Abstract
Physical activity and muscle strengthening are essential for preventing and managing metabolic syndrome. This study was conducted to investigate the relationship between the prevalence of metabolic syndrome and meeting the guidelines for aerobic physical activity (APA), muscle strengthening exercise (MSE), and combined exercise. We used data from 22,467 Koreans aged 40 years or older, who participated in in the Korea National Health and Nutrition Examination Survey (KNHANES) 2014–2019. We used the Global Physical Activity Questionnaire (GPAQ) to measure physical activity and surveyed frequency of MSE through a questionnaire. Metabolic syndrome was defined according to the American heart association and the National Heart, Lung, and Blood Institute. Compared with none exercise group, odds ratios of APA, MSE, and combined exercise group (CEG) on metabolic syndrome prevalence were 0.85 (95% confidence interval (CI), 0.74–0.98), 0.81 (95% CI, 0.67–0.99), and 0.65 (95% CI, 0.54–0.78) among men, respectively. Among women, ORs of APA, MSE, and CEG were 0.83 (95% CI, 0.73–0.93), 0.73 (95% CI, 0.58–0.91), and 0.74 (95% CI, 0.58–0.93), respectively. This study showed that meeting guidelines for APA and MSE was associated with lower prevalence of metabolic syndrome. Furthermore, subjects who met both APA and MSE had the lowest metabolic syndrome prevalence.
Keywords:
exercise; metabolic syndrome; obesity; blood pressure; glucose; triglycerides; cholesterol 1. Introduction
Metabolic syndrome, expressed through insulin-resistant diabetes, hypertension, abdominal obesity, and dyslipidemia, is a risk factor for cardiovascular disease and is known as a precursor of several chronic diseases [1,2]. The prevalence of metabolic syndrome has gradually increased worldwide [3,4]. In Korean men, the prevalence of metabolic syndrome increased from 2008 to 2017, whereas that in women stable [5].
It is necessary to actively manage chronic diseases such as metabolic syndrome to prevent cardiovascular disease. We recommend physical activity and exercise to prevent and manage diabetes mellitus and metabolic syndrome [6,7]. According to physical activity guidelines, aerobic physical activity (APA) is recommended at least 150 min/week of moderate-intensity or 75 min/week of high-intensity aerobic activity. Muscle-strengthening exercise (MSE) is recommended for at least 2 days a week [8]. In addition, both APA and MSE are recommended for diabetes [9]. Among Japanese adults, 35.1% of men and 27.4% of women met physical activity guidelines in 2016 [10]. Among Chinese adults, the proportion of meeting physical activity guidelines increased from 17.2% in 2000 to 22.8% in 2014, but it was still low [11]. According to the Korea National Health and Nutrition Examination Survey (KNHANES), Korean adults meeting guidelines for APA decreased from 58.3% in 2014 to 45.6% in 2020. In contrast, the proportion of Korean adults meeting guidelines for MSE has increased from 21.0% in 2014 to 24.7% in 2020. Only 16.9% of Korean adults met guidelines for both APA and MSE in 2020 [12].
Previous studies have examined the effects of physical activity and exercise on managing metabolic syndrome. According to a meta-analysis, aerobic exercise improves metabolic syndrome components reducing fasting plasma glucose (FPG), waist circumference, blood pressure and triglycerides [13]. In addition, muscle-strengthening training reduces waist circumference, glycated hemoglobin (HbA1c) and systolic blood pressure (SBP) in obese and diabetic patients [14]. In the general population, aerobic exercise has been consistently shown to benefit the management of metabolic syndrome components in some studies [15,16]. However, the effects of MSE and combined aerobic and resistance exercise on metabolic syndrome were inconsistent between studies [16]. Moreover, previous studies have mainly focused on Americans or Europeans and the studies on Asian population are insufficient.
