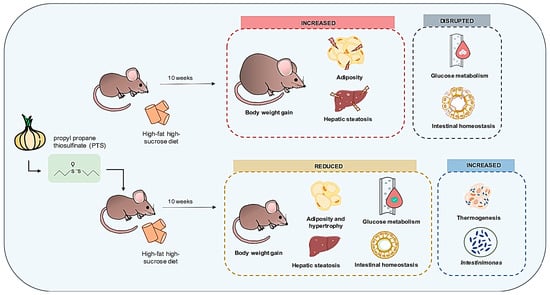
The Allium Derivate Propyl Propane Thiosulfinate Exerts Anti-Obesogenic Effects in a Murine Model of Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Treatments
2.2. Oral Glucose Tolerance Test
2.3. Intestinal Permeability
2.4. Biochemical Analyses
2.5. Immune Parameters
2.6. Gene Expression Analyses
2.7. Histological Analysis
2.8. Gut Microbiota Composition
2.9. Statistical Analyses
3. Results
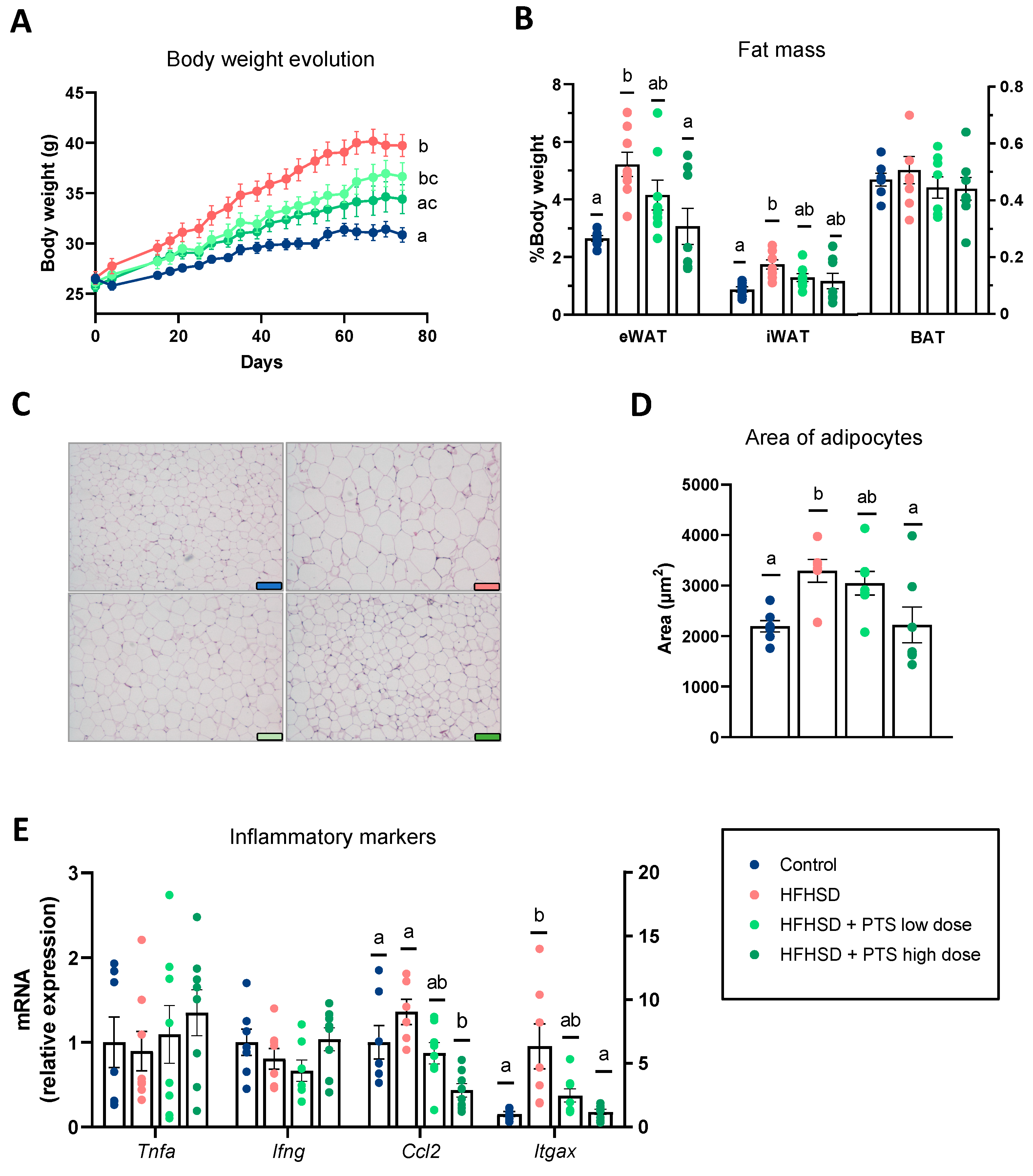
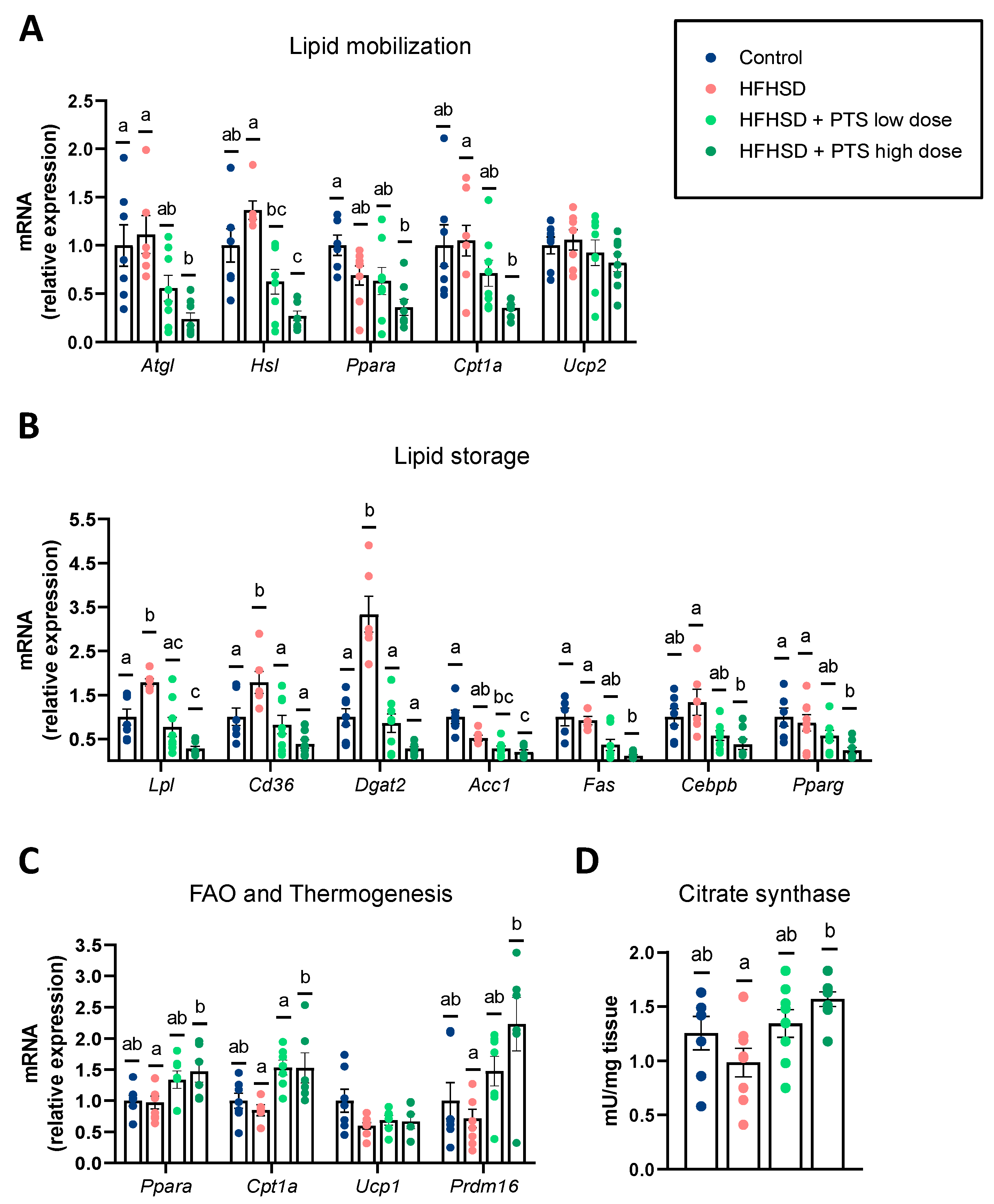
3.1. PTS Curbed Obesity by Influencing the White and Brown Adipose Tissue Metabolism in a Dose-Dependent Manner
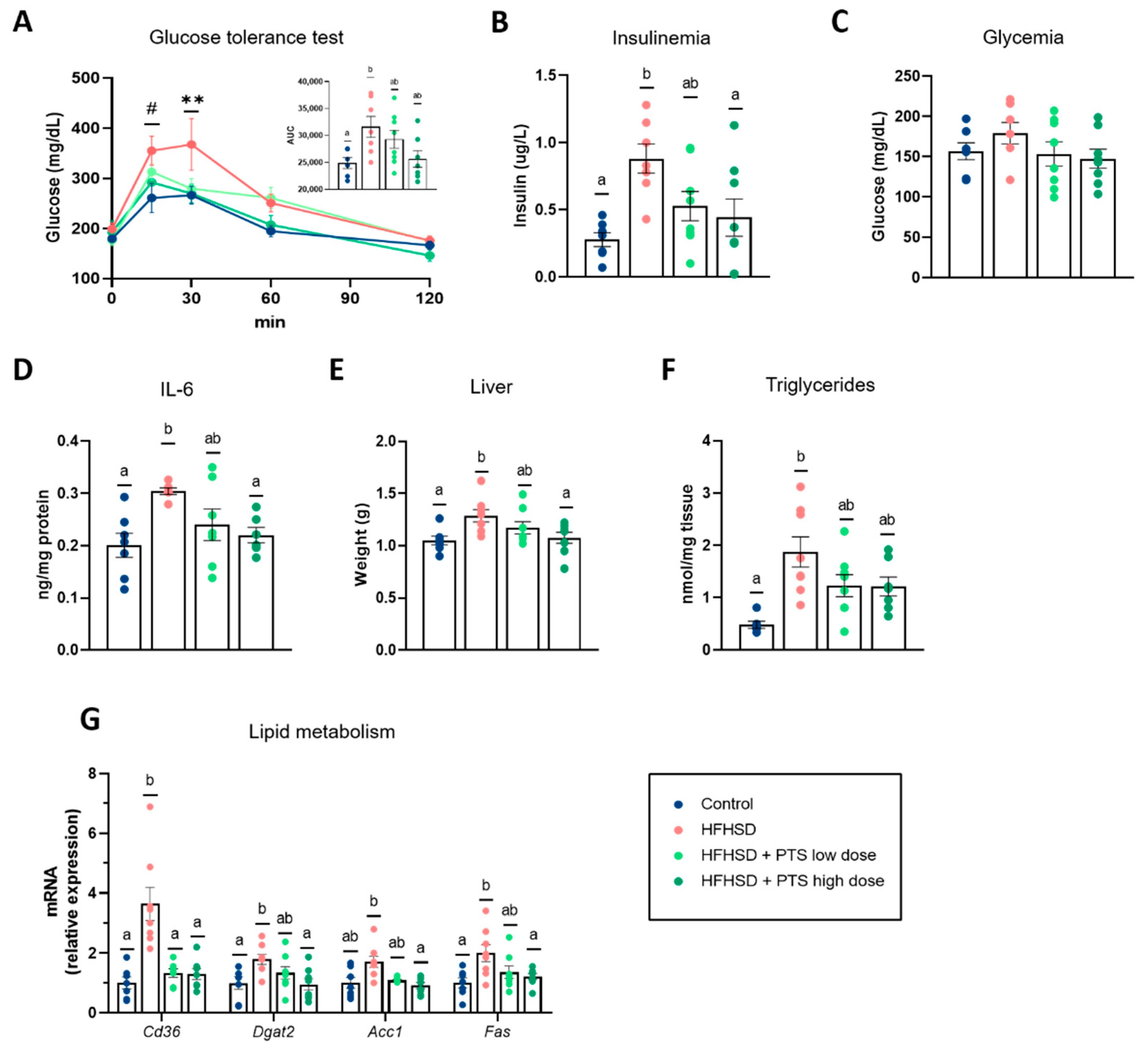
3.2. PTS Improved Systemic Glucose Homeostasis, Hepatic Metabolism and Inflammatory Response
3.3. PTS Had Minor Effects on the Intestinal Permeability and the Gut Barrier Function
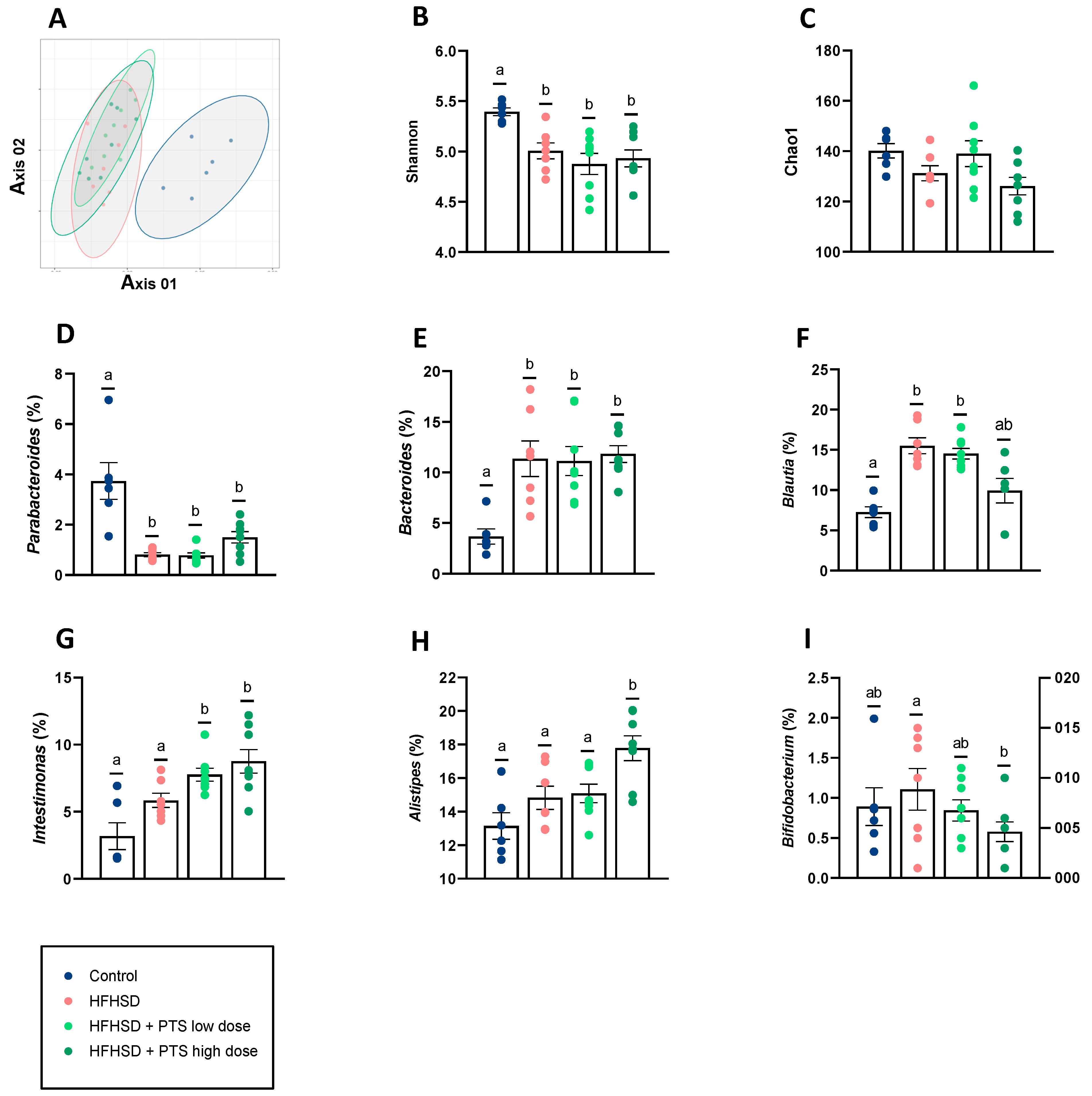
3.4. PTS Caused No Differences in Gut Microbiota Diversity and Only Minor Taxonomic Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity n.d. Available online: https://www.who.int/westernpacific/health-topics/obesity (accessed on 26 April 2021).
- Goodarzi, M.O. Genetics of obesity: What genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef]
- Rohde, K.; Keller, M.; la Cour Poulsen, L.; Blüher, M.; Kovacs, P.; Böttcher, Y. Genetics and epigenetics in obesity. Metabolism 2019, 92, 37–50. [Google Scholar] [CrossRef] [Green Version]
