plans may take 1–2 weeks before the patient can start radiotherapy in a manner that treats tumor and minimizes radiation to nearby normal tissue |
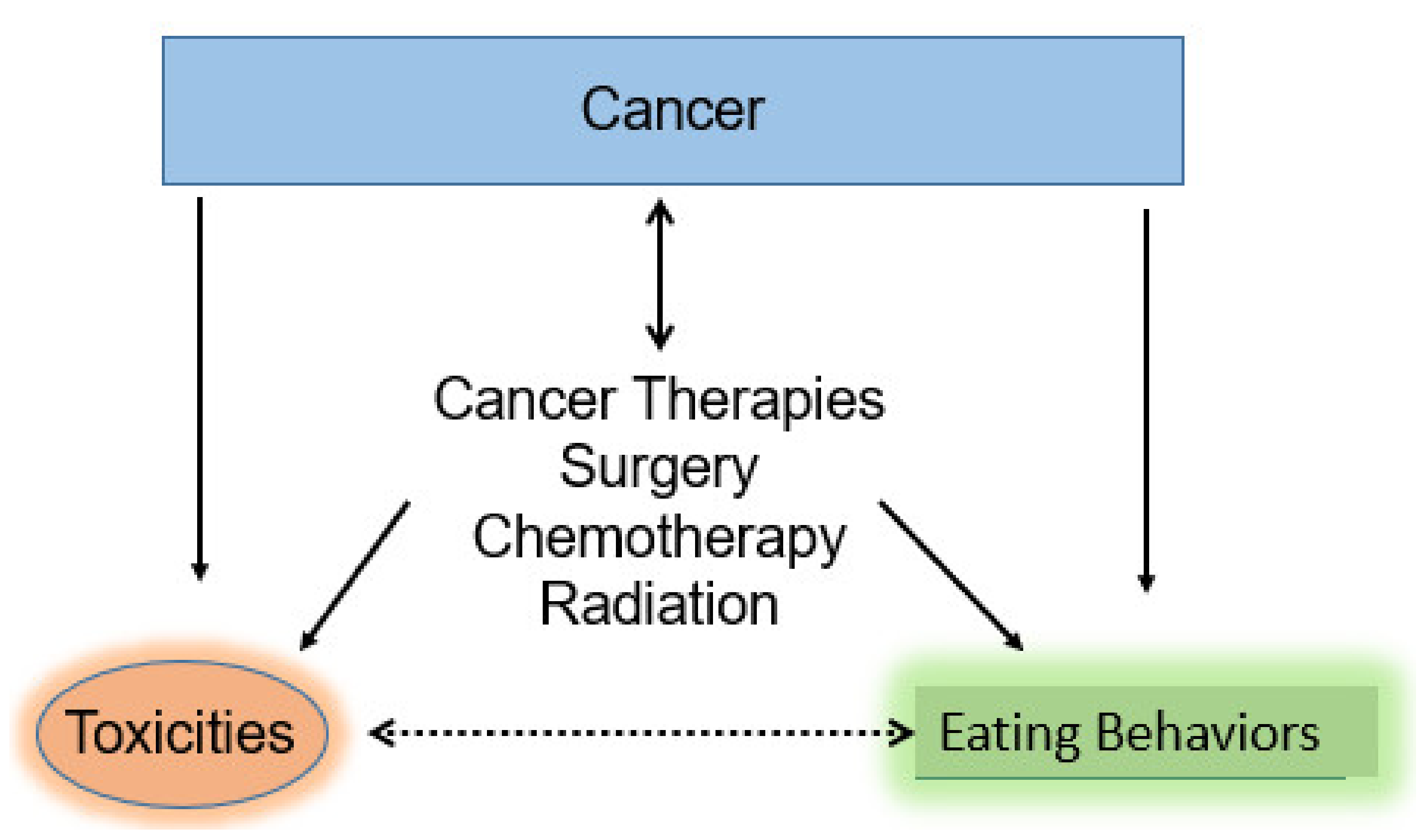
2. Review of Strategies to Improve Eating Behaviors While Receiving Chemotherapy and/or Radiation
2.1. Nausea as a Source of Poor Appetite: Approaches to Reduce Nausea
| Cancer Therapy N/V Cause and Associations | Anti-Emetic Agents and Mechanisms of Action: Generic Name (Brand Name) | Strategy, Some Practical Considerations, and References |
|---|---|---|
| Immediate N/V from chemotherapy agents: chemoreceptor trigger zone | Selective serotonin receptor (5HT) antagonists:
| MASCC + ASCO anti-emetic guidelines for chemotherapy and radiation [19,21]; ondansetron has more drug interactions. Granisetron is an oral or transdermal patch. Palonosetron is IV, has fewer drug interactions and longest half-life [84,86,87]. |
| Dysmotility | Dopamine agonists:
| Use with caution in children. Dopamine agonists can cause extrapyramidal symptoms including dystonic reactions. |
| Inflammation | Corticosteroids act on both immune cells and tumor microenvironment:
| Excellent for 1–7 days; high-doses can increase appetite and eating, but cannot use long term because of chronic issues, including: infection, appearance, skin thinning, blood pressure, diabetes, osteoporosis and avascular necrosis of shoulder, hip, and knee joints |
| Delayed N/V: Many hours to days after starting chemotherapy | Neurokinin receptor antagonists:
| Few drug interactions; especially effective with cisplatin. Can give fosaprepitaint intravenously on days 1 and 4 of each 5-day cycle |
| Anticipatory N/V | Help change context of N/V
| Change environment or routine Take meds before coming to clinic Oral or IV possible; some sedation is associated with these agents which may be an undesirable side effect especially if it impedes nutritional intake |
| Sleep deprevation | Promote routine sleep
| At bedtime melatonin 2–10 mg per day olanzapine may also help mood sedating |
| Decreased appetite | Central acting
| Oral before meals THC also has antiemetic activity Both pills and liquid available |
| Motion sickness | Scopolamine (Transderm Scop) | Patch for 3 days |
| Multiple causes | Anti-emetic combinations often more effective than single agents | MASCC, ESMO, ASCO, and NCCN guidelines [14,15,16,17,18,19,20,21] |
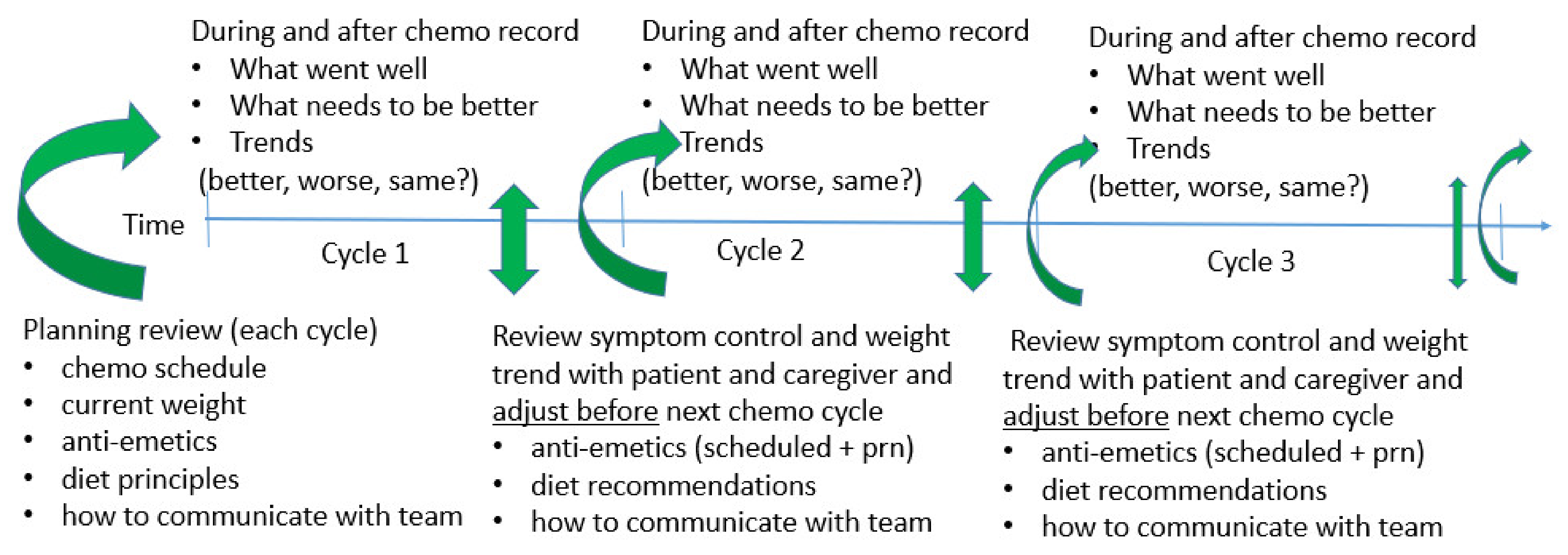
2.2. Proactive Approaches to Toxicity Reduction for Better Eating Behaviors (Forward Observation)
2.3. Mucosal and Skin Injury
| Agent | Type of Injury | Reduction Strategies |
|---|---|---|
| Melphalan | High dose alkylator (peak effect) | Cryotherapy (ice chips) [24,25,26,27] Keratinocyte growth factor (palifermin) [97,98,99,100,101] |
| Gemcitabine | Cytotoxic injury of mucosal cells | A 30 min infusion is less toxic than a 90 min infusion. Avoid daily dosing. Often used weekly × 2 weeks (day 1, day 8) and then 1 week off |
| 5-Flurouracil | Cytotoxic injury to mouth | Cryotherapy; change schedule |
| Doxorubicin | Cytotoxic injury, radiation recall | Use dexrazoxane, then a short infusion of doxorubicin, instead of continuous infusion to reduce heart injury; to reduce mucositis use glutamine + trehalose (Healios, [89]) |
| Mammalian target of rapamycin (mTOR) inhibitors | Cytotoxic injury by sirolimus, temsirolimus, everolimus | Follow blood levels and adjust dose; use glutamine + trehalose [89] |
| Vascular endothelial growth factor (VEGF) inhibitors: bevacizumab, pazopanib, cabozantinib, regorafenib | Less of an ability to form new blood vessels: wounds and radiation injured tissues heal slowly | Dose reduction or drug holiday utilizing intermittent dosing of oral agents (e.g., three weeks on then one week off) |
| Irinotecan | Active metabolite of irinotecan in the intestinal lumen, SN-38, causes intestinal mucosa injury | Reduction of immediate cramping, diarrhea, and GI upset: loperamide +/− atropine. For intermediate and delayed diarrhea octreotide and/or glutamine + trehalose (Healios [89]). If eating solid food is difficult, liquid nutritional supplements can be helpful |
| Yeast | Broad spectrum antibiotics reduce normal bacterial flora | Anti-fungal antibiotics, yogurt, kefir; limit anti-bacterial antibiotics unless suspected or known infection(s) |
| Radiation | Harms rapidly dividing cells in the renewing tissues (crypts) lining the mouth, esophagus, intestines, rectum | Protein to heal and/or glutamine + trehalose; review radiation plan (dose/schedule) to allow some healing (e.g., weekends off); boost radiation to tumor volume only. Keep skin and mucosal surfaces clean. If whole abdominal radiation therapy (WART): g-tube for additional enteral nutrition |
| Cause of Damage | Type of Injury and Consequence(s) | Reduction Strategies |
|---|---|---|
| Radiation | Single and double-stranded DNA breaks: cell death | Anticipatory guidance that damage may last longer than radiation treatment. Glutamine seems protective for some tissues, especially intestines [30,89,102,103,104,105] |
| Radiotherapy (RT) or surgery and VEGF inhibitors | Tissue may heal slowly after VEGF inhibitors due to decrease in vasculature | Use the oral tyrosine kinase inhibitor (TKI) with shorter half-lives so if there are symptoms, these can be stopped, and then restarted sooner (when the wound or injury is improved) |
| Corticosteroids: prednisone methylprednisolone dexamethasone | Thinning of skin Delayed wound healing Increased risk of infections | Use short 1–5 day pulses. If prolonged use, then taper to hydrocortisone in physiologic doses (e.g., 15 to 20 mg am and 10 mg pm) |
| Opiates | Slower GI motility causes nausea, constipation, hard stool with rectal fissures and perirectal inflammation may recur with each cycle of chemotherapy when patient may experience tissue damage and subsequent infection due to low neutrophil counts | Increase liquid in diet and physical activity as tolerated for patient. When appropriate consults to PT/OT. Kefir, stool softeners such as docusate, lactulose, polyethylene glycol powder 3350 (MiraLax), and senna can be helpful. Use of a hand-held shower and baby wipes or cotton-balls with lotion may clean rectal tissues with less damage |
| Total parenteral nutrition (TPN) | Prolonged use is associated with villous atrophy of intestine lining and liver toxicity | Some trophic enteral nutrition is needed to increase absorptive surface of intestines and to avoid liver toxicity |
| Abdominal surgery then RT to abdomen especially whole abdominal radiotherapy (WART) | Less GI motility post-op delays eating increases radiation enteritis. Effects of radiation can cause future small bowel obstruction | Gastrostomy tube (g-tube) to facilitate hospital discharge and eating sooner with better enteral nutrition before, during, and after whole abdominal radiotherapy (WART). PT/OT and dietitian consults |
