Feasibility and Acceptability of a Dietary Intervention to Reduce Salt Intake and Increase High-Nitrate Vegetable Consumption in Malaysian Middle-Aged and Older Adults with Elevated Blood Pressure: Findings from the DePEC-Nutrition Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Recruitment
2.3. Dietary Interventions
- Group nutritional counselling was delivered during the baseline clinic visit by a trained medical officer using PowerPoint slides and practical activities. Each session lasted between 1–1.5 hours. The topics addressed at the counselling session included health benefits, sources, recommended intakes and practical tips related to the allocated intervention of increasing dietary nitrate and/or reducing salt intake. Participants in the low salt group were advised to consume less than 5 g of salt in accordance with the recommendations from the World Health Organization (WHO) [50]. Advice was delivered on how to reduce salt intake during home cooking, eating out and shopping for food (reading food labels). Participants in the high-nitrate groups were recommended to consume vegetables rich in dietary nitrate at least three times per week or more to achieve an intake of approximately 1000–1500 mg of dietary nitrate per week. A list of commonly consumed nitrate-rich vegetables (i.e., broccoli, cabbage, spinach, cauliflower, lettuce and eggplant) was provided in the information booklet, along with the recommended portion size. In addition, practical tips for increasing dietary nitrate intake and retaining the nitrate content in the vegetables when cooking were also provided.
- Information booklets were provided during the group counselling sessions. In addition to the detailed information discussed during the sessions, the booklet contained recipes of low salt and/or high-nitrate meals that participants could prepare at home. The booklet also provided several sources and links for further information.
- A salt measuring spoon (Atila GmbH, Neidenstein, Germany, see the picture in the Online Supplementary Material) was provided to the participants who were randomised into the salt interventions (low salt and combined high-nitrate vegetable plus low salt consumption groups) to support them in understanding portion sizes and measuring salt intake. The measuring spoon had a dual side with nine adjustable scales (0.5, 1, 2, 3.5, 5, 7, 9, 11 and 13 g). Participants were taught how to use the spoon during the group counselling sessions.
- Biweekly text messages were sent to all participants in the intervention groups. The text messages included educational messages and reminders of the key dietary behaviour changes to encourage adherence.
- Reinforcement video messages were delivered to the participants in the intervention groups at the interim visits to remind participants of the key dietary advice that was discussed during the baseline counselling sessions. The reinforcement videos were hosted on the YouTube platform and only viewable to those who had access to the video link. Video clip links were sent to the participants via WhatsApp (WhatsApp Inc., Mountain View, CA, USA).
2.4. Randomisation
2.5. Study Procedures
2.6. Outcomes
2.7. Protocol Changes Implemented during the COVID-19 Outbreak
- Methodology of data collectionThe data collection at interim 1, interim 2 and the end of study visits was initially planned to be conducted in person at the participant’s home. However, due to the COVID-19 outbreak, the need for social distancing and the movement restriction imposed by the Malaysian government, data collection by home visit was not possible. Hence, to continue the study during the COVID-19 pandemic, the data collection at interim 1, 2 and the end of study visits were conducted via telephone interview. The collection of information related to the primary outcomes of evaluating the acceptability and feasibility of the intervention was prioritised. The measurement of the secondary outcomes (i.e., physical assessment and biological samples) was not performed.