Despite the Korean Health Policy to improve physical activity rate [17], the proportion of meeting exercise guidelines was still low and the prevalence of metabolic syndrome has increased. There are not enough studies about effect of MSE or combined exercise. This study was conducted to investigate the relationship between the prevalence of metabolic syndrome and meeting the guidelines for APA, MSE, and combined exercise using a nationally representative sample of Korean population.
2. Materials and Methods
2.1. Subjects
We used data from KNHANES, 2014–2019. A two-stage stratified cluster sampling designed the KNHANES to represent the Korean population. Data consists of the health interview survey (medical history, household survey, socioeconomic status, and health behavior survey) and health examination (anthropometric measurement, physical examination, blood pressure, biochemical measurement).
Among 47,309 subjects who participated in KNHANES, 2014–2019, we selected the study subjects as adults over 40 years of age since the prevalence of metabolic syndrome increased in subjects over 40 years [18]. Subjects with missing data on the physical activity questionnaires, physical measurements, high-density lipoprotein cholesterol (HDL-C), triglyceride or FPG were excluded. In addition, we excluded subjects with medical history of stroke, angina, or myocardial infarction, pregnant women, and subjects who had their blood drawn without fasting for more than 8 h. Finally, 22,467 Korean adults (9670 men and 12,797 women) were included for the study analysis. There was no statistical significant difference in the prevalence of metabolic syndrome and the components of metabolic syndrome between subjects who were excluded from the study and those who were included. The study protocol was approved by institutional review board at the Aerospace Medical Center, Republic of Korea Air Force (No. 136367-202202-HR-02-00).
2.2. Definition of Metabolic Syndrome and Metabolic Syndrome Components
Trained medical staffs measured waist circumference at the midpoint between the inferior margin of the last rib and the iliac crest, according to World Health Organization guidelines. The participant’s blood pressure was measured three times, after they had been quietly seated for 10 min, and the average of the second and third measurements was used. After fasting for 8 h or more, blood samples were taken to measure total cholesterol, triglycerides, HDL-C, and FPG. We defined hypertension as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or taking anti-hypertensive drugs diabetes as FPG ≥ 126 mg/dL, a glycated hemoglobin level ≥ 6.5%, taking oral diabetes medications or using insulin therapy and hypercholesterolemia as total cholesterol ≥240 mg/dL or taking lipid-lowering medications.
We defined metabolic syndrome according to the American heart association and the National Heart, Lung, and Blood Institute [19] and the diagnostic criteria of abdominal obesity for Koreans [20]. Metabolic syndrome was diagnosed as presenting with three or more of the following criteria: (1) high waist circumference (≥90 cm for men or ≥85 cm for women), (2) elevated triglycerides (≥150 mg/dL), (3) low HDL-C (<40 mg/dL for men or 50 mg/dL for women), (4) elevated FPG (≥100 mg/dL) or a diabetic patient, and (5) high blood pressure (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg) or a hypertensive patient.
2.3. Assessment of Physical Activity and Exercise Guidelines
We used the Global Physical Activity Questionnaire (GPAQ) to measure physical activity. GPAQ comprises 16 questions to survey various physical activities such as work, transportation, leisure, and recreation. Through the questionnaire, we surveyed whether study participants were active, how many days per week and how many minutes per day were spent on moderate or high-intensity leisure or physical activity for at least 10 min over the past week. These questions divided physical activity into five categories: high-intensity work, moderate-intensity work, moving to a location, high-intensity recreation, and moderate-intensity recreation.
We surveyed frequency of MSE through a questionnaire. For example, have you done sit-ups, push-ups, pull-ups, barbells or dumbbells in the past week? Weekly frequencies ranging from “not at all” to “more than 5 days” were used.