| Head and neck RT C-spine or T-spine RT (with or without concurrent chemotherapy) | Often associated with severe mucositis including mouth sores (stomatitis) and oropharyngeal and esophageal mucositis | MASCC guidelines [15,16,17,20]. Pain medicine before eating and OT consult; glutamine + trehaose suspension (Healios) swish 10 s then spit or swallow [89] |
| High-dose chemotherapy +/− RT (preparative regimens for bone marrow transplant (BMT) | Very high incidence of mucositis. Extra inflammation may predispose to graft versus host disease (skin, mouth, and GI toxicities). Toxicities can cause eating and activity issues | Palifermin [97] and/or a very high level of supportive care (TPN, G-tube or NG tube) [27,85,90,106,107,108]. PT/OT and education to maintain “trophic” enteral can be helpful. MASCC guidelines [15,16,17,20] |
2.4. Deconditioning and Fatigue
2.5. Improved Information
3. Discussion
4. Summary and Conclusions
5. Patents
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Spelling | Context and Comment |
| SBRT | Sterotactic body radiotherapy | Very precise radiation given in 1–5 treatment sessions |
| MASCC | Multinational Association for Supportive Care in Cancer | Organization which includes experts that provide guidelines for improving side effects of cancer and cancer treatment |
| ESMO | European Society of Clinical Oncology | Organization of cancer professionals to share information and guidelines to develop better ways to treat cancer in Europe and other regions. The academic journal is ESMO Open |
| ASCO | American Society of Clinical Oncology | World’s largest organization of cancer professionals to share information and guidelines to develop better ways to treat cancer. Academic journal is the Journal of Clinical Oncology |
| NCCN | National Comprehensive Cancer Network | An organization devoted to providing guidelines for treatment of specific cancers and issues related to cancer treatment |
| PG-SGA | Patient Generated Subjective Global Assessment | A tool to help identify malnutrition and cachexia in cancer patients at risk for sarcopenia |
| OBD | Optimal biologic dose | Dose of an agent that achieves best effect against a target with acceptable toxicity (for example effect on immune activation) |
| MTD | Maximally tolerated dose | Dose of a drug (e.g., chemotherapy drug) for which an increase dose would have unacceptable toxicity. Once the MTD is determined in a phase I clinical trial, this becomes the recommended phase 2 dose of an anti-cancer agent |
| AUC | Area under the curve | A pharmacokinetic/pharmacodynamic concept that reflects the graph of drug concentration over time. Usually, an inverted U shape because of absorption, distribution, and metabolism, and elimination of an agent |
| VEGF | Vascular endothelial growth factor | A protein involved in generation of new blood vessels to heal wounds or injury or associated with tumors growing new blood vessels. Some anti-cancer drugs block VEGF |
| NG | Naso-gastric | Usually refers to thin tube that extends from the nose to the stomach to provide liquid nutrition, suspensions of drugs, or fluids without swallowing |
| G-tube | Gastrostomy tube | A tube that goes directly from abdominal skin through the muscles and lining of the abdomen into the stomach. Same use as NG but without the discomfort of a tube in the nose or back of the throat |
| SRS | Stereotactic radiosurgery | Ultraprecise radiation that may require a “halo” device or anesthesia to give the dose to tumor only in the brain or near the spinal cord |
| N/V | Nausea and/or vomiting | The most common side effect of cancer chemotherapy |
| 5HT | 5-hydroxytryptamine (serotonin) | The 5-HT3 receptor is triggered in the brain chemoreceptor trigger zone to cause drug associated N/V. Inhibitors of 5-HT are very useful as anti-emetics to reduce or prevent chemotherapy associated N/V |
| TPN | Total parenteral nutrition | An intravenous solution containing glucose, amino acids, vitamins, and sometimes lipids that is used when patients cannot eat or drink adequate amounts of nutrients |
| TKI | Tyrosine kinase inhibitors | A class of drugs that act to block cancer-associated tyrosine kinase enzyme(s) in cancer cells that facilitate cancer growth |
| WART | Whole abdominal radiotherapy | A radiation technique to provide a moderately high dose of radiation to the entire abdomen with relative sparing of liver and kidneys and treating intestines and intestinal lining to tolerance dose. |
| RT | Radiotherapy | Use of radiation as a treatment modality for cancer |
| BMT | Bone marrow transplant | High-dose chemotherapy followed by infusion of marrow or blood stem cells to allow recovery of blood cell production |
| G-CSF | Granulocyte colony stimulating factor | A subcutaneous injection given after chemotherapy to increase granulocyte (neutrophil) production by the bone marrow to make chemotherapy safer |
| KPS | Karnofsky performance scale | A scale from 0% (dead) to 100% (full activity without limitation) to indicate how active a cancer patient is and whether or not activity (performance) is limited by symptoms of drugs, radiation, or cancer |
| PT/OT | Physical therapy and/or occupational therapy | PT involves improving function, exercise, and strength training. OT involves learning how to do activities of daily living better (e.g., climbing stairs, opening a jar, buttoning a shirt) |
| GNRI | Geriatric Nutrition Risk Index | A composite compilation of risk factors to help with malnutritional assessment in older people |
| MNA | Mini-Nutritional Assessment | A composite compilation of risk factors to help with malnutritional assessment |
| PRO | Patient reported outcome | A self-assessment form (if electronic it is an ePRO) |
| BMI | Body mass index | A calculation involving height and weight that can give a number to indicate thin, normal or obese (e.g., BMI > 30) |
References
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.A.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual report to the nation on the status of cancer, Part 1: National cancer statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Bradley, C.J.; Yabroff, K.R.; Zafar, S.Y.; Shih, Y.T. Time to add screening for financial hardship as a quality measure? CA Cancer J. Clin. 2021, 71, 100–106. [Google Scholar] [CrossRef]
- Warren, J.L.; Yabroff, K.R.; Meekins, A.; Topor, M.; Lamont, E.B.; Brown, M.L. Evaluation of trends in the cost of initial cancer treatment. J. Natl. Cancer Inst. 2008, 100, 888–897. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Davis, W.W.; Lamont, E.B.; Fahey, A.; Topor, M.; Brown, M.L.; Warren, J.L. Patient time costs associated with cancer care. J. Natl. Cancer Inst. 2007, 99, 14–23. [Google Scholar] [CrossRef]
- Pisu, M.; Henrikson, N.B.; Banegas, M.P.; Yabroff, K.R. Costs of cancer along the care continuum: What we can expect based on recent literature. Cancer 2018, 124, 4181–4191. [Google Scholar] [CrossRef]