- TLG & Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Dixit, K.; Kamath, D.V.; Alluri, K.V.; Davis, B.A. Efficacy of a novel herbal formulation for weight loss demonstrated in a 16-week randomized, double-blind, placebo-controlled clinical trial with healthy overweight adults. Diabetes Obes. Metab. 2018, 20, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, A.; Daguet, D.; Calame, W.; Dhoye, P.; Kodimule, S.P. Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2019, 11, 1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Luck, H.; Khan, S.; Schneeberger, P.H.H.; Tsai, S.; Clemente-Casares, X.; Lei, H.; Leu, Y.L.; Chan, Y.T.; Chen, H.-Y.; et al. Aryl hydrocarbon receptor agonist indigo protects against obesity-related insulin resistance through modulation of intestinal and metabolic tissue immunity. Int. J. Obes. 2019, 43, 2407–2421. [Google Scholar] [CrossRef] [Green Version]
- Régnier, M.; Rastelli, M.; Morissette, A.; Suriano, F.; Le Roy, T.; Pilon, G.; Delzenne, N.M.; Marette, A.; Van Hul, M.; Cani, P.D. Rhubarb Supplementation Prevents Diet-Induced Obesity and Diabetes in Association with Increased Akkermansia muciniphila in Mice. Nutrients 2020, 12, 2932. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Subramanian, M.S.; Nandagopal, M.S.G.; Amin Nordin, S.; Thilakavathy, K.; Joseph, N. Prevailing Knowledge on the Bioavailability and Biological Activities of Sulphur Compounds from Alliums: A Potential Drug Candidate. Molecules 2020, 25, 4111. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Sheng, Y.; Yang, C.; Xu, J.; Zheng, S.; Liu, J.; Xu, W.; Luo, Y.; Huang, K. Allicin-induced host-gut microbe interactions improves energy homeostasis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 10682–10698. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients 2019, 11, 2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Baños, A.; Guillamon, E.; Garcia, F.; Rodriguez-Nogales, A.; Galvez, J. The Immunomodulatory Properties of Propyl-Propane Thiosulfonate Contribute to its Intestinal Anti-Inflammatory Effect in Experimental Colitis. Mol. Nutr Food Res. 2019, 63, 1800653. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Garrido-Mesa, J.; Diez-Echave, P.; Hidalgo-García, L.; Ruiz-Malagón, A.J.; García, F.; Sánchez, M.; Toral, M.; Romero, M.; Duarte, J.; et al. Allium-Derived Compound Propyl Propane Thiosulfonate (PTSO) Attenuates Metabolic Alterations in Mice Fed a High-Fat Diet through Its Anti-Inflammatory and Prebiotic Properties. Nutrients 2021, 13, 2595. [Google Scholar] [CrossRef] [PubMed]
- Rabelo-Ruiz, M.; Teso-Pérez, C.; Peralta-Sánchez, J.M.; Ariza, J.J.; Martín-Platero, A.M.; Casabuena-Rincón, Ó.; Rabelo-Ruiz, M.; Teso-Pérez, C.; Peralta-Sánchez, J.M.; Ariza, J.J.; et al. Allium Extract Implements Weaned Piglet’s Productive Parameters by Modulating Distal Gut Microbiota. Antibiotics 2021, 10, 269. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, F. In Vitro Antibacterial Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate Derived from Allium spp. against Gram-Negative and Gram-Positive Multidrug-Resistant Bacteria Isolated from Human Samples. BioMed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2013, 109, 76–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascajosa-Lira, A.; Puerto, M.; Prieto, A.I.; Pichardo, S.; Díez-Quijada Jiménez, L.; Baños, A.; Guillamón, E.; Moyano, R.; Molina-Hernández, V.; Jos, A.; et al. Genotoxicity Evaluation of Propyl-Propane-Thiosulfinate (PTS) from Allium genus Essential Oils by a Combination of Micronucleus and Comet Assays in Rats. Foods 2021, 10, 989. [Google Scholar] [CrossRef]
- Olivares, M.; Rodriguez, J.; Pötgens, S.A.; Neyrinck, A.M.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. The Janus Face of Cereals: Wheat-Derived Prebiotics Counteract the Detrimental Effect of Gluten on Metabolic Homeostasis in Mice Fed a High-Fat/High-Sucrose Diet. Mol. Nutr. Food Res. 2019, 63, e1900632. [Google Scholar] [CrossRef] [Green Version]
- López-Almela, I.; Romaní-Pérez, M.; Bullich-Vilarrubias, C.; Benítez-Páez, A.; Pulgar, E.M.G.D.; Francés, R. Bacteroides uniformis combined with fiber amplifies metabolic and immune benefits in obese mice. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Yi, T.; Lu, T.T.; Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 2020, 217, e20192195. [Google Scholar] [CrossRef] [PubMed]