The Korean guideline for physical activity adopts the same basic principles with the US guideline. Recently, the US guideline was revised. According to the US guideline, APA is recommended at least 150–300 min/week of moderate-intensity or 75–150 min/week of high-intensity, and MSE is recommended for at least 2 days/week [9]. The study participants were categorized into four groups; (1) none exercise group (NEG, meeting neither APA and MSE recommendation), (2) APA group (meeting only recommendation for APA), (3) MSE group (meeting only recommendation for MSE), and (4) combined exercise group (CEG, meeting both APA and MSE).
2.4. Covariates
We selected covariates according to two previous studies [21,22]. Weight and height were measured up to 0.1 kg and 0.1 cm, and body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2). Smoking history was divided into current smoking and non-smoking, and alcohol consumption was categorized as none (1 or less per year) mild (less than 1 per month), or heavy (2 per month or more). The locality of residence was divided into urban and rural, and household equivalent income was categorized into quartiles. Educational level was divided into 4 categories; elementary school or lower, middle school, high school, and college or higher graduates.
2.5. Statistical Analyses
We performed statistical analyses through the R program 5.4.3 (R foundation, Vienna, Austria) using the Survey library and the Gtsummary library. We incorporated sampling weight considering the multistage probability sampling design and the non-responses to be representative of the Korean population. Since 6 years of data were integrated, a weight of 1/6 was applied to each analysis year.
The general characteristics of the study subjects were presented as a mean and standard deviation (SD) for continuous variables through a t-test, and were presented as percentage (%) and standard error (SE) for categorical variables through the chi-squared test.
We used multivariate logistic regression models to estimate the difference in the prevalence of metabolic syndrome according to meeting guidelines for APA and MSE. The NEG was considered as reference group. All analyses were stratified according to sex. The odds ratios (ORs) and 95% confidence intervals (CIs) for the prevalence of metabolic syndrome were measured after adjusting for age in model 1, adjusting for age, alcohol consumption, smoking, and BMI in model 2, and adjusting for age, alcohol consumption, smoking, BMI, education level, household income, and residence locality in model 3. In addition, we used general linear regression models to estimate waist circumference, triglycerides, SBP, DBP, HDL-C, and FPG. Statistical significance was considered using a two-sided p-value < 0.05.
3. Results
3.1. Baseline Characteristics
Among 22,467 subjects, 43.0% were men. The mean age of men and women was 55.3 ± 10.5 years and 56.6 ± 11.2 years, respectively. Men had a higher prevalence of metabolic syndrome than women (33.0 ± 0.6% vs. 24.8 ± 0.5%, p < 0.001). The proportions of each exercise group were 42.4 ± 0.6% for NEG, 10.6 ± 0.4% for MSE, 29.7 ± 0.6% for APA and 17.3 ± 0.5% for CEG in men and 52.6 ± 0.6% for NEG, 5.7 ± 0.3% for MSE, 33.5 ± 0.5% for APA and 8.2 ± 0.3% for CEG in women, respectively (Table 1).

Table 1.
General characteristics of study participants.
Among women, all other exercise groups were likely to have lower BMI, waist circumference, triglyceride, SBP, FPG and higher HDL-C than NEG. (all p < 0.001 except p = 0.001 for BMI in APA) Among men, CEG was likely to have lower waist circumference and triglyceride and higher HDL-C compared to NEG. (all p < 0.001) In addition, APA was likely to have a higher HDL-C (p < 0.001), and MSE was likely to have a lower triglyceride (p = 0.007) (Table 2).

Table 2.
Metabolic profiles of study participants according to exercise groups.
3.2. Metabolic Syndrome Prevalence (Table 3)
The prevalence of metabolic syndrome was significantly lower in all other exercise groups than NEG in both sexes. Among men, CEG had a lower proportion of waist circumference, triglyceride, HDL-C and FPG meeting metabolic syndrome criteria in than NEG (all p < 0.001). APA had a lower proportion of low HDL-C category and high FPG category and MSE had a lower proportion of elevated triglycerides category and low HDL-C category. Among women, all other exercise groups had a lower proportion of all metabolic components compared to NEG.

Table 3.