- Mariotto, A.B.; Yabroff, K.R.; Shao, Y.; Feuer, E.J.; Brown, M.L. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 2011, 103, 117–128. [Google Scholar] [CrossRef]
- Banegas, M.P.; Yabroff, K.R. Out of pocket, out of sight? An unmeasured component of the burden of cancer. J. Natl. Cancer Inst. 2013, 105, 252–253. [Google Scholar] [CrossRef][Green Version]
- Yabroff, K.R.; Bradley, C.J.; Mariotto, A.B.; Brown, M.L.; Feuer, E.J. Estimates and projections of value of life lost from cancer deaths in the United States. J. Natl. Cancer Inst. 2008, 100, 1755–1762. [Google Scholar] [CrossRef]
- Yabroff, K.R.; Lund, J.; Kepka, D.; Mariotto, A. Economic burden of cancer in the United States: Estimates, projections, and future research. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2006–2014. [Google Scholar] [CrossRef]
- Thomas, T.; Hughes, T.; Mady, L.; Belcher, S.M. Financial toxicity: A review of the literature and nursing opportunities. Clin. J. Oncol. Nurs. 2019, 23, 5–13. [Google Scholar] [CrossRef]
- Bowen, J.M.; Elad, S.; Hutchins, R.D.; Lalla, R.V.; Mucositis study group of the multinational association of supportive care in cancer/international society of Oral Oncology. Methodology for the MASCC/ISOO mucositis clinical practice guidelines update. Support. Care Cancer 2013, 21, 303–308. [Google Scholar] [CrossRef]
- Elad, S. The MASCC/ISOO mucositis guidelines 2019: The second set of articles and future directions. Support. Care Cancer 2020, 28, 2445–2447. [Google Scholar] [CrossRef]
- Elad, S.; Bowen, J.; Zadik, Y.; Lalla, R.V.; Mucositis study group of the multinational association of supportive care in cancer/international society of Oral Oncology. Development of the MASCC/ISOO clinical practice guidelines for mucositis: Considerations underlying the process. Support. Care Cancer 2013, 21, 309–312. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Lalla, R.V. The MASCC/ISOO mucositis guidelines update: Introduction to the first set of articles. Support. Care Cancer 2013, 21, 301–302. [Google Scholar] [CrossRef]
- McKenzie, E.; Zaki, P.; Raman, S.; Olson, R.; McFarlane, T.; DeAngelis, C.; Chan, S.; Pidduck, W.; Razvi, Y.; Bushehri, A.; et al. Radiation-induced nausea and vomiting: A comparison between MASCC/ESMO, ASCO, and NCCN antiemetic guidelines. Support. Care Cancer 2019, 27, 783–791. [Google Scholar] [CrossRef]
- Ranna, V.; Cheng, K.K.F.; Castillo, D.A.; Porcello, L.; Vaddi, A.; Lalla, R.V.; Bossi, P.; Elad, S.; Mucositis study group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology. Development of the MASCC/ISOO clinical practice guidelines for mucositis: An overview of the methods. Support. Care Cancer 2019, 27, 3933–3948. [Google Scholar] [CrossRef]
- Razvi, Y.; Chan, S.; McFarlane, T.; McKenzie, E.; Zaki, P.; DeAngelis, C.; Pidduck, W.; Bushehri, A.; Chow, E.; Jerzak, K.J. ASCO, NCCN, MASCC/ESMO: A comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support. Care Cancer 2019, 27, 87–95. [Google Scholar] [CrossRef]
- Meazza, C.; Casanova, M.; Luksch, R.; Podda, M.; Favini, F.; Cefalo, G.; Massimino, M.; Ferrari, A. Prolonged 14-day continuous infusion of high-dose ifosfamide with an external portable pump: Feasibility and efficacy in refractory pediatric sarcoma. Pediatr. Blood Cancer 2010, 55, 617–620. [Google Scholar] [CrossRef]
- Anderson, P. Continuously improving ifosfamide/mesna: A winning combination. Pediatr. Blood Cancer 2010, 55, 599–600. [Google Scholar] [CrossRef]
- Peterson, D.E.; Ohrn, K.; Bowen, J.; Fliedner, M.; Lees, J.; Loprinzi, C.; Mori, T.; Osaguona, A.; Weikel, D.S.; Elad, S.; et al. Systematic review of oral cryotherapy for management of oral mucositis caused by cancer therapy. Support. Care Cancer 2013, 21, 327–332. [Google Scholar] [CrossRef]
- Correa, M.E.P.; Cheng, K.K.F.; Chiang, K.; Kandwal, A.; Loprinzi, C.L.; Mori, T.; Potting, C.; Rouleau, T.; Toro, J.J.; Ranna, V.; et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 28, 2457–2472. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, H.S. Meta-analysis of oral cryotherapy in preventing oral mucositis associated with cancer therapy. Int. J. Nurs. Pract. 2019, 25, e12759. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Z.; Zhai, R.; Zhao, S.; Luo, L.; Li, D.; Zhao, X.; Wei, H.; Pang, Z.; Wang, L.; et al. Efficacy of oral cryotherapy on oral mucositis prevention in patients with hematological malignancies undergoing hematopoietic stem cell transplantation: A meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0128763. [Google Scholar] [CrossRef]
- Todorova, V.K.; Harms, S.A.; Kaufmann, Y.; Luo, S.; Luo, K.Q.; Babb, K.; Klimberg, V.S. Effect of dietary glutamine on tumor glutathione levels and apoptosis-related proteins in DMBA-induced breast cancer of rats. Breast Cancer Res. Treat. 2004, 88, 247–256. [Google Scholar] [CrossRef]
- Rouse, K.; Nwokedi, E.; Woodliff, J.E.; Epstein, J.; Klimberg, V.S. Glutamine enhances selectivity of chemotherapy through changes in glutathione metabolism. Ann. Surg. 1995, 221, 420–426. [Google Scholar] [CrossRef]
- Rubio, I.; Suva, L.J.; Todorova, V.; Bhattacharyya, S.; Kaufmann, Y.; Maners, A.; Smith, M.; Klimberg, V.S. Oral glutamine reduces radiation morbidity in breast conservation surgery. JPEN J. Parenter. Enter. Nutr. 2013, 37, 623–630. [Google Scholar] [CrossRef]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Sedor, G.; Ellsworth, P.; Scarborough, J.A.; Ahmed, K.A.; Oliver, D.E.; Eschrich, S.A.; Kattan, M.W.; Torres-Roca, J.F. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): A cohort-based pooled analysis. Lancet Oncol. 2021, 22, 1221–1229. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Poortmans, P.; Salgado, R. Genomic-adjusted radiation dose to personalise radiotherapy. Lancet Oncol. 2021, 22, 1200–1201. [Google Scholar] [CrossRef]
- Scott, J.G.; Harrison, L.B.; Torres-Roca, J.F. Genomic-adjusted radiation dose—Authors’ reply. Lancet Oncol. 2017, 18, e129. [Google Scholar] [CrossRef][Green Version]
- De Sousa, I.M.; Silva, F.M.; de Carvalho, A.L.M.; da Rocha, I.M.G.; Fayh, A.P.T. Accuracy of isolated nutrition indicators in diagnosing malnutrition and their prognostic value to predict death in patients with gastric and colorectal cancer: A prospective study. JPEN J. Parenter. Enter. Nutr. 2021, 1–9. [Google Scholar] [CrossRef]
- Deftereos, I.; Djordjevic, A.; Carter, V.M.; McNamara, J.; Yeung, J.M.; Kiss, N. Malnutrition screening tools in gastrointestinal cancer: A systematic review of concurrent validity. Surg. Oncol. 2021, 38, 101627. [Google Scholar] [CrossRef]
- Gascon-Ruiz, M.; Casas-Deza, D.; Torres-Ramon, I.; Zapata-Garcia, M.; Alonso, N.; Sesma, A.; Lambea, J.; Alvarez-Alejandro, M.; Quilez, E.; Isla, D.; et al. Comparation of different malnutrition screening tools according to GLIM criteria in cancer outpatients. Eur. J. Clin. Nutr. 2021, 9, 1898. [Google Scholar] [CrossRef]