- TimeframeInitially, the planned duration of the DePEC-Nutrition feasibility study was six months, with interim 1 in the second month and interim 2 in the fourth month after the baseline. The shift from face-to-face data collection to telephone-based data collection required an extension of the study in order to implement the necessary changes to the protocol, including (1) obtaining approval from the ethics committee, SEACO and Monash University Malaysia on the amendment of the study protocol, (2) the development of the telephone-based data collection protocol, including the adaptation of the questionnaires, and (3) the re-training of the data collectors. Therefore, the total duration of the study was extended to 10 months and an additional interim 3 was added at month eight.
- Intervention deliveryThe reinforcement was originally planned to be delivered by the data collectors during the interim 1 and 2 visits. Due to the COVID-19 outbreak and the need for social distancing, video reinforcements were instead sent to each participant through WhatsApp. Participants without a mobile phone number were informed of the video content by the data collectors or had their close relative receive the video message on their behalf. In addition, the videos were also copied onto a compact disc and posted to participants who did not have access to a mobile phone. The reinforcement video included a comprehensive review of the key dietary messages delivered through nutritional counselling at the start of the study and provided specific information on adapting and making dietary changes during the pandemic.
2.8. Sample Size
2.9. Data Presentation and Statistical Analysis
3. Results
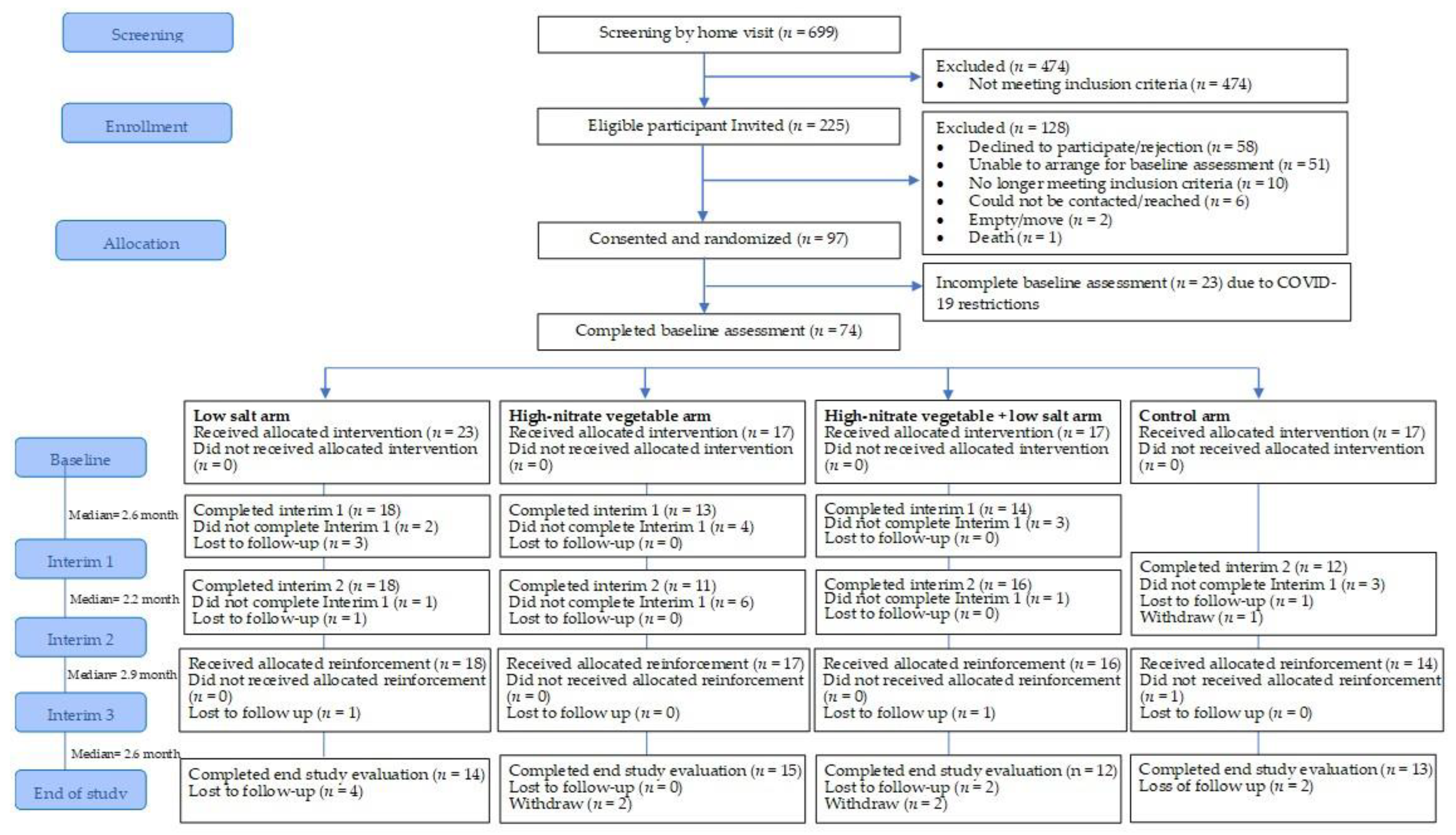
3.1. Recruitment, Follow-Up Response and Retention
3.1.1. Recruitment
3.1.2. Follow-Up Response Rate and Retention
3.1.3. Characteristics of Study Participants
3.2. Suitability and Acceptability of Data Collection Procedures and Outcomes Measures
3.2.1. Study Visits
3.2.2. Questionnaire
“Phone interviews were quite challenging when the respondent can’t imagine or even understand the score and questions given as the choice of the answer was confusing”.(Data collector 3)
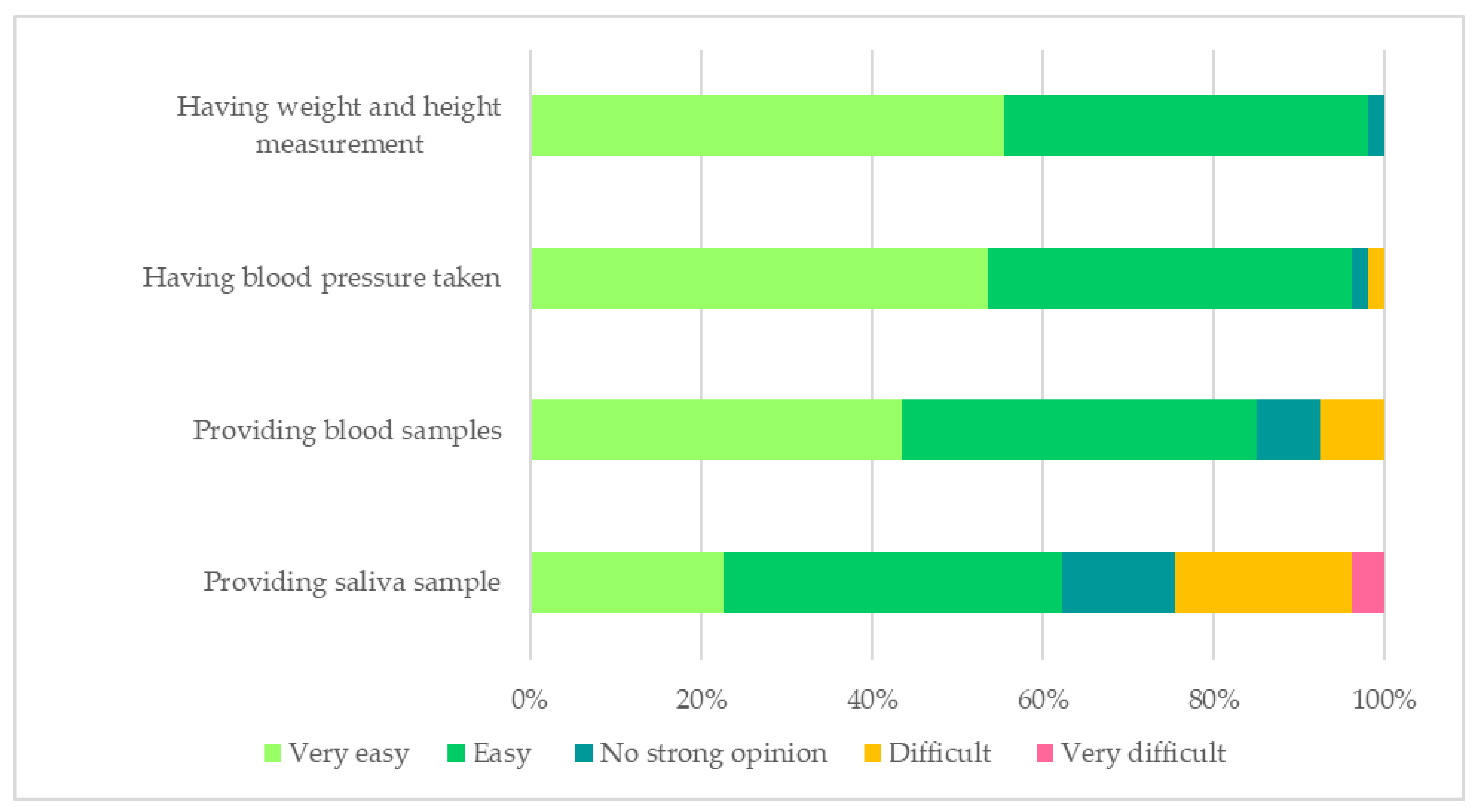
3.2.3. Biological Sample Collection and Physical Measurements
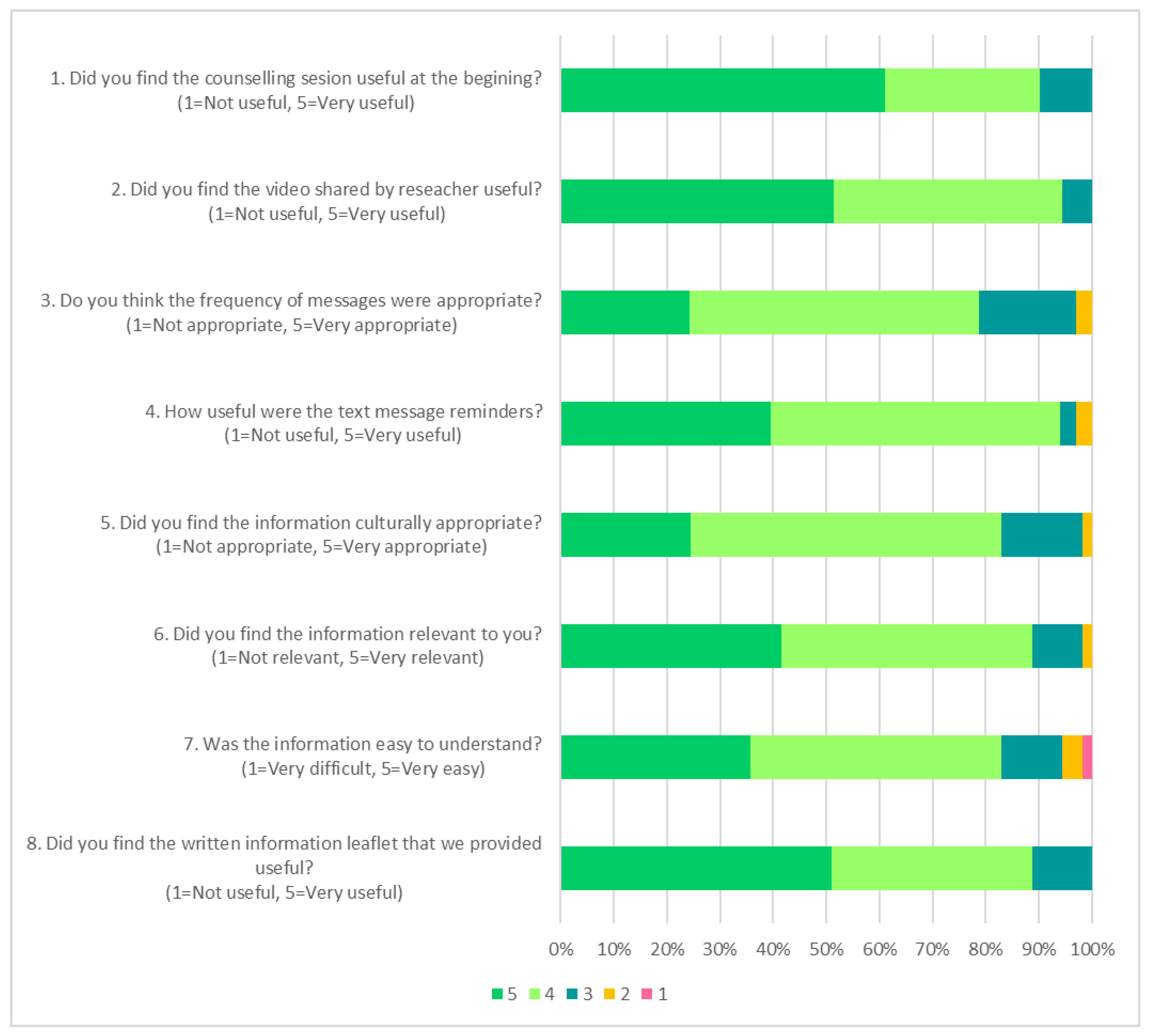
3.3. Feasibility and Acceptability of Various Nutrition Education Strategies
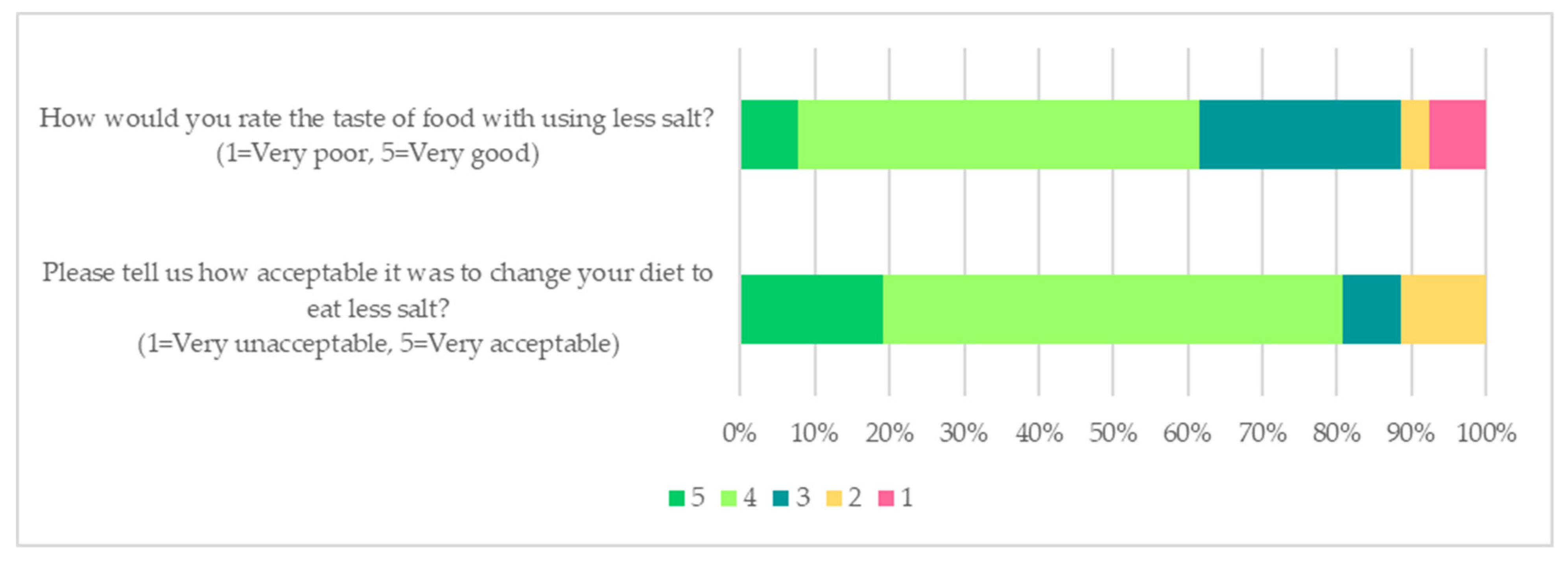
3.4. Acceptability of Low Salt and High-Nitrate Vegetable Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Inclusion criteria |
|
| Exclusion criteria |
|
| Outcome Measures | Description of Measures Used |
|---|---|
| Primary outcomes | |
| Screening, recruitment, follow-up rate and retention | Number screened: The number of people assessed for eligibility using inclusion/exclusion criteria. Eligibility rate: The number of people screened for eligibility by the number who met inclusion criteria. Reason for exclusion: Reason for ineligibility recorded by data collectors as in the field notes. Consented rate: The number of people who are eligible by the number who consented to participate in the study. Reason for declining: Reason provided by participants who declined to participate was recorded by data collectors in the field notes. |
| Follow-up rate: The number of participants who were assessed during interim follow-ups. Retention rate: The number of participants who remained in the study. Reason for dropped out: Reason provided by participants for withdrawing recorded by data collectors in the field notes. Motivation to participation: Reason provided by participants for participation in the study during the end of study evaluation. | |
| Suitability and acceptability of data collection procedures and outcomes measures | Self-rating appropriateness and suitability of intervention timing and location Feedback from data collectors on home visits and clinic visits Missing data from questionnaires: Number of participants with invalid data for specific instruments. Number of participants provided biological sample and physical measurements (blood pressure and anthropometric measurement) Participants’ self-rating acceptability of providing biological samples and physical measurement Data collectors’ feedback on the acceptability of collecting biological samples and physical measurement |