The prevalence of metabolic syndrome and its components according to exercise groups.
Table 3.
The prevalence of metabolic syndrome and its components according to exercise groups.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| NEG (Ref) | APA | MSE | CEG | NEG (Ref) | APA | MSE | CEG | |
| Waist Circumference meeting for Mets | 36.5 (1.2)% | 33.5 (1.2)% | 35.4 (1.7)% | 29.7 (1.7)% *** | 34.0 (0.8)% | 22.5 (0.9)% *** | 27.2 (1.7)% *** | 16.5 (1.7)% *** |
| Triglyceride meeting for Mets | 45.1 (1.2)% | 39.0 (1.2)% | 45.8 (1.8)% ** | 37.5 (1.8)% *** | 27.0 (0.7)% | 20.3 (0.7)% *** | 20.6 (1.7)% *** | 17.1 (1.7)% *** |
| HDL-C meeting for Mets | 30.3 (0.9)% | 26.6 (1.0)% *** | 24.7 (1.6)% * | 21.0 (1.6)% *** | 43.6 (0.7)% | 36.8 (0.9)% *** | 36.9 (2.0)% ** | 28.5 (2.0)% *** |
| Blood Pressure meeting for Mets | 41.0 (1.0)% | 39.2 (1.0)% | 40.8 (1.1)% | 40.1 (1.2)% | 33.1 (0.7)% | 29.3 (0.7)% *** | 25.5 (0.8)% *** | 23.4 (0.8)% *** |
| FPG meeting for Mets | 48.2 (1.8)% | 45.2 (1.9)% * | 44.6 (1.5)% | 44.4 (1.4)% * | 35.1 (1.8)% | 29.6 (2.0)% *** | 29.2 (1.6)% ** | 23.6 (1.6)% *** |
| Metabolic Syndrome | 35.8 (0.9)% | 30.4 (1.0)% * | 33.0 (1.7)% ** | 27.3 (1.7)% *** | 29.2 (0.7)% | 18.4 (0.8)% *** | 21.5 (1.5)% *** | 14.6 (1.5)% *** |
Values are presented as weighted proportions (with standard errors). Abbreviations: NEG, none exercise group; APA, aerobic physical activity group; MSE, muscle strengthening exercise group; CEG, combined exercise group; Mets, metabolic syndrome; HDL-C, high-density lipoprotein cholesterol; FPG, fating plasma glucose. * p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
3.3. ORs on the Prevalence of Metabolic Syndrome
The difference in the prevalence of metabolic syndrome according to meeting guidelines for APA and MSE. In both sexes, all other exercise groups had lower likelihood of diagnosis of metabolic syndrome compared to NEG in all models. Among men, ORs of APA, MSE, and CEG were 0.85 (95% CI, 0.74–0.98), 0.81 (95% CI, 0.67–0.99), and 0.65 (95% CI, 0.54–0.78) in model 3, respectively. Among women, ORs of APA, MSE, and CEG were 0.83 (95% CI, 0.73–0.93), 0.73 (95% CI, 0.58–0.91), and 0.74 (95% CI, 0.58–0.93) in model 3, respectively (Figure 1).
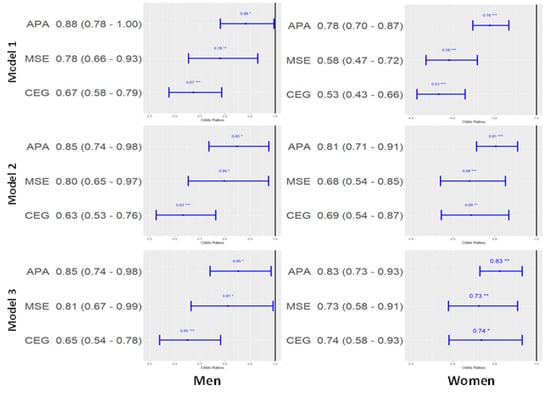
Figure 1.