- Darooghegi Mofrad, M.; Milajerdi, A.; Koohdani, F.; Surkan, P.J.; Azadbakht, L. Garlic Supplementation Reduces Circulating C-reactive Protein, Tumor Necrosis Factor, and Interleukin-6 in Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Nutr. 2019, 149, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Liébana-García, R.; Olivares, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Romaní-Pérez, M.; Sanz, Y. The gut microbiota as a versatile immunomodulator in obesity and associated metabolic disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101542. [Google Scholar] [CrossRef]
- Suriano, F.; Neyrinck, A.; Verspreet, J.; Olivares, M.; Leclercq, S.; Van de Wiele, T.; Courtin, C.M.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Particle size determines the anti-inflammatory effect of wheat bran in a model of fructose over-consumption: Implication of the gut microbiota. J. Funct. Foods 2018, 41, 155–162. [Google Scholar] [CrossRef]
- Xu, E.; Pereira, M.M.A.; Karakasilioti, I.; Theurich, S.; Al-Maarri, M.; Rappl, G.; Waisman, A.; Wunderlich, F.T.; Brüning, J.C. Temporal and tissue-specific requirements for T-lymphocyte IL-6 signalling in obesity-associated inflammation and insulin resistance. Nat. Commun. 2017, 8, 14803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- König, M.; Bulik, S.; Holzhütter, H.-G. Quantifying the Contribution of the Liver to Glucose Homeostasis: A Detailed Kinetic Model of Human Hepatic Glucose Metabolism. PLoS Comput. Biol. 2012, 8, e1002577. [Google Scholar] [CrossRef] [Green Version]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongiovanni, P.; Stender, S.; Pietrelli, A.; Mancina, R.M.; Cespiati, A.; Petta, S.; Pelusi, S.; Pingitore, P.; Badiali, S.; Maggioni, M.; et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J. Intern. Med. 2018, 283, 356–370. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Poher, A.-L.; Altirriba, J.; Veyrat-Durebex, C.; Rohner-Jeanrenaud, F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front. Physiol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvester, A.J.; Aseer, K.R.; Yun, J.W. Dietary polyphenols and their roles in fat browning. J. Nutr. Biochem. 2019, 64, 1–12. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P.; et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 2020, 12, eaaz8664. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Roos, J.; et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef]
- Dixon, B.R.E.A.; Radin, J.N.; Piazuelo, M.B.; Contreras, D.C.; Algood, H.M.S. IL-17a and IL-22 Induce Expression of Antimicrobials in Gastrointestinal Epithelial Cells and May Contribute to Epithelial Cell Defense against Helicobacter pylori. PLoS ONE 2016, 11, e0148514. [Google Scholar] [CrossRef] [PubMed]
- Leyrolle, Q.; Cserjesi, R.; Mulders, M.D.G.H.; Zamariola, G.; Hiel, S.; Gianfrancesco, M.A.; Rodriguez, J.; Portheaulte, D.; Amadieua, C.; Leclercq, S.; et al. Specific gut microbial, biological, and psychiatric profiling related to binge eating disorders: A cross-sectional study in obese patients. Clin. Nutr. 2021, 40, 2035–2044. [Google Scholar] [CrossRef]