| Feasibility and acceptability of the intervention strategies | Engagement with the intervention: Meaningful engagement was determined by whether the information booklet provided was read by the participants. For the text messages and reinforcement video, engagement was determined by whether the participants opened, read and responded to the text messages and video. Participants self-rating of usefulness of interventions strategies or materials |
| Low salt and nitrate intervention | Participant self-rated acceptability of low salt diet Participant self-reported use of salt measuring spoon and easiness of use Number of participants interviewed who will continue to follow the recommended diet Number of participants interviewed who will recommend participation in a similar study to their family and friends |
| Outcomes for a definitive trial * | |
| Demographic and medical history | Demographic, medical and medication history |
| Health and lifestyle | Global Physical Activity Questionnaire (GPAQ), Global Activity Limitation Indicator (GALI), smoking and alcohol use, Geriatric Depression Scale (GDS) (short form) |
| Cognitive assessment | Montreal Cognitive Assessment (MoCA) test, Auditory Verbal Learning Test (AVLT), Trail Making Test Part B, Animal Naming Test |
| Blood pressure | Three consecutive measurements of resting blood pressure readings in a sitting position using OMRON automated monitor (OMRON HEM 907, OMRON Healthcare, Milton Keynes, UK) |
| Anthropometry and body composition | Height (m), weight (kg), BMI (kg/m2) and body composition were measured using a portable bioelectrical impedance analysis (BIA) scale (Tanita DC-430MA Body Composition Analyzer, Tanita Corporation, Japan) with a 0.1 kg precision |
| Functional performance | Hand grip strength dynamometer, 4-metre gait speed test, Timed Up and Go (TUG) test |
| Dietary assessment | Food Frequency Questionnaire, 24-h diet recall |
| Biological sample collection | 15 mL whole venous blood, 24-h urine sample, spot urine sample, whole saliva collected using passive drool technique, salivary strips and dried blood spot |
References
- Robinson, L.; Tang, E.; Taylor, J.P. Dementia: Timely diagnosis and early intervention. BMJ (Clin. Res. Ed.) 2015, 350, h3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, L.; Sikkes, S.A.M.; van den Hout, A.; Handels, R.; Bos, I.; van der Flier, W.M.; Kern, S.; Ousset, P.-J.; Maruff, P.; Skoog, I.; et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimer’s Dement. 2019, 15, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C. Wolrd Alzheimer Report 2018; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Grande, G.; Qiu, C.; Fratiglioni, L. Prevention of dementia in an ageing world: Evidence and biological rationale. Ageing Res. Rev. 2020, 64, 101045. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- McGrath, E.R.; Beiser, A.S.; DeCarli, C.; Plourde, K.L.; Vasan, R.S.; Greenberg, S.M.; Seshadri, S. Blood pressure from mid- to late life and risk of incident dementia. Neurology 2017, 89, 2447–2454. [Google Scholar] [CrossRef]
- Pase, M.P.; Beiser, A.; Enserro, D.; Xanthakis, V.; Aparicio, H.; Satizabal, C.L.; Himali, J.J.; Kase, C.S.; Vasan, R.S.; DeCarli, C.; et al. Association of Ideal Cardiovascular Health with Vascular Brain Injury and Incident Dementia. Stroke 2016, 47, 1201–1206. [Google Scholar] [CrossRef] [Green Version]
- Abell, J.G.; Kivimäki, M.; Dugravot, A.; Tabak, A.G.; Fayosse, A.; Shipley, M.; Sabia, S.; Singh-Manoux, A. Association between systolic blood pressure and dementia in the Whitehall II cohort study: Role of age, duration, and threshold used to define hypertension. Eur. Heart J. 2018, 39, 3119–3125. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Bundy, J.D.; Li, C.; Stuchlik, P.; Bu, X.; Kelly, T.N.; Mills, K.T.; He, H.; Chen, J.; Whelton, P.K.; He, J. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017, 2, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.J.; Blumenthal, J.A.; Babyak, M.A.; Craighead, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Strauman, T.A.; Sherwood, A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 2010, 55, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Smith, P.J.; Mabe, S.; Hinderliter, A.; Lin, P.H.; Liao, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Kraus, W.E.; Doraiswamy, P.M.; et al. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology 2019, 92, e212–e223. [Google Scholar] [CrossRef] [Green Version]