Odds ratio and 95% confidence interval of each exercise group on the prevalence of metabolic syndrome (reference: none exercise group). Model 1: adjusted by age (continuous), Model 2: adjusted by age (continued), smoking (present or not), alcohol (<1/year, <1/month, ≥2/month), BMI (continuous), Model 3: adjusted by age (continuous), smoking (present or not), alcohol (<1/year, <1/month, ≥2/month), BMI (continuous), locality of dwelling (urban or rural), education level (under elementary school, elementary school graduate or middle school less, middle school graduate or high school less, university undergraduate or graduate) and household income (lower, middle, middle upper, and upper). Abbreviations: APA, aerobic physical activity group; MSE, muscle strengthening exercise group; CEG, combined exercise group.* p-value < 0.05, ** p-value < 0.01, *** p-value < 0.001.
BMI, which was included as an adjustment variable in the main analysis, may have multicollinearity with waist circumference, a component of metabolic syndrome. However, excluding BMI from the model did not result in statistically significant differences in the main results.
3.4. Generalized Linear Model on Metabolic Components
Among men, CEG had statistically significantly lower levels of waist circumference and triglycerides and higher level of HDL-C compared to NEG after adjusting by age, lifestyle habits, BMI, and socioeconomic status. Also, APA had lower level of waist circumference and higher level of HDL-C and MSE had lower level of waist circumference.
Among women, CEG and APA had lower levels of waist circumference, triglycerides, and FPG and higher level of HDL-C than NEG. MSE also had lower levels of waist circumference, triglycerides, and SBP and higher level of HDL-C (Table 4).

Table 4.
Generalized linear model of each exercise group on metabolic components (reference: NEG).
4. Discussion
In this study, we found that meeting guidelines for APA and MSE was independently associated with a lower prevalence of metabolic syndrome and had favorable association with various metabolic syndrome components. Subjects who met both APA and MSE had the lowest prevalence of metabolic syndrome. APA and MSE also had likelihood of lower prevalence of metabolic syndrome compared to NEG. These findings were consistent with previous studies results that physical activity had beneficial effects on lowering risk of developing metabolic syndrome [23,24]. Among subjects with metabolic syndrome, APA, MSE and CEG improved health outcomes significantly [25]. Even though the relationship between MSE and metabolic syndrome was not consistent [26], we ascertained that MSE had an association with lower prevalence of metabolic syndrome.
In previous meta-analysis studies, APA was beneficial effects in reducing waist circumference, SBP and DBP and increasing HDL [27]. In subjects with metabolic syndrome, APA improved waist circumference, TG, DBP and FPG [13,26]. In obese subjects, APA was effective to manage metabolic syndrome, but not in MSE [16]. In this study, APA was associated to lower levels of waist circumference, triglycerides, FPG and higher level of HDL-C in women. In men, APA had relationship with lower level of waist circumference and higher level of HDL-C. However, significant relationship of APA with triglycerides and FPG was not found in men.
Unlike in the current study, MSE did not reduce body mass index and waist circumference in some studies [26,28], but other studies showed that MSE had beneficial effects on reducing waist circumference [14,29]. In impaired glucose tolerance and diabetic patients, MSE significantly reduced glycated hemoglobin [14,29]. For participants with Type 2DM or obesity, MSE significantly reduced SBP [14,30]. MSE had beneficial effects on managing blood pressure. However, in the general population, there was no beneficial effects on metabolic parameters [26]. In addition, metabolic effect of MSE was different according to intensity of exercise and age. In this study, MSE was associated to lower levels of waist circumference, SBP, triglycerides and higher level of HDL-C in women. In men, MSE had relationship with lower level of waist circumference.
