Nutritional Impact and Eating Pattern Changes in Schizophrenic Spectrum Disorders after Health Education Program on Symbiotic Dietary Modulation Offered by Specialised Psychiatric Nursing–Two-Arm Randomised Clinical Trial
Abstract
1. Introduction
Background
2. Materials and Methods