- Williamson, J.D.; Pajewski, N.M.; Auchus, A.P.; Bryan, R.N.; Chelune, G.; Cheung, A.K.; Cleveland, M.L.; Coker, L.H.; Crowe, M.G.; Cushman, W.C.; et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019, 321, 553–561. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Siervo, M.; Shannon, O.M.; Llewellyn, D.J.; Stephan, B.C.; Fontana, L. Mediterranean diet and cognitive function: From methodology to mechanisms of action. Free Radic. Biol. Med. 2021, 176, 105–117. [Google Scholar] [CrossRef]
- Duplantier, S.C.; Gardner, C.D. A Critical Review of the Study of Neuroprotective Diets to Reduce Cognitive Decline. Nutrients 2021, 13, 2264. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.C.; Feelisch, M.; Horowitz, J.D.; Frenneaux, M.P.; Madhani, M. Pharmacology and therapeutic role of inorganic nitrite and nitrate in vasodilatation. Pharmacol. Ther. 2014, 144, 303–320. [Google Scholar] [CrossRef] [Green Version]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [Green Version]
- Blekkenhorst, L.C.; Bondonno, C.P.; Lewis, J.R.; Devine, A.; Woodman, R.J.; Croft, K.D.; Lim, W.H.; Wong, G.; Beilin, L.J.; Prince, R.L.; et al. Association of dietary nitrate with atherosclerotic vascular disease mortality: A prospective cohort study of older adult women. Am. J. Clin. Nutr. 2017, 106, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Bonilla Ocampo, D.A.; Paipilla, A.F.; Marín, E.; Vargas-Molina, S.; Petro, J.L.; Pérez-Idárraga, A. Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules 2018, 8, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siervo, M.; Scialò, F.; Shannon, O.M.; Stephan, B.C.M.; Ashor, A.W. Does dietary nitrate say NO to cardiovascular ageing? Current evidence and implications for research. Proc. Nutr. Soc. 2018, 77, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, M.J.; Ble, A.; Melzer, D.; Winyard, P.G.; Benjamin, N.; Shore, A.C.; Gilchrist, M. Relationship Between Urinary Nitrate Excretion and Blood Pressure in the InChianti Cohort. Am. J. Hypertens. 2017, 30, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siervo, M.; Shannon, O.; Kandhari, N.; Prabhakar, M.; Fostier, W.; Köchl, C.; Rogathi, J.; Temu, G.; Stephan, B.C.M.; Gray, W.K.; et al. Nitrate-Rich Beetroot Juice Reduces Blood Pressure in Tanzanian Adults with Elevated Blood Pressure: A Double-Blind Randomized Controlled Feasibility Trial. J. Nutr. 2020, 150, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.; Mitchell, K.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. High-nitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public Health Nutr. 2015, 18, 2669–2678. [Google Scholar] [CrossRef] [Green Version]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Bondonno, C.P.; Croft, K.D.; Puddey, I.B.; Woodman, R.J.; Rich, L.; Ward, N.C.; Vita, J.A.; Hodgson, J.M. Effects of a nitrate-rich meal on arterial stiffness and blood pressure in healthy volunteers. Nitric Oxide Biol. Chem. 2013, 35, 123–130. [Google Scholar] [CrossRef]