In this study, CEG was associated with a reduced prevalence of metabolic syndrome, consistent with previous studies [13,26]. We found that CEG was associated with the lowest prevalence of metabolic syndrome. Among obese or overweight participants, CEG had no difference in reducing waist circumference compared with APA, but among normal participants, CEG had more beneficial effects [13,28]. As in the current study, APA and CEG were associated with decreased FPG in women, and there was no difference between APA and CEG [13,26]. In type 2 diabetic patients, APA and CEG were more effective in managing and preventing metabolic syndrome than MSE [15]. CEG had more beneficial effects in reducing glycated hemoglobin than other exercises groups [31]. APA and CEG were associated with increased HDL-C and decreased triglycerides [13,26,32]. Among healthy and young people, exercise did not affect cholesterol, but for old or obese people. APA and CEG were associated with reducing cholesterol [33].
Insulin resistance and chronic inflammation are essential mechanisms for the pathogenesis of metabolic syndrome [34]. APA is associated with higher energy expenditure than MSE during the same exercise period [29,35], reducing chronic inflammation [25], and increasing the expression of PGC-1a, which protects against mitochondrial disorders such as apoptosis or oxidative damage [36]. Increased skeletal muscle was associated with higher resting metabolic rate (RMR), as well as higher use of glycogen and fatty acids [29]. MSE is superior to aerobic exercise in increasing skeletal muscle mass. An increase of 1 kg muscle mass should result in an RMR increase of 21 kcal/kg of new muscle. Thus MSE elevates RMR [37]. In addition, APA and MSE are associated with increased glucose uptake and decreased visceral fat [25]. Different mechanisms mediate these metabolic changes. In CEG, we considered both mechanisms beneficial in managing metabolic syndrome.
Previous studies showed that aerobic exercise was more effective to decrease the prevalence of metabolic syndrome than MSE [15,16]. In previous studies, participants were overweight or obese [33] or had type 2 diabetes or metabolic syndrome [13,15]. In contrast, the participants of this study were the community dwelling population including normal weight subjects and subjects without type 2 diabetes or metabolic syndrome. In previous studies, aerobic exercise was defined as using the treadmill, jogging, or cycling [15,31]. We used the terms aerobic physical activity instead aerobic exercise to include a broader range of daily activities. We defined aerobic activity based on physical activity through work, recreation, or moving a location, including treadmill, jogging, or cycling. The metabolic effects of APA were consistent. Most of muscle-strengthening studies focused on machine-based weight training [32,37]; however, few studies include free weights or bodyweight exercises [15]. In this study, MSE included machine-based weight training, free weights, and body weight exercise.
Our study has several limitations. First, since this study was a cross-sectional study, the causality of physical activity and metabolic syndrome could not be drawn. Underlying diseases with metabolic syndrome could negatively affect physical activity even though subjects with cardiovascular diseases were excluded. Second, because this study was a secondary data analysis of national health surveys, there may be residual confounding. For example, diet quality and sleep duration could act as a confounder, but was not included in the analysis. Moreover, the subjects’ detailed history of drug intake was not investigated. Third, since self-reported questionnaires were used to assess physical activity, there may be reporting bias or misclassification. Therefore, we think that longitudinal or interventional studies are warranted to elaborate the association between meeting exercise guidelines and the prevalence of metabolic syndrome.
Exercise prescription is a cost-effective strategy to prevent and manage metabolic syndrome [38,39]. Furthermore, we found that all exercise groups have beneficial effect on managing metabolic syndrome and its components. In Korea, the National Health Promotion Plan was developed to improve aerobic physical activity; establishment of physical activity programs or creating a physical activity-friendly environment such as running road and bike path [17]. However, the proportion of Korean adults meeting MSE recommendation was still low and the proportion of those meeting APA has decreased [12,40]. Face-to-face interventions and counseling physical activity was effective to improve physical activity [12]. In Korean medical status, because of too many patients, there was not enough clinic hours to counsel physical activity. Despite insufficient clinic hours, clinicians counsel patients to meet exercise guidelines not only APA but also MSE and encourage combined exercise to improve metabolic health.