- Gebremedhin, T.K.; Cherie, A.; Tolera, B.D.; Atinafu, B.T.; Demelew, T.M. Prevalence and risk factors of malnutrition among adult cancer patients receiving chemotherapy treatment in cancer center, Ethiopia: Cross-sectional study. Heliyon 2021, 7, e07362. [Google Scholar] [CrossRef]
- Hunter, M.; Kellett, J.; Toohey, K.; D’Cunha, N.M.; Isbel, S.; Naumovski, N. Toxicities caused by head and neck cancer treatments and their influence on the development of malnutrition: Review of the literature. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 66. [Google Scholar] [CrossRef]
- Kenny, E.; Samavat, H.; Touger-Decker, R.; Parrott, J.S.; Byham-Gray, L.; August, D.A. Adverse perioperative outcomes among patients undergoing gastrointestinal cancer surgery: Quantifying attributable risk from malnutrition. JPEN J. Parenter. Enteral. Nutr. 2021. [Google Scholar] [CrossRef]
- Kose, E.; Wakabayashi, H.; Yasuno, N. Polypharmacy and malnutrition management of elderly perioperative patients with cancer: A systematic review. Nutrients 2021, 13, 1961. [Google Scholar] [CrossRef]
- Levonyak, N.S.; Hodges, M.P.; Haaf, N.; Brown, T.J.; Hardy, S.; Mhoon, V.; Kainthla, R.; Beg, M.S.; Kazmi, S.M. Importance of addressing malnutrition in cancer and implementation of a quality improvement project in a gastrointestinal cancer clinic. Nutr. Clin. Pract. 2021, 107, 2411–2502. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Tang, M.; Song, M.; Zhang, Q.; Zhang, K.; Ruan, G.; Zhang, X.; Ge, Y.; Yang, M.; et al. Different muscle mass indices of the global leadership initiative on malnutrition in diagnosing malnutrition and predicting survival of patients with gastric cancer. Nutrition 2021, 89, 111286. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Corsaro, E.; Molfino, A. Awareness of cancer-related malnutrition and its management: Analysis of the results from a survey conducted among medical oncologists. Front. Oncol. 2021, 11, 682999. [Google Scholar] [CrossRef]
- Nakyeyune, R.; Ruan, X.; Shen, Y.; Shao, Y.; Niu, C.; Zang, Z.; Liu, F. Diagnostic performance of SGA, PG-SGA and MUST for malnutrition assessment in adult cancer patients: A systematic literature review and hierarchical bayesian meta-analysis. Nutr. Cancer 2021, 1–13. [Google Scholar] [CrossRef]
- Pingili, S.; Ahmed, J.; Sujir, N.; Shenoy, N.; Ongole, R. Evaluation of malnutrition and quality of life in patients treated for oral and oropharyngeal cancer. Sci. World J. 2021, 2021, 9936715. [Google Scholar] [CrossRef]
- Sanchez-Torralvo, F.J.; Ruiz-Garcia, I.; Contreras-Bolivar, V.; Gonzalez-Almendros, I.; Ruiz-Vico, M.; Abuin-Fernandez, J.; Barrios, M.; Alba, E.; Olveira, G. CT-determined sarcopenia in GLIM-defined malnutrition and prediction of 6-month mortality in cancer inpatients. Nutrients 2021, 13, 2647. [Google Scholar] [CrossRef]
- Wang, P.; Chen, X.; Liu, Q.; Liu, X.; Li, Y. Good performance of the global leadership initiative on malnutrition criteria for diagnosing and classifying malnutrition in people with esophageal cancer undergoing esophagectomy. Nutrition 2021, 91–92, 111420. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, S.; Jia, J.; Yang, J.; Song, Y.; Duan, H. Exploring the malnutrition status and impact of total parenteral nutrition on the outcome of patients with advanced stage ovarian cancer. BMC Cancer 2021, 21, 799. [Google Scholar] [CrossRef]
- Yin, L.; Fan, Y.; Lin, X.; Zhang, L.; Li, N.; Liu, J.; Guo, J.; Zhang, M.; He, X.; Liu, L.; et al. Fat mass assessment using the triceps skinfold thickness enhances the prognostic value of the global leadership initiative on malnutrition criteria in patients with lung cancer. Br. J. Nutr. 2021, 1–11. [Google Scholar] [CrossRef]
- Zhang, K.P.; Tang, M.; Fu, Z.M.; Zhang, Q.; Zhang, X.; Guo, Z.Q.; Xu, H.X.; Song, C.H.; Braga, M.; Cederholm, T.; et al. Global leadership initiative on malnutrition criteria as a nutrition assessment tool for patients with cancer. Nutrition 2021, 91–92, 111379. [Google Scholar] [CrossRef]
- Anderson, P.M.; Hanna, R. Defining moments: Making time for virtual visits and catalyzing better cancer care. Health Commun. 2020, 35, 787–791. [Google Scholar] [CrossRef]
- O’Hanlon, C.E.; Lindvall, C.; Giannitrapani, K.F.; Garrido, M.; Ritchie, C.; Asch, S.; Gamboa, R.C.; Canning, M.; Lorenz, K.A.; Walling, A.M.; et al. Expert stakeholder prioritization of process quality measures to achieve patient—and family-centered palliative and end-of-life cancer care. J. Palliat. Med. 2021, 24, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, A.P.; Baltich Nelson, B.; Delgado, D.; Mages, K.C.; Martin, L.; Stribling, J.C. Involvement of information professionals in patient- and family-centered care initiatives: A scoping review. J. Med. Libr. Assoc. 2019, 107, 314–322. [Google Scholar] [CrossRef]
- Crespo, C.; Santos, S.; Tavares, A.; Salvador, A. “Care that matters”: Family-centered care, caregiving burden, and adaptation in parents of children with cancer. Fam. Syst. Health 2016, 34, 31–40. [Google Scholar] [CrossRef]
- Michael, N.; O’Callaghan, C.; Baird, A.; Hiscock, N.; Clayton, J. Cancer caregivers advocate a patient—and family-centered approach to advance care planning. J. Pain Symptom Manag. 2014, 47, 1064–1077. [Google Scholar] [CrossRef]
- Oshima, S.M.; Tait, S.D.; Thomas, S.M.; Fayanju, O.M.; Ingraham, K.; Barrett, N.J.; Hwang, E.S. Association of smartphone ownership and internet use with markers of health literacy and access: Cross-sectional survey study of perspectives from project PLACE (Population Level Approaches to Cancer Elimination). J. Med. Internet Res. 2021, 23, e24947. [Google Scholar] [CrossRef]
- Keaver, L.; Yiannakou, I.; Folta, S.C.; Zhang, F.F. Perceptions of oncology providers and cancer survivors on the role of nutrition in cancer care and their views on the “NutriCare” program. Nutrients 2020, 12, 1277. [Google Scholar] [CrossRef]
- Barreira, J.V. The role of nutrition in cancer patients. Nutr. Cancer 2020, 1–2. [Google Scholar] [CrossRef]
- Barrett, M.; Ui Dhuibhir, P.; Njoroge, C.; Wickham, S.; Buchanan, P.; Aktas, A.; Walsh, D. Diet and nutrition information on nine national cancer organisation websites: A critical review. Eur. J. Cancer Care 2020, 29, e13280. [Google Scholar] [CrossRef] [PubMed]
- Haskins, C.P.; Champ, C.E.; Miller, R.; Vyfhuis, M.A.L. Nutrition in cancer: Evidence and equality. Adv. Radiat. Oncol. 2020, 5, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Arensberg, M.B.; Thomas, S.; Kerr, K.W.; Hegazi, R.; Bastasch, M. Impact of early incorporation of nutrition interventions as a component of cancer therapy in adults: A review. Nutrients 2020, 12, 3403. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.S.; Rice, N.; Kingston, E.; Kelly, A.; Reynolds, J.V.; Feighan, J.; Power, D.G.; Ryan, A.M. A national survey of oncology survivors examining nutrition attitudes, problems and behaviours, and access to dietetic care throughout the cancer journey. Clin. Nutr. ESPEN 2021, 41, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.L.; Moore, J.B.; Corfe, B.M. Nutrition and cancer: Evidence gaps and opportunities for improving knowledge. Proc. Nutr. Soc. 2020, 79, 367–372. [Google Scholar] [CrossRef]