- Jackson, J.K.; Patterson, A.J.; MacDonald-Wicks, L.K.; Oldmeadow, C.; McEvoy, M.A. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: A systematic review and meta-analysis of human evidence. Nutr. Rev. 2018, 76, 348–371. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Jones, D.A.; Rathod, K.; Primus, C.; Massimo, G.; Fukuto, J.M.; Ahluwalia, A. The Noncanonical Pathway for In Vivo Nitric Oxide Generation: The Nitrate-Nitrite-Nitric Oxide Pathway. Pharm. Rev 2020, 72, 692–766. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Babateen, A.; Shannon, O.M.; Capper, T.; Ashor, A.; Stephan, B.; Robinson, L.; O’Hara, J.P.; Mathers, J.C.; Stevenson, E.; et al. Effects of inorganic nitrate and nitrite consumption on cognitive function and cerebral blood flow: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Presley, T.D.; Morgan, A.R.; Bechtold, E.; Clodfelter, W.; Dove, R.W.; Jennings, J.M.; Kraft, R.A.; King, S.B.; Laurienti, P.J.; Rejeski, W.J.; et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide Biol. Chem. 2011, 24, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Thompson, K.G.; Blackwell, J.R.; Winyard, P.G.; Forster, J.; Jones, A.M.; Kennedy, D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015, 149, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [Green Version]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Ah, S.T.L.; Wei, L.; Diaz, R.; et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: A community-level prospective epidemiological cohort study. Lancet 2018, 392, 496–506. [Google Scholar] [CrossRef]
- He, F.J.; MacGregor, G.A. Role of salt intake in prevention of cardiovascular disease: Controversies and challenges. Nat. Rev. Cardiol. 2018, 15, 371–377. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013, 346, f1325. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.; Judge, C.; Murphy, R.; Loughlin, E.; Costello, M.; Whiteley, W.; Bosch, J.; O’Donnell, M.J.; Canavan, M. Association of Blood Pressure Lowering With Incident Dementia or Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA 2020, 323, 1934–1944. [Google Scholar] [CrossRef]
- Fiocco, A.J.; Shatenstein, B.; Ferland, G.; Payette, H.; Belleville, S.; Kergoat, M.J.; Morais, J.A.; Greenwood, C.E. Sodium intake and physical activity impact cognitive maintenance in older adults: The NuAge Study. Neurobiol. Aging 2012, 33, 829.e21–829.e28. [Google Scholar] [CrossRef]
- Haring, B.; Wu, C.; Coker, L.H.; Seth, A.; Snetselaar, L.; Manson, J.E.; Rossouw, J.E.; Wassertheil-Smoller, S. Hypertension, Dietary Sodium, and Cognitive Decline: Results From the Women’s Health Initiative Memory Study. Am. J. Hypertens 2016, 29, 202–216. [Google Scholar] [CrossRef] [Green Version]
- Nowak, K.L.; Fried, L.; Jovanovich, A.; Ix, J.; Yaffe, K.; You, Z.; Chonchol, M. Dietary Sodium/Potassium Intake Does Not Affect Cognitive Function or Brain Imaging Indices. Am. J. Nephrol. 2018, 47, 57–65. [Google Scholar] [CrossRef]
- Rush, T.M.; Kritz-Silverstein, D.; Laughlin, G.A.; Fung, T.T.; Barrett-Connor, E.; McEvoy, L.K. Association between dietary sodium intake and cognitive function in older adults. J. Nutr. Health Aging 2017, 21, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Yap, K.H.; Reidpath, D.; Soh, Y.C.; McGrattan, A.; Stephan, B.C.M.; Robinson, L.; Chaiyakunapruk, N.; Siervo, M. Link Between Dietary Sodium Intake, Cognitive Function, and Dementia Risk in Middle-Aged and Older Adults: A Systematic Review. J. Alzheimer’s Dis. JAD 2020, 76, 1347–1373. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.; van Aller, C.; Narytnyk, A.; Reidpath, D.; Keage, H.; Mohan, D.; Su, T.T.; Stephan, B.; Robinson, L.; Siervo, M. Nutritional interventions for the prevention of cognitive impairment and dementia in developing economies in East-Asia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.; Mohan, D.; Chua, P.W.; Mat Hussin, A.; Soh, Y.C.; Alawad, M.; Bin Kassim, Z.; Bin Mohd Ghazali, A.N.; Stephan, B.; Allotey, P.; et al. Feasibility and acceptability of a dietary intervention study to reduce salt intake and increase high-nitrate vegetable consumption among middle-aged and older Malaysian adults with elevated blood pressure: A study protocol. BMJ Open 2020, 10, e035453. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.M.; Shohaimi, S.; Chong, H.T.; Rahman, A.H.; Razali, R.; Esther, E.; Basri, H.B. Validation study of the Mini-Mental State Examination in a Malay-speaking elderly population in Malaysia. Dement. Geriatr. Cogn. Disord. 2009, 27, 247–253. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- National Coordinating Committee on Food and Nutrition. Malaysian Dietary Guidelines; Ministry of Health Malaysia: Putrajaya, Malaysia, 2010.