5. Conclusions
This study showed that meeting guidelines for APA and MSE was associated with lower prevalence of metabolic syndrome. Furthermore, CEG was associated with the lowest metabolic syndrome prevalence. We suggest that both APA and MSE should be educated and combined exercise should be encouraged to improve metabolic health.
Author Contributions
D.H.K. contributed to interpret the study results and wrote the paper. Y.G.C. contributed to the study concept, and design, analysis and interpretation of the data and manuscript writing; H.A.P. contributed to the study concept and design and gave comments on manuscript; H.S.K. contributed to the study concept, analysis and interpretation of the data, and manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Aerospace Medical Center, Republic of Korea Air Force (No. 136367-202202-HR-02-00).
Informed Consent Statement
Informed consent was waived because the Korea National Health and Nutrition Examination Survey (KNHANES) data were constructed after anonymization by rigorous confidently guidelines.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the Korea National Health and Nutrition Examination Survey (KNHANES) official website.at https://knhanes.kdca.go.kr/knhanes/eng/index.do (accessed on 1 April 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef]
- Kim, M.-h.; Lee, S.-h.; Shin, K.-S.; Son, D.-Y.; Kim, S.-H.; Joe, H.; Yoo, B.-W.; Hong, S.-H.; Cho, C.-Y.; Shin, H.-S. The change of metabolic syndrome prevalence and its risk factors in Korean adults for decade: Korea National Health and Nutrition Examination Survey for 2008–2017. Korean J. Fam. Pract. 2020, 10, 44–52. [Google Scholar] [CrossRef]
- Yamaoka, K.; Tango, T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med. 2012, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, M.J.; Blair, S.N.; Church, T.S. Physical activity and diabetes prevention. J. Appl. Physiol. 2005, 99, 1205–1213. [Google Scholar] [CrossRef]
- Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010.
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The physical activity guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Tanaka, S. Status of physical activity in Japanese adults and children. Ann. Hum. Biol. 2019, 46, 305–310. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, C.; Wang, M.; Cai, R.; Zhang, Y.; He, Z.; Wang, H.; Wu, D.; Wang, F.; Liu, X. BMI, leisure-time physical activity, and physical fitness in adults in China: Results from a series of national surveys, 2000–2014. Lancet Diabetes Endocrinol. 2016, 4, 487–497. [Google Scholar] [CrossRef]
- Seo, Y.B.; Oh, Y.H.; Yang, Y.J. Current Status of Physical Activity in South Korea. Korean J. Fam. Med. 2022, 43, 209. [Google Scholar] [CrossRef] [PubMed]
- Ostman, C.; Smart, N.; Morcos, D.; Duller, A.; Ridley, W.; Jewiss, D. The effect of exercise training on clinical outcomes in patients with the metabolic syndrome: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2017, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Siebert, U.; Schobersberger, W. Resistance training in the treatment of the metabolic syndrome. A Systematic Review and Meta-Analysis of the Effect of Resistance Training on Metabolic Clustering in Patients with Abnormal Glucose Metabolism. Sport. Med. 2010, 40, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Johannsen, N.M.; Swift, D.L.; Gillison, F.B.; Mikus, C.R.; Lucia, A.; Kramer, K.; Lavie, C.J.; Church, T.S.J.M. Aerobic and strength training in concomitant metabolic syndrome and type 2 diabetes. Med. Sci. Sport Exerc. 2014, 46, 1293. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.A.; Slentz, C.A.; Willis, L.H.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention Through Defined Exercise-STRRIDE-AT/RT). Am. J. Cardiol. 2011, 108, 838–844. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. Integrated Health Promotion Program [Internet]; Korea Health Promotion Institute: Seoul, Korea, 2021; Available online: https://www.khealth.or.kr/board?menuId=MENU00829&siteId=null- (accessed on 27 March 2022).