- Trehan, A.; Viani, K.; da Cruz, L.B.; Sagastizado, S.Z.; Ladas, E.J. The importance of enteral nutrition to prevent or treat undernutrition in children undergoing treatment for cancer. Pediatr. Blood Cancer 2020, 67 (Suppl. 3), e28378. [Google Scholar] [CrossRef]
- Koppold-Liebscher, D.; Kessler, C.S.; Steckhan, N.; Bahr, V.; Kempter, C.; Wischnewsky, M.; Hubner, M.; Kunz, B.; Paul, M.; Zorn, S.; et al. Short-term fasting accompanying chemotherapy as a supportive therapy in gynecological cancer: Protocol for a multicenter randomized controlled clinical trial. Trials 2020, 21, 854. [Google Scholar] [CrossRef]
- Sadeghian, M.; Rahmani, S.; Khalesi, S.; Hejazi, E. A review of fasting effects on the response of cancer to chemotherapy. Clin. Nutr. 2021, 40, 1669–1681. [Google Scholar] [CrossRef]
- Tang, X.; Li, G.; Shi, L.; Su, F.; Qian, M.; Liu, Z.; Meng, Y.; Sun, S.; Li, J.; Liu, B. Combined intermittent fasting and ERK inhibition enhance the anti-tumor effects of chemotherapy via the GSK3beta-SIRT7 axis. Nat. Commun. 2021, 12, 5058. [Google Scholar] [CrossRef]
- Cong, M.; Song, C.; Xu, H.; Song, C.; Wang, C.; Fu, Z.; Ba, Y.; Wu, J.; Xie, C.; Chen, G.; et al. The patient-generated subjective global assessment is a promising screening tool for cancer cachexia. BMJ Support. Palliat. Care 2020. [Google Scholar] [CrossRef]
- Jager-Wittenaar, H.; de Bats, H.F.; Welink-Lamberts, B.J.; Gort-van Dijk, D.; van der Laan, B.; Ottery, F.D.; Roodenburg, J.L.N. Self-completion of the patient-generated subjective global assessment short form is feasible and is associated with increased awareness on malnutrition risk in patients with head and neck cancer. Nutr. Clin. Pract. 2020, 35, 353–362. [Google Scholar] [CrossRef]
- De Groot, L.M.; Lee, G.; Ackerie, A.; van der Meij, B.S. Malnutrition screening and assessment in the cancer care ambulatory setting: Mortality predictability and validity of the patient-generated subjective global assessment short form (PG-SGA SF) and the GLIM criteria. Nutrients 2020, 12, 2287. [Google Scholar] [CrossRef]
- De Oliveira Pereira, F.; Pereira Mota, A.; Azevedo Aredes, M.; Villaca Chaves, G.; Ramos Cardoso, I.C. Association between scored patient-generated subjective global assessment and skeletal muscle determined by computed tomography in patients with cervical cancer. Nutr. Cancer 2020, 72, 595–601. [Google Scholar] [CrossRef]
- Cavalcante Martins, F.F.; de Pinho, N.B.; de Carvalho Padilha, P.; Martucci, R.B.; Rodrigues, V.D.; Sales, R.C.; Ferreira Peres, W.A. Patient-generated subjective global assessment predicts cachexia and death in patients with head, neck and abdominal cancer: A retrospective longitudinal study. Clin. Nutr. ESPEN 2019, 31, 17–22. [Google Scholar] [CrossRef]
- Pana, A.; Sourtzi, P.; Kalokairinou, A.; Velonaki, V.S. Sarcopenia and polypharmacy among older adults: A scoping review of the literature. Arch. Gerontol. Geriatr. 2021, 98, 104520. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Sugiyama, K. Sarcopenia and Nutrition Support in the Treatment of Gastric Cancer. Gan Kagaku Ryoho 2020, 47, 1542–1546. [Google Scholar]
- Williams, G.R.; Deal, A.M.; Muss, H.B.; Weinberg, M.S.; Sanoff, H.K.; Nyrop, K.A.; Pergolotti, M.; Shachar, S.S. Skeletal muscle measures and physical function in older adults with cancer: Sarcopenia or myopenia? Oncotarget 2017, 8, 33658–33665. [Google Scholar] [CrossRef]
- Klassen, P.; Baracos, V.; Gramlich, L.; Nelson, G.; Mazurak, V.; Martin, L. Computed-tomography body composition analysis complements pre-operative nutrition screening in colorectal cancer patients on an enhanced recovery after surgery pathway. Nutrients 2020, 12, 3745. [Google Scholar] [CrossRef]
- Neoh, M.K.; Abu Zaid, Z.; Mat Daud, Z.A.; Md Yusop, N.B.; Ibrahim, Z.; Abdul Rahman, Z.; Jamhuri, N. Changes in nutrition impact symptoms, nutritional and functional status during head and neck cancer treatment. Nutrients 2020, 12, 1225. [Google Scholar] [CrossRef]
- Opanga, Y.; Kaduka, L.; Bukania, Z.; Mutisya, R.; Korir, A.; Thuita, V.; Mwangi, M.; Muniu, E.; Mbakaya, C. Nutritional status of cancer outpatients using scored patient generated subjective global assessment in two cancer treatment centers, Nairobi, Kenya. BMC Nutr. 2017, 3, 63. [Google Scholar] [CrossRef]
- Guo, X.; Peters, M.D.J.; Lu, Z. Management of skin toxicity caused by epidermal growth factor receptor inhibitors: An evidence-based implementation project. JBI Database Syst. Rev. Implement Rep. 2017, 15, 2815–2829. [Google Scholar] [CrossRef]
- Bechtel, A.S.; Indelicato, D.J.; Sandler, E. Enteral nutrition in pediatric high-risk head and neck cancer patients receiving proton therapy: Identifying risk factors and quality of life concerns to optimize care. J. Pediatr. Hematol. Oncol. 2019, 41, e247–e253. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Cai, X.; Cao, Y.; Yang, G.; Huang, P. Impact of total parenteral nutrition standardization led by pharmacist on quality in postoperative patients with colorectal cancer. Eur. J. Clin. Nutr. 2019, 73, 243–249. [Google Scholar] [CrossRef]
- Suehiro, M.; Kojima, Y.; Takahashi, M.; Ito, Y.; Keira, T.; Ikegawa, K.; Minatogawa, H.; Tsugawa, K.; Tanaka, T. Antiemetic efficacy of adding olanzapine 5 mg to aprepitant, palonosetron and dexamethasone-sparing after day two for cancer patients receiving anthracycline and cyclophosphamide. Cancer Manag. Res. 2021, 13, 1617–1624. [Google Scholar] [CrossRef]
- Cabanillas Stanchi, K.M.; Willier, S.; Vek, J.; Schlegel, P.; Queudeville, M.; Rieflin, N.; Klaus, V.; Gansel, M.; Rupprecht, J.V.; Flaadt, T.; et al. Antiemetic prophylaxis with fosaprepitant and 5-HT3-receptor antagonists in pediatric patients undergoing autologous hematopoietic stem cell transplantation. Drug Des. Devel. Ther. 2020, 14, 3915–3927. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chen, C.Y.; Tam, K.W.; Hsu, C.Y. Effectiveness of palonosetron versus granisetron in preventing chemotherapy-induced nausea and vomiting: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2021, 77, 1597–1609. [Google Scholar] [CrossRef]
- Van der Vorst, M.; Toffoli, E.C.; Beusink, M.; van Linde, M.E.; van Voorthuizen, T.; Brouwer, S.; van Zweeden, A.A.; Vrijaldenhoven, S.; Berends, J.C.; Berkhof, J.; et al. Metoclopramide, dexamethasone, or palonosetron for prevention of delayed chemotherapy-induced nausea and vomiting after moderately emetogenic chemotherapy (MEDEA): A randomized, phase III, noninferiority trial. Oncologist 2021, 26, e173–e181. [Google Scholar] [CrossRef]
- Willier, S.; Cabanillas Stanchi, K.M.; von Have, M.; Binder, V.; Blaeschke, F.; Feucht, J.; Feuchtinger, T.; Doring, M. Efficacy, safety and feasibility of fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in pediatric patients receiving moderately and highly emetogenic chemotherapy—Results of a non-interventional observation study. BMC Cancer 2019, 19, 1118. [Google Scholar] [CrossRef]