- Uschner, D.; Schindler, D.; Hilgers, R.-D.; Heussen, N. randomizeR: An R package for the assessment and implementation of randomization in clinical trials. J. Stat. Softw. 2018, 85, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Sahathevan, R.; Mohd Ali, K.; Ellery, F.; Mohamad, N.F.; Hamdan, N.; Mohd Ibrahim, N.; Churilov, L.; Cumming, T.B. A Bahasa Malaysia version of the Montreal Cognitive Assessment: Validation in stroke. Int. Psychogeriatr. 2014, 26, 781–786. [Google Scholar] [CrossRef]
- Jamaluddin, R.; Othman, Z.; Musa, K.I.; Alwi, M.N.M. Validation of the Malay version of auditory verbal learning test (MVAVLT) among schizophrenia patients in hospital Universiti Sains Malaysia (HUSM), Malaysia. ASEAN J. Psychiatry 2009, 10, 54–74. [Google Scholar]
- Institute for Public Health (IPH). National Health and Morbidity Survey 2014: Malaysian Adult Nutrition Survey. Vol. 1: Methodology and General Findings; Institute for Public Health, Ministry of Health Malaysia: Kuala Lumpur, Malaysia, 2014.
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2015, 25, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Forsat, N.D.; Palmowski, A.; Palmowski, Y.; Boers, M.; Buttgereit, F. Recruitment and Retention of Older People in Clinical Research: A Systematic Literature Review. J. Am. Geriatr. Soc. 2020, 68, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Fiordelli, M.; Fadda, M.; Amati, R.; Albanese, E. Older adults’ motivations to participate or not in epidemiological research. Qualitative inquiry on a study into dementia in Switzerland. PLoS ONE 2021, 16, e0247141. [Google Scholar] [CrossRef]
- Bardach, S.H.; Yarbrough, M.; Walker, C.; Alfred, D.L.; Ighodaro, E.; Kiviniemi, M.T.; Jicha, G.A. Insights From African American Older Adults on Brain Health Research Engagement: “Need to See the Need”. J. Appl. Gerontol. 2020, 40, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, A.L.; Lambe, S.; Chaisson, C.; Palmisano, J.; Horvath, K.J.; Karlawish, J. Clinical Research Participation among Aging Adults Enrolled in an Alzheimer’s Disease Center Research Registry. J. Alzheimer’s Dis. 2011, 23, 443–452. [Google Scholar] [CrossRef]
- Treweek, S.; Lockhart, P.; Pitkethly, M.; Cook, J.A.; Kjeldstrøm, M.; Johansen, M.; Taskila, T.K.; Sullivan, F.M.; Wilson, S.; Jackson, C.; et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013, 3, e002360. [Google Scholar] [CrossRef] [Green Version]
- Holzer, J.K.; Ellis, L.; Merritt, M.W. Why we need community engagement in medical research. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2014, 62, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Hinton, L.; Carter, K.; Reed, B.R.; Beckett, L.; Lara, E.; DeCarli, C.; Mungas, D. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis. Assoc. Disord. 2010, 24, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Romero, H.R.; Welsh-Bohmer, K.A.; Gwyther, L.P.; Edmonds, H.L.; Plassman, B.L.; Germain, C.M.; McCart, M.; Hayden, K.M.; Pieper, C.; Roses, A.D. Community engagement in diverse populations for Alzheimer disease prevention trials. Alzheimer Dis. Assoc. Disord. 2014, 28, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Wallace, R.; Lo, J.; Devine, A. Tailored Nutrition Education in the Elderly Can Lead to Sustained Dietary Behaviour Change. J. Nutr. Health Aging 2016, 20, 8–15. [Google Scholar] [CrossRef]
- Young, K.; Bunn, F.; Trivedi, D.; Dickinson, A. Nutritional education for community dwelling older people: A systematic review of randomised controlled trials. Int. J. Nurs. Stud. 2011, 48, 751–780. [Google Scholar] [CrossRef] [Green Version]
- Lyons, B.P. Nutrition education intervention with community-dwelling older adults: Research challenges and opportunities. J. Community Health 2014, 39, 810–818. [Google Scholar] [CrossRef]
- Shahar, S.; Adznam, S.N.A.; Rahman, S.A.; Yusoff, N.A.M.; Yassin, Z.; Arshad, F.; Sakian, N.I.M.; Salleh, M.; Samah, A.A. Development and analysis of acceptance of a nutrition education package among a rural elderly population: An action research study. BMC Geriatr. 2012, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiloglou, M.F.; Fletcher, J.; Poulia, K.-A. Challenges and Perspectives in Nutritional Counselling and Nursing: A Narrative Review. J. Clin. Med. 2019, 8, 1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, N.; Surender, R.; Bobrow, K.; Muller, J.; Farmer, A. Improving treatment adherence for blood pressure lowering via mobile phone SMS-messages in South Africa: A qualitative evaluation of the SMS-text Adherence SuppoRt (StAR) trial. BMC Fam. Pract. 2015, 16, 80. [Google Scholar] [CrossRef] [Green Version]
- Hacking, D.; Haricharan, H.J.; Brittain, K.; Lau, Y.K.; Cassidy, T.; Heap, M. Hypertension Health Promotion via Text Messaging at a Community Health Center in South Africa: A Mixed Methods Study. JMIR Mhealth Uhealth 2016, 4, e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.; Chong, M.C.; Khoo, S.; Wong, L.P.; Chung, I.; Tan, M.P. Virtual Group Exercises and Psychological Status among Community-Dwelling Older Adults during the COVID-19 Pandemic—A Feasibility Study. Geriatrics 2021, 6, 31. [Google Scholar] [CrossRef]