- Lee, S.-H.; Tao, S.; Kim, H.-S. The prevalence of metabolic syndrome and its related risk complications among Koreans. Nutrients 2019, 11, 1755. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Am. Heart Assoc. 2005, 112, 2735–2752. [Google Scholar]
- Lee, S.Y.; Park, H.S.; Kim, D.J.; Han, J.H.; Kim, S.M.; Cho, G.J.; Kim, D.Y.; Kwon, H.S.; Kim, S.R.; Lee, C.B. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res. Clin. Pract. 2007, 75, 72–80. [Google Scholar] [CrossRef]
- Yi, D.; Khang, A.R.; Lee, H.W.; Son, S.M.; Kang, Y.H. Relative handgrip strength as a marker of metabolic syndrome: The Korea National Health and Nutrition Examination Survey (KNHANES) VI (2014–2015). Diabetes Metab. Syndr. Obes. 2018, 11, 227. [Google Scholar] [CrossRef]
- Metzger, J.S.; Catellier, D.J.; Evenson, K.R.; Treuth, M.S.; Rosamond, W.D.; Siega-Riz, A.M. Associations between patterns of objectively measured physical activity and risk factors for the metabolic syndrome. Am. J. Health Promot. 2010, 24, 161–169. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Jeon, J.Y. Association between physical activity and the prevalence of metabolic syndrome: From the Korean National Health and Nutrition Examination Survey, 1999–2012. SpringerPlus 2016, 5, 1870. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Santamaria, M.; Fernandez-Montero, A.; Martinez-Gonzalez, M.A.; Moreno-Galarraga, L.; Sanchez-Villegas, A.; Barrio-Lopez, M.T.; Bes-Rastrollo, M. Exercise intensity and incidence of metabolic syndrome: The SUN Project. Am. J. Prev. Med. 2017, 52, e95–e101. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.A.; Thom, J.M.; Rye, K.-A.; Parmenter, B.J. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Lemes, Í.R.; Turi-Lynch, B.C.; Cavero-Redondo, I.; Linares, S.N.; Monteiro, H.L. Aerobic training reduces blood pressure and waist circumference and increases HDL-c in metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Am. Soc. Hypertens. 2018, 12, 580–588. [Google Scholar] [CrossRef]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef]
- Strasser, B.; Schobersberger, W. Evidence for resistance training as a treatment therapy in obesity. J. Obes. 2011, 2011, 482564. [Google Scholar] [CrossRef]
- Lemes, Í.R.; Ferreira, P.H.; Linares, S.N.; Machado, A.F.; Pastre, C.M.; Netto, J. Resistance training reduces systolic blood pressure in metabolic syndrome: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sport. Med. 2016, 50, 1438–1442. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Boulé, N.G.; Wells, G.A.; Prud’homme, D.; Fortier, M.; Reid, R.D.; Tulloch, H.; Coyle, D.; Phillips, P.; et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 357–369. [Google Scholar] [CrossRef]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sport. Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Doewes, R.I.; Gharibian, G.; Zaman, B.A.; Akhavan-Sigari, R. An updated systematic review on the effects of aerobic exercise on human blood lipid profile. Curr. Probl. Cardiol. 2022; 101108, in press. [Google Scholar] [CrossRef] [PubMed]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Drenowatz, C.; Grieve, G.L.; DeMello, M.M. Change in energy expenditure and physical activity in response to aerobic and resistance exercise programs. SpringerPlus 2015, 4, 798. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef] [PubMed]
- Braith, R.W.; Stewart, K.J. Resistance exercise training: Its role in the prevention of cardiovascular disease. Circulation 2006, 113, 2642–2650. [Google Scholar] [CrossRef]
- Lakka, T.A.; Laaksonen, D.E. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine–evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport. 2015, 25, 1–72. [Google Scholar] [CrossRef]
- Yang, Y.J. An overview of current physical activity recommendations in primary care. Korean J. Fam. Med. 2019, 40, 135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).