- Anderson, P.M.; Lalla, R.V. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients 2020, 12, 1675. [Google Scholar] [CrossRef]
- Anderson, P.M.; Ramsay, N.K.; Shu, X.O.; Rydholm, N.; Rogosheske, J.; Nicklow, R.; Weisdorf, D.J.; Skubitz, K.M. Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transpl. 1998, 22, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Schroeder, G.; Skubitz, K.M. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer 1998, 83, 1433–1439. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Anderson, P.M. Oral glutamine to prevent chemotherapy induced stomatitis: A pilot study. J. Lab. Clin. Med. 1996, 127, 223–228. [Google Scholar] [CrossRef]
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R.; et al. Emerging evidence on the pathobiology of mucositis. Support. Care Cancer 2013, 21, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Lai, Y.H.; Huang, B.S.; Lin, C.Y.; Fan, K.H.; Chang, J.T. Changes and predictors of radiation-induced oral mucositis in patients with oral cavity cancer during active treatment. Eur. J. Oncol. Nurs. 2015, 19, 214–219. [Google Scholar] [CrossRef]
- Lalla, R.V.; Saunders, D.P.; Peterson, D.E. Chemotherapy or radiation-induced oral mucositis. Dent. Clin. N. Am. 2014, 58, 341–349. [Google Scholar] [CrossRef]
- Tobin, J.L.; Thomas, S.M.; Freyer, D.R.; Hamilton, A.S.; Milam, J.E. Estimating cancer treatment intensity from SEER cancer registry data: Methods and implications for population-based registry studies of pediatric cancers. Cancer Causes Control 2020, 31, 881–890. [Google Scholar] [CrossRef]
- Beaven, A.W.; Shea, T.C. The effect of palifermin on chemotherapyand radiation therapy-induced mucositis: A review of the current literature. Support. Cancer Ther. 2007, 4, 188–197. [Google Scholar] [CrossRef]
- Sonis, S.T. Efficacy of palifermin (keratinocyte growth factor-1) in the amelioration of oral mucositis. Core Evid. 2010, 4, 199–205. [Google Scholar] [CrossRef]
- Reddy, G.K.; Jain, V.K. Palifermin is efficacious in reducing oral mucositis induced by intensive cancer therapy. Support. Cancer Ther. 2005, 2, 84–85. [Google Scholar] [CrossRef]
- Le, Q.T.; Kim, H.E.; Schneider, C.J.; Murakozy, G.; Skladowski, K.; Reinisch, S.; Chen, Y.; Hickey, M.; Mo, M.; Chen, M.G.; et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: A randomized, placebo-controlled study. J. Clin. Oncol. 2011, 29, 2808–2814. [Google Scholar] [CrossRef]
- Vadhan-Raj, S.; Trent, J.; Patel, S.; Zhou, X.; Johnson, M.M.; Araujo, D.; Ludwig, J.A.; O’Roark, S.; Gillenwater, A.M.; Bueso-Ramos, C.; et al. Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: A randomized trial. Ann. Intern. Med. 2010, 153, 358–367. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leung, S.W.; Wang, C.J.; Chen, H.C.; Sun, L.M.; Fang, F.M.; Yeh, S.A.; Hsu, H.C.; Hsiung, C.Y. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 535–539. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Salloum, R.M.; Kasper, M.; Plumley, D.A.; Dolson, D.J.; Hautamaki, R.D.; Mendenhall, W.R.; Bova, F.C.; Bland, K.I.; Copeland, E.M.; et al. Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch. Surg. 1990, 125, 1040–1045. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Souba, W.W.; Dolson, D.J.; Salloum, R.M.; Hautamaki, R.D.; Plumley, D.A.; Mendenhall, W.M.; Bova, F.J.; Khan, S.R.; Hackett, R.L.; et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer 1990, 66, 62–68. [Google Scholar] [CrossRef]
- Souba, W.W.; Klimberg, V.S.; Hautamaki, R.D.; Mendenhall, W.H.; Bova, F.C.; Howard, R.J.; Bland, K.I.; Copeland, E.M. Oral glutamine reduces bacterial translocation following abdominal radiation. J. Surg. Res. 1990, 48, 1–5. [Google Scholar] [CrossRef]
- Aquino, V.M.; Harvey, A.R.; Garvin, J.H.; Godder, K.T.; Nieder, M.L.; Adams, R.H.; Jackson, G.B.; Sandler, E.S. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: A pediatric blood and marrow transplant consortium study. Bone Marrow Transpl. 2005, 36, 611–616. [Google Scholar] [CrossRef]
- Schloerb, P.R.; Skikne, B.S. Oral and parenteral glutamine in bone marrow transplantation: A randomized, double-blind study. JPEN J. Parenter. Enter. Nutr. 1999, 23, 117–122. [Google Scholar] [CrossRef]
- Wingard, J.R.; Niehaus, C.S.; Peterson, D.E.; Jones, R.J.; Piantadosi, S.; Levin, L.S.; Saral, R.; Santos, G.W. Oral mucositis after bone marrow transplantation. A marker of treatment toxicity and predictor of hepatic veno-occlusive disease. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 419–424. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- De Angulo, G.; Yuen, C.; Palla, S.L.; Anderson, P.M.; Zweidler-McKay, P.A. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: Implications for risk stratification and future studies. Cancer 2008, 112, 407–415. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Campbell, P.; Zhou, Y.; Sandlund, J.T.; Jeha, S.; Ribeiro, R.C.; Inaba, H.; Bhojwani, D.; Relling, M.V.; Howard, S.C.; et al. Prognostic impact of absolute lymphocyte counts at the end of remission induction in childhood acute lymphoblastic leukemia. Cancer 2013, 119, 2061–2066. [Google Scholar] [CrossRef]
- Anderson, P.M. Immune therapy for sarcomas. Adv. Exp. Med. Biol. 2017, 995, 127–140. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef]
- Anderson, P.; Kaye, L. The therapeutic alliance: Adapting to the unthinkable with better information. Health Commun. 2009, 24, 775–778. [Google Scholar] [CrossRef]
- Anderson, P.; Salazar-Abshire, M. Improving outcomes in difficult bone cancers using multimodality therapy, including radiation: Physician and nursing perspectives. Curr. Oncol. Rep. 2006, 8, 415–422. [Google Scholar] [CrossRef]
- Aleixo, G.F.P.; Williams, G.R.; Nyrop, K.A.; Muss, H.B.; Shachar, S.S. Muscle composition and outcomes in patients with breast cancer: Meta-analysis and systematic review. Breast Cancer Res. Treat. 2019, 177, 569–579. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Williams, G.R.; Deal, A.M.; Muss, H.B.; Weinberg, M.S.; Sanoff, H.K.; Guerard, E.J.; Nyrop, K.A.; Pergolotti, M.; Shachar, S.S. Frailty and skeletal muscle in older adults with cancer. J. Geriatr. Oncol. 2018, 9, 68–73. [Google Scholar] [CrossRef]
- Lacey, J.M.; Wilmore, D.W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 1990, 48, 297–309. [Google Scholar] [CrossRef]
- Smith, R.J.; Wilmore, D.W. Glutamine nutrition and requirements. JPEN J. Parenter. Enter. Nutr. 1990, 14, 94S–99S. [Google Scholar] [CrossRef]
- Souba, W.W.; Smith, R.J.; Wilmore, D.W. Glutamine metabolism by the intestinal tract. JPEN J. Parenter. Enter. Nutr. 1985, 9, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W.; Shabert, J.K. Role of glutamine in immunologic responses. Nutrition 1998, 14, 618–626. [Google Scholar] [CrossRef]