- Ridda, I.; MacIntyre, C.R.; Lindley, R.I.; Tan, T.C. Difficulties in recruiting older people in clinical trials: An examination of barriers and solutions. Vaccine 2010, 28, 901–906. [Google Scholar] [CrossRef]
- Sano, M.; Egelko, S.; Zhu, C.W.; Li, C.; Donohue, M.C.; Ferris, S.; Kaye, J.; Mundt, J.C.; Sun, C.-K.; Aisen, P.S.; et al. Participant satisfaction with dementia prevention research: Results from Home-Based Assessment trial. Alzheimer’s Dement. 2018, 14, 1397–1405. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, M.J.; Kim, S. Alternative type of the trail making test in nonnative English-speakers: The trail making test-black & white. PLoS ONE 2014, 9, e89078. [Google Scholar] [CrossRef]
- Hashimoto, R.; Meguro, K.; Lee, E.; Kasai, M.; Ishii, H.; Yamaguchi, S. Effect of age and education on the Trail Making Test and determination of normative data for Japanese elderly people: The Tajiri Project. Psychiatry Clin. Neurosci. 2006, 60, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Ivery, J.M.; Benton, L.; Harrison, A.; Paul, M.; Cortés, M. The DASH Pilot Project: Developing Community-Based Nutrition Education for Older Adults. J. Gerontol. Soc. Work 2017, 60, 286–299. [Google Scholar] [CrossRef] [PubMed]
| Characteristics a | Total (n = 74) | Low Salt (n = 23) | High-Nitrate Vegetable (n = 17) | High-Nitrate Vegetable + Low Salt (n = 17) | Control (n = 17) | p-Value |
|---|---|---|---|---|---|---|
| Age, years | 61.6 ± 6.7 | 62.6 ± 7.2 | 61.9 ± 6.7 | 60.4 ± 7.3 | 61.0 ± 5.8 | 0.752 |
| Ethnicity | ||||||
| Malay | 51 (68.9) | 17 (73.9) | 9 (52.9) | 13 (76.5) | 12 (70.6) | 0.457 |
| Chinese | 23 (31.1) | 6 (26.1) | 8 (47.1) | 4 (23.5) | 5 (29.4) | |
| Sex: Male | 31 (41.9) | 7 (30.4) | 9 (52.9) | 9 (52.9) | 6 (35.3) | 0.386 |
| Employment status | ||||||
| Working, full-time/self-employed | 26 (35.1) | 6 (26.1) | 5 (29.4) | 11 (64.7) | 4 (23.5) | 0.075 |
| Working, part-time | 9 (12.2) | 5 (21.7) | 1 (5.9) | 0 (0.0) | 3 (17.6) | |
| Retired/Unemployed /Homemaker | 39 (52.7) | 12 (52.2) | 11 (64.7) | 6 (35.3) | 10 (58.8) | |
| Highest education level | ||||||
| No formal education | 6 (8.1) | 1 (4.3) | 2 (11.8) | 1 (5.9) | 2 (11.8) | 0.465 |
| Primary | 27 (36.5) | 10 (43.5) | 8 (47.1) | 3 (17.6) | 6 (35.3) | |
| Secondary | 36 (48.6) | 11 (47.8) | 6 (35.3) | 10 (58.8) | 9 (52.9) | |
| Tertiary and others | 5 (6.8) | 1 (4.3) | 1 (5.9) | 3 (17.6) | 0 (0.0) | |
| Marital status | ||||||
| Married | 62 (83.8) | 18 (78.3) | 14 (82.4) | 16 (94.1) | 14 (82.4) | 0.643 |
| Never married/Divorced/Widow/Widower | 12 (16.2) | 5 (21.7) | 3 (17.6) | 1 (5.9) | 3 (17.6) | |
| Current smoking (Yes) | 12 (16.2) | 4 (17.4) | 4 (23.5) | 2 (11.8) | 2 (11.8) | 0.841 |
| Current use of alcohol (Yes) | 8 (10.8) | 2 (8.7) | 4 (23.5) | 1 (5.9) | 1 (5.9) | 0.456 |
| BMI, kg/m2 | 27.5 ± 4.6 | 28.3 ± 5.5 | 27.2 ± 4.6 | 27.5 ± 3.4 | 26.9 ± 4.4 | 0.806 |
| Systolic blood pressure, mmHg | 135.9 ± 14.1 | 135.0 ± 15.3 | 136.6 ± 10.5 | 137.5 ± 16.5 | 134.9 ± 13.9 | 0.934 |
| Diastolic blood pressure, mmHg | 80.3 ± 9.7 | 81.1 ± 10.6 | 80.8 ± 5.8 | 79.5 ±12.3 | 79.6 ± 9.5 | 0.940 |
| Geriatric Depression Scale score | 4.0 (2.0, 5.0) | 4.0 (2.0, 5.5) | 4.0 (2.0, 5.0) | 3.0 (1.0, 4.0) | 2.5 (2.0, 4.3) | 0.227 |
| Grip strength, mm | 26.7 ± 9.5 | 26.2 ± 9.6 | 25.3 ± 9.1 | 29.0 ± 10.3 | 26.5 ± 9.3 | 0.698 |
| Physical activity, MET–mins/week | 2880 (710, 5415) | 2960 (660, 3840) | 1920 (420, 4620) | 5040 (760, 8840) | 3900 (1560, 10875) | 0.605 |
| Gait speed test, m/s (n = 67) b | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.9 ± 0.3 | 0.122 |
| Timed up and go, s (n = 70) b | 9.8 (8.8, 11.8) | 9.6 (8.6, 11.5) | 9.3 (8.6, 11.0) | 11.4 (8.9, 12.1) | 10.2 (9.1, 10.2) | 0.283 |
| MoCA total score | 20.8 ± 4.0 | 20.3 ± 4.4 | 21.5 ± 4.0 | 20.6 ± 3.8 | 20.9 ± 3.9 | 0.834 |
| Trail Making Test B, s | 207 ± 86 | 221 ± 87 | 222 ± 95 | 194 ± 80 | 188 ± 83 | 0.518 |
| Animal Naming (n = 73) b | 14.9 ± 4.2 | 15.8 ± 3.5 | 13.8 ± 3.5 | 14.1 ± 3.4 | 15.5 ± 5.8 | 0.337 |
| AVLT Trial 8, A7 (Delayed recall) | 6.7 ± 3.4 | 6.7 ± 2.8 | 5.9 ± 4.9 | 6.9 ± 2.6 | 7.3 ± 3.3 | 0.684 |
| Category | Quantitative Data | Qualitative Data-Representative Quotes from Respondents and Data Collectors |
|---|---|---|
| Number of visits | Appropriate: 90.7% (49/54) Too many: 5.6% (3/54) Too few: 3.7% (2/54) | “…many respondents complained that there were too many visits to their house and calls during baseline, clinic visit, interim 1, interim 2 and end study assessments. It made them lose interest and not want to continue the project” (Data collector 3) “…always the same (questions) only…” (Participant #20) “Asking many questions difficult to answer” (Participant #60) “Because sometimes not free. Got other works” (Participant #62) “Come (to my house) too less (frequent)” (Participant #33) |
| Duration of visit | Appropriate: 84.9% (45/53) Too long: 11.3% (6/53) Too short: 3.8% (2/53) | “Time for the counselling at the clinic was too short” (Participant #51) “A home/clinic visit used up to 2 h and too many assessments, questionnaires and samples to be done, it is too tiring for the elderly” (Data collector 5) |