- Ruiz, R.; García, M.P.; Lara, A.; Rubio, L.A. Garlic derivatives (PTS and PTS-O) differently affect the ecology of swine faecal microbiota in vitro. Vet. Microbiol. 2010, 144, 110–117. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liébana-García, R.; Olivares, M.; Rodríguez-Ruano, S.M.; Tolosa-Enguís, V.; Chulia, I.; Gil-Martínez, L.; Guillamón, E.; Baños, A.; Sanz, Y. The Allium Derivate Propyl Propane Thiosulfinate Exerts Anti-Obesogenic Effects in a Murine Model of Diet-Induced Obesity. Nutrients 2022, 14, 440. https://doi.org/10.3390/nu14030440
Liébana-García R, Olivares M, Rodríguez-Ruano SM, Tolosa-Enguís V, Chulia I, Gil-Martínez L, Guillamón E, Baños A, Sanz Y. The Allium Derivate Propyl Propane Thiosulfinate Exerts Anti-Obesogenic Effects in a Murine Model of Diet-Induced Obesity. Nutrients. 2022; 14(3):440. https://doi.org/10.3390/nu14030440
Chicago/Turabian StyleLiébana-García, Rebeca, Marta Olivares, Sonia M. Rodríguez-Ruano, Verónica Tolosa-Enguís, Isabel Chulia, Lidia Gil-Martínez, Enrique Guillamón, Alberto Baños, and Yolanda Sanz. 2022. "The Allium Derivate Propyl Propane Thiosulfinate Exerts Anti-Obesogenic Effects in a Murine Model of Diet-Induced Obesity" Nutrients 14, no. 3: 440. https://doi.org/10.3390/nu14030440
APA StyleLiébana-García, R., Olivares, M., Rodríguez-Ruano, S. M., Tolosa-Enguís, V., Chulia, I., Gil-Martínez, L., Guillamón, E., Baños, A., & Sanz, Y. (2022). The Allium Derivate Propyl Propane Thiosulfinate Exerts Anti-Obesogenic Effects in a Murine Model of Diet-Induced Obesity. Nutrients, 14(3), 440. https://doi.org/10.3390/nu14030440