- Kaufmann, Y.; Spring, P.; Klimberg, V.S. Oral glutamine prevents DMBA-induced mammary carcinogenesis via upregulation of glutathione production. Nutrition 2008, 24, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Lim, V.; Korourian, S.; Todorova, V.K.; Kaufmann, Y.; Klimberg, V.S. Glutamine prevents DMBA-induced squamous cell cancer. Oral Oncol. 2009, 45, 148–155. [Google Scholar] [CrossRef]
- Klimberg, S. Prevention of radiogenic side effects using glutamine-enriched elemental diets. Recent Results Cancer Res. 1991, 121, 283–285. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Nwokedi, E.; Hutchins, L.F.; Pappas, A.A.; Lang, N.P.; Broadwater, J.R.; Read, R.C.; Westbrook, K.C. Glutamine facilitates chemotherapy while reducing toxicity. JPEN J. Parenter. Enter. Nutr. 1992, 16, 83S–87S. [Google Scholar] [CrossRef]
- Todorova, V.; Vanderpool, D.; Blossom, S.; Nwokedi, E.; Hennings, L.; Mrak, R.; Klimberg, V.S. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition 2009, 25, 812–817. [Google Scholar] [CrossRef]
- Barlow, A.; Prusak, E.S.; Barlow, B.; Nightingale, G. Interventions to reduce polypharmacy and optimize medication use in older adults with cancer. J. Geriatr. Oncol. 2021, 12, 863–871. [Google Scholar] [CrossRef]
- Chen, L.J.; Trares, K.; Laetsch, D.C.; Nguyen, T.N.M.; Brenner, H.; Schottker, B. Systematic review and meta-analysis on the associations of polypharmacy and potentially inappropriate medication with adverse outcomes in older cancer patients. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1044–1052. [Google Scholar] [CrossRef]
- Flannery, M.A.; Mohile, S.; Culakova, E.; Norton, S.; Kamen, C.; Dionne-Odom, J.N.; DiGiovanni, G.; Griggs, L.; Bradley, T.; Hopkins, J.O.; et al. Completion of patient-reported outcome questionnaires among older adults with advanced cancer. J. Pain Symptom Manag. 2021. [Google Scholar] [CrossRef]
- Guven, D.C.; Kavgaci, G.; Aktepe, O.H.; Yildirim, H.C.; Sahin, T.K.; Aksoy, S.; Erman, M.; Kilickap, S.; Yalcin, S. The burden of polypharmacy and drug-drug interactions in older cancer patients treated with immunotherapy. J. Oncol. Pharm. Pract. 2021. [Google Scholar] [CrossRef]
- Nightingale, G.; Mohamed, M.R.; Holmes, H.M.; Sharma, M.; Ramsdale, E.; Lu-Yao, G.; Chapman, A. Research priorities to address polypharmacy in older adults with cancer. J. Geriatr. Oncol. 2021, 12, 964–970. [Google Scholar] [CrossRef]
- Abd-El-Gawad, W.M.; Abou-Hashem, R.M.; El Maraghy, M.O.; Amin, G.E. The validity of geriatric nutrition risk index: Simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with mini nutritional assessment. Clin. Nutr. 2014, 33, 1108–1116. [Google Scholar] [CrossRef]
- Hung, C.Y.; Hsueh, S.W.; Lu, C.H.; Chang, P.H.; Chen, P.T.; Yeh, K.Y.; Wang, H.M.; Tsang, N.M.; Huang, P.W.; Hung, Y.S.; et al. A prospective nutritional assessment using mini nutritional assessment-short form among patients with head and neck cancer receiving concurrent chemoradiotherapy. Support. Care Cancer 2021, 29, 1509–1518. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, M.; McKoy, J.M.; Bhulani, N.N.A.; Valero, V.; Barcenas, C.H.; Popat, U.R.; Sri, M.K.; Shah, J.B.; Dinney, C.P.; et al. Malnutrition in older patients with cancer: Appraisal of the mini nutritional assessment, weight loss, and body mass index. J. Geriatr. Oncol. 2018, 9, 81–83. [Google Scholar] [CrossRef]
- Stover, A.M.; Kurtzman, R.; Walker Bissram, J.; Jansen, J.; Carr, P.; Atkinson, T.; Ellis, C.T.; Freeman, A.T.; Turner, K.; Basch, E.M. Stakeholder perceptions of key aspects of high-quality cancer care to assess with patient reported outcome measures: A systematic review. Cancers 2021, 13, 3628. [Google Scholar] [CrossRef]
- Basch, E.; Becker, C.; Rogak, L.J.; Schrag, D.; Reeve, B.B.; Spears, P.; Smith, M.L.; Gounder, M.M.; Mahoney, M.R.; Schwartz, G.K.; et al. Composite grading algorithm for the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Clin. Trials 2021, 18, 104–114. [Google Scholar] [CrossRef]
- Denis, F.; Krakowski, I. How should oncologists choose an electronic patient-reported outcome system for remote monitoring of patients with cancer? J. Med. Internet Res. 2021, 23, e30549. [Google Scholar] [CrossRef]
- Meryk, A.; Kropshofer, G.; Hetzer, B.; Riedl, D.; Lehmann, J.; Rumpold, G.; Haid, A.; Holzner, B.; Crazzolara, R. Implementation of daily patient-reported outcome measurements to support children with cancer. Pediatr. Blood Cancer 2021, 68, e29279. [Google Scholar] [CrossRef]
- Peltola, M.K.; Poikonen-Saksela, P.; Mattson, J.; Parkkari, T. A novel digital patient-reported outcome platform (Noona) for clinical use in patients with cancer: Pilot study assessing suitability. JMIR Form. Res. 2021, 5, e16156. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.L.; Kim, H.; Soto-Perez-de-Celis, E.; Chung, V.; Koczywas, M.; Fakih, M.; Chao, J.; Cabrera Chien, L.; Charles, K.; et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer: A randomized clinical trial. JAMA Oncol. 2021, 7, e214158. [Google Scholar] [CrossRef]
- Ando, Y.; Hayashi, T.; Shiouchi, H.; Tanaka, C.; Ito, K.; Nishibe, S.; Miyata, N.; Horiba, R.; Yanagi, H.; Fujii, T.; et al. Effect of obesity on hematotoxicity induced by carboplatin and paclitaxel combination therapy in patients with gynecological cancer. Biol. Pharm. Bull. 2020, 43, 669–674. [Google Scholar] [CrossRef]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef]
- Barone, I.; Caruso, A.; Gelsomino, L.; Giordano, C.; Bonofiglio, D.; Catalano, S.; Ando, S. Obesity and endocrine therapy resistance in breast cancer: Mechanistic insights and perspectives. Obes. Rev. 2021, e13358. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef]
- Incio, J.; Ligibel, J.A.; McManus, D.T.; Suboj, P.; Jung, K.; Kawaguchi, K.; Pinter, M.; Babykutty, S.; Chin, S.M.; Vardam, T.D.; et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci. Transl. Med. 2018, 10, eaag0945. [Google Scholar] [CrossRef]
- Koneru, H.; Cyr, R.; Feng, L.R.; Bae, E.; Danner, M.T.; Ayoob, M.; Yung, T.M.; Lei, S.; Collins, B.T.; Saligan, L.; et al. The impact of obesity on patient reported outcomes following stereotactic body radiation therapy for prostate cancer. Cureus 2016, 8, e669. [Google Scholar] [CrossRef]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, R.; Mandal, C.C. Anti-obesity medications in cancer therapy: A comprehensive insight. Curr. Cancer Drug Targets 2021, 21, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Sirin, O.; Kolonin, M.G. Treatment of obesity as a potential complementary approach to cancer therapy. Drug Discov. Today 2013, 18, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Pan, K.; Mortimer, J.; Chlebowski, R.T.; Luo, J.; Yan, J.E.; Yost, S.E.; Kroenke, C.H.; Adams-Campbell, L.; Nassir, R.; et al. Metabolic syndrome risk components and mortality after triple-negative breast cancer diagnosis in postmenopausal women in the Women’s Health Initiative. Cancer 2021, 127, 1658–1667. [Google Scholar] [CrossRef]