| Location of the clinic | Convenient: 96.2% (50/52) Inconvenient: 3.8% (2/52) | “The differences are while at their home, they might feel more comfortable since we are at their house. Compared to in the clinic, the situation might be more stressful since there are also other patients in the clinic. They also needed to move from one station to another, where the distance was quite far because we used two different buildings” (Data collector 2) |
| Intervention Strategy | Engagement with Intervention | Perceived Usefulness of Intervention Strategies (Qualitative Data) | |
|---|---|---|---|
| Theme Generated | Representative Quote’s | ||
| Group counselling sessions | All participants from the intervention groups (n = 47) attended the group counselling sessions |
| “It can give awareness to us and can increase our knowledge about health” (Participant #4) “For uncle, it is good for people aged 45, 50, 60, to remind about healthy food habit” (Participant #64) “Can face-to-face ask doctor question. If there is doctor it will be more confident” (Participant #18) “I can’t change my diet immediately, it takes time, the explanation from the doctor was good” (Participant #070) |
| Bi-weekly text message | Interim 1: 49% (22/45) claimed they read the text messages Interim 2: 69% (31/45) claimed they read the text messages End study: 80% (33/41) claimed they read the text messages |
| “Because it reminds me” (Participant #3, Interim 1) “Give advice and show things that healthy” (Participant #26, Interim 2) “I didn’t mean that that (message) not help, based on what I had read, yes if really to be very helpful, if I was the one who cooked” (Participant #40, Interim 1) “Elderly not familiar with the technology even on using WhatsApp or open the messages, they also claimed on not received any messages or videos while our record shown they have received” (Data collector 6) |
| Reinforcement video | Interim 1: Reinforcement delivered through home visit (n = 18); the video was sent to 85% of participants (23/27) Interim 2: The video was sent to 74% (31/42) of participants; reinforcement delivered by telephone call (n = 11) |
| “Only minority has watched the videos/messages (even mostly claimed that they have watched) respondents not familiar with using technology. Respondents claimed that they did not receive videos/messages, the records show they have received it” (Data collector 5) |
| Interim 3: The video was sent to 86% of participants (55/64); reinforcement delivered by phone call (n = 7) or posted compact discs (n = 2) End study: 85% (35/41) claimed they had watched the video | |||
| Information booklet | At interim 2, at least 14 (31%) of 45 participants who completed the assessment admitted that they did not read the information booklet At the end of the study, only about half of the participants (51%; 21/41) claimed that they tried the recipes in the booklet provided |
| “Reduce salt in cooking according to recipe book” (Participant #29, Salt, Malay, Interim 2) “Always remind me to reduce salt in cooking” (Participant #42, combined, Malay, Interim 1) “I can know the type, type of food that rich in nitrate. Sometimes we don’t know that source where it comes from. Other than that, it very for knowledge only. Like change this” (Participant #18, Nitrate, Chinese, Interim 1) “It provides the information in the type of vegetables and we know which one to choose” (Participant #18, Nitrate, Chinese, Interim 2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.S.; McGrattan, A.; Soh, Y.C.; Alawad, M.; Su, T.T.; Palanisamy, U.D.; Hussin, A.M.; Kassim, Z.b.; Mohd Ghazali, A.N.b.; Christa Maree Stephan, B.; et al. Feasibility and Acceptability of a Dietary Intervention to Reduce Salt Intake and Increase High-Nitrate Vegetable Consumption in Malaysian Middle-Aged and Older Adults with Elevated Blood Pressure: Findings from the DePEC-Nutrition Trial. Nutrients 2022, 14, 430. https://doi.org/10.3390/nu14030430
Lee SS, McGrattan A, Soh YC, Alawad M, Su TT, Palanisamy UD, Hussin AM, Kassim Zb, Mohd Ghazali ANb, Christa Maree Stephan B, et al. Feasibility and Acceptability of a Dietary Intervention to Reduce Salt Intake and Increase High-Nitrate Vegetable Consumption in Malaysian Middle-Aged and Older Adults with Elevated Blood Pressure: Findings from the DePEC-Nutrition Trial. Nutrients. 2022; 14(3):430. https://doi.org/10.3390/nu14030430
Chicago/Turabian StyleLee, Siew Siew, Andrea McGrattan, Yee Chang Soh, Mawada Alawad, Tin Tin Su, Uma Devi Palanisamy, Azizah Mat Hussin, Zaid bin Kassim, Ahmad Nizal bin Mohd Ghazali, Blossom Christa Maree Stephan, and et al. 2022. "Feasibility and Acceptability of a Dietary Intervention to Reduce Salt Intake and Increase High-Nitrate Vegetable Consumption in Malaysian Middle-Aged and Older Adults with Elevated Blood Pressure: Findings from the DePEC-Nutrition Trial" Nutrients 14, no. 3: 430. https://doi.org/10.3390/nu14030430
APA StyleLee, S. S., McGrattan, A., Soh, Y. C., Alawad, M., Su, T. T., Palanisamy, U. D., Hussin, A. M., Kassim, Z. b., Mohd Ghazali, A. N. b., Christa Maree Stephan, B., Allotey, P., Reidpath, D. D., Robinson, L., Mohan, D., & Siervo, M. (2022). Feasibility and Acceptability of a Dietary Intervention to Reduce Salt Intake and Increase High-Nitrate Vegetable Consumption in Malaysian Middle-Aged and Older Adults with Elevated Blood Pressure: Findings from the DePEC-Nutrition Trial. Nutrients, 14(3), 430. https://doi.org/10.3390/nu14030430