- Quagliariello, V.; Bonelli, A.; Caronna, A.; Conforti, G.; Iovine, M.; Carbone, A.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 infection and cardioncology: From cardiometabolic risk factors to outcomes in cancer patients. Cancers 2020, 12, 3316. [Google Scholar] [CrossRef]
- Gardiner, J.; Oben, J.; Sutcliffe, A. Obesity as a driver of international differences in COVID-19 death rates. Diabetes Obes. Metab. 2021, 23, 1463–1470. [Google Scholar] [CrossRef]
- Pena, J.E.; Rascon-Pacheco, R.A.; Ascencio-Montiel, I.J.; Gonzalez-Figueroa, E.; Fernandez-Garate, J.E.; Medina-Gomez, O.S.; Borja-Bustamante, P.; Santillan-Oropeza, J.A.; Borja-Aburto, V.H. Hypertension, diabetes and obesity, major risk factors for death in patients with COVID-19 in Mexico. Arch. Med. Res. 2021, 52, 443–449. [Google Scholar] [CrossRef]
- Rottoli, M.; Bernante, P.; Belvedere, A.; Balsamo, F.; Garelli, S.; Giannella, M.; Cascavilla, A.; Tedeschi, S.; Ianniruberto, S.; Rosselli Del Turco, E.; et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur. J. Endocrinol. 2020, 183, 389–397. [Google Scholar] [CrossRef]
- Kiwata, J.L.; Dorff, T.B.; Schroeder, E.T.; Gross, M.E.; Dieli-Conwright, C.M. A review of clinical effects associated with metabolic syndrome and exercise in prostate cancer patients. Prostate Cancer Prostatic Dis. 2016, 19, 323–332. [Google Scholar] [CrossRef]
- Bandera, E.V.; Alfano, C.M.; Qin, B.; Kang, D.W.; Friel, C.P.; Dieli-Conwright, C.M. Harnessing nutrition and physical activity for breast cancer prevention and control to reduce racial/ethnic cancer health disparities. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, 1–17. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Norris, M.K.; Fox, F.S.; Buchanan, T.A.; Spicer, D.; Bernstein, L.; Tripathy, D. Aerobic and resistance exercise improve patient-reported sleep quality and is associated with cardiometabolic biomarkers in Hispanic and non-Hispanic breast cancer survivors who are overweight or obese: Results from a secondary analysis. Sleep 2021, 44, zsab111. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Sami, N.; Norris, M.K.; Wan, J.; Kumagai, H.; Kim, S.J.; Cohen, P. Effect of aerobic and resistance exercise on the mitochondrial peptide MOTS-c in Hispanic and Non-Hispanic White breast cancer survivors. Sci. Rep. 2021, 11, 16916. [Google Scholar] [CrossRef]
- Kiwata, J.L.; Dorff, T.B.; Todd Schroeder, E.; Salem, G.J.; Lane, C.J.; Rice, J.C.; Gross, M.E.; Dieli-Conwright, C.M. A pilot randomised controlled trial of a periodised resistance training and protein supplementation intervention in prostate cancer survivors on androgen deprivation therapy. BMJ Open 2017, 7, e016910. [Google Scholar] [CrossRef]
- Lee, K.; Kang, I.; Mack, W.J.; Mortimer, J.; Sattler, F.; Salem, G.; Dieli-Conwright, C.M. Effect of high intensity interval training on matrix metalloproteinases in women with breast cancer receiving anthracycline-based chemotherapy. Sci. Rep. 2020, 10, 5839. [Google Scholar] [CrossRef]
- Lee, K.; Sami, N.; Tripathy, D.; Demark-Wahnefried, W.; Norris, M.K.; Courneya, K.S.; Dieli-Conwright, C.M. Aerobic and resistance exercise improves Reynolds risk score in overweight or obese breast cancer survivors. Cardiooncology 2020, 6, 27. [Google Scholar] [CrossRef]
- Lee, K.; Zhou, J.; Norris, M.K.; Chow, C.; Dieli-Conwright, C.M. Prehabilitative exercise for the enhancement of physical, psychosocial, and biological outcomes among patients diagnosed with cancer. Curr. Oncol. Rep. 2020, 22, 71. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Waliany, S.; Dieli-Conwright, C.M.; Patel, S.K.; Hurria, A.; Chao, J.; Tiep, B.; Behrendt, C.E. Objective physical and mental markers of self-reported fatigue in women undergoing (neo)adjuvant chemotherapy for early-stage breast cancer. Cancer 2017, 123, 1810–1816. [Google Scholar] [CrossRef]
- De Oliveira Faria, S.; Hurwitz, G.; Kim, J.; Liberty, J.; Orchard, K.; Liu, G.; Barbera, L.; Howell, D. Identifying patient-reported outcome measures (PROMs) for routine surveillance of physical and emotional symptoms in head and neck cancer populations: A systematic review. J. Clin. Med. 2021, 10, 4162. [Google Scholar] [CrossRef]
- Efficace, F.; Cella, D.; Aaronson, N.K.; Calvert, M.; Cottone, F.; Di Maio, M.; Perrone, F.; Sparano, F.; Gamper, E.M.; Vignetti, M.; et al. Impact of blinding on patient-reported outcome differences between treatment arms in cancer randomized controlled trials. J. Natl. Cancer Inst. 2021, 44, zsab111. [Google Scholar] [CrossRef]
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M.; et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef]
- Vyas, A.; Alghaith, G.; Hufstader-Gabriel, M. Psychotropic polypharmacy and its association with health-related quality of life among cancer survivors in the USA: A population-level analysis. Qual. Life Res. 2020, 29, 2029–2037. [Google Scholar] [CrossRef]
| Oncology Professionals | Others in the Clinic and Hospital | Community Resources |
|---|---|---|
| Medical Oncologist | Dietitian (nutritionist) | Primary caregiver |
| Pediatric Oncologist | Social worker | Other family |
| Radiation Oncologist | Psychological support (coping skills) | Friends |
| Oncology Surgeon | Physical therapy (PT) | Peer support (disease-specific groups) |
| Nurse Educator | Occupational therapy (OT) | Facebook and other internet sites |
| Oncology Pharmacist | Art therapy | On-line consults |
| Oncology Navigator | Music therapy | Insurance case manager |
| Oncology Nurse Practioner | Scheduling | Faith community |
| Oncology Physician Assistant | Lab (e.g., phlebotomy) personnel | School support |
| Chemotherapy Nurse Virtual Oncologist [52] | Radiology personnel Quality teams Child life specialists | Employee support Neighbors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, P.M.; Thomas, S.M.; Sartoski, S.; Scott, J.G.; Sobilo, K.; Bewley, S.; Salvador, L.K.; Salazar-Abshire, M. Strategies to Mitigate Chemotherapy and Radiation Toxicities That Affect Eating. Nutrients 2021, 13, 4397. https://doi.org/10.3390/nu13124397
Anderson PM, Thomas SM, Sartoski S, Scott JG, Sobilo K, Bewley S, Salvador LK, Salazar-Abshire M. Strategies to Mitigate Chemotherapy and Radiation Toxicities That Affect Eating. Nutrients. 2021; 13(12):4397. https://doi.org/10.3390/nu13124397
Chicago/Turabian StyleAnderson, Peter M., Stefanie M. Thomas, Shauna Sartoski, Jacob G. Scott, Kaitlin Sobilo, Sara Bewley, Laura K. Salvador, and Maritza Salazar-Abshire. 2021. "Strategies to Mitigate Chemotherapy and Radiation Toxicities That Affect Eating" Nutrients 13, no. 12: 4397. https://doi.org/10.3390/nu13124397
APA StyleAnderson, P. M., Thomas, S. M., Sartoski, S., Scott, J. G., Sobilo, K., Bewley, S., Salvador, L. K., & Salazar-Abshire, M. (2021). Strategies to Mitigate Chemotherapy and Radiation Toxicities That Affect Eating. Nutrients, 13(12), 4397. https://doi.org/10.3390/nu13124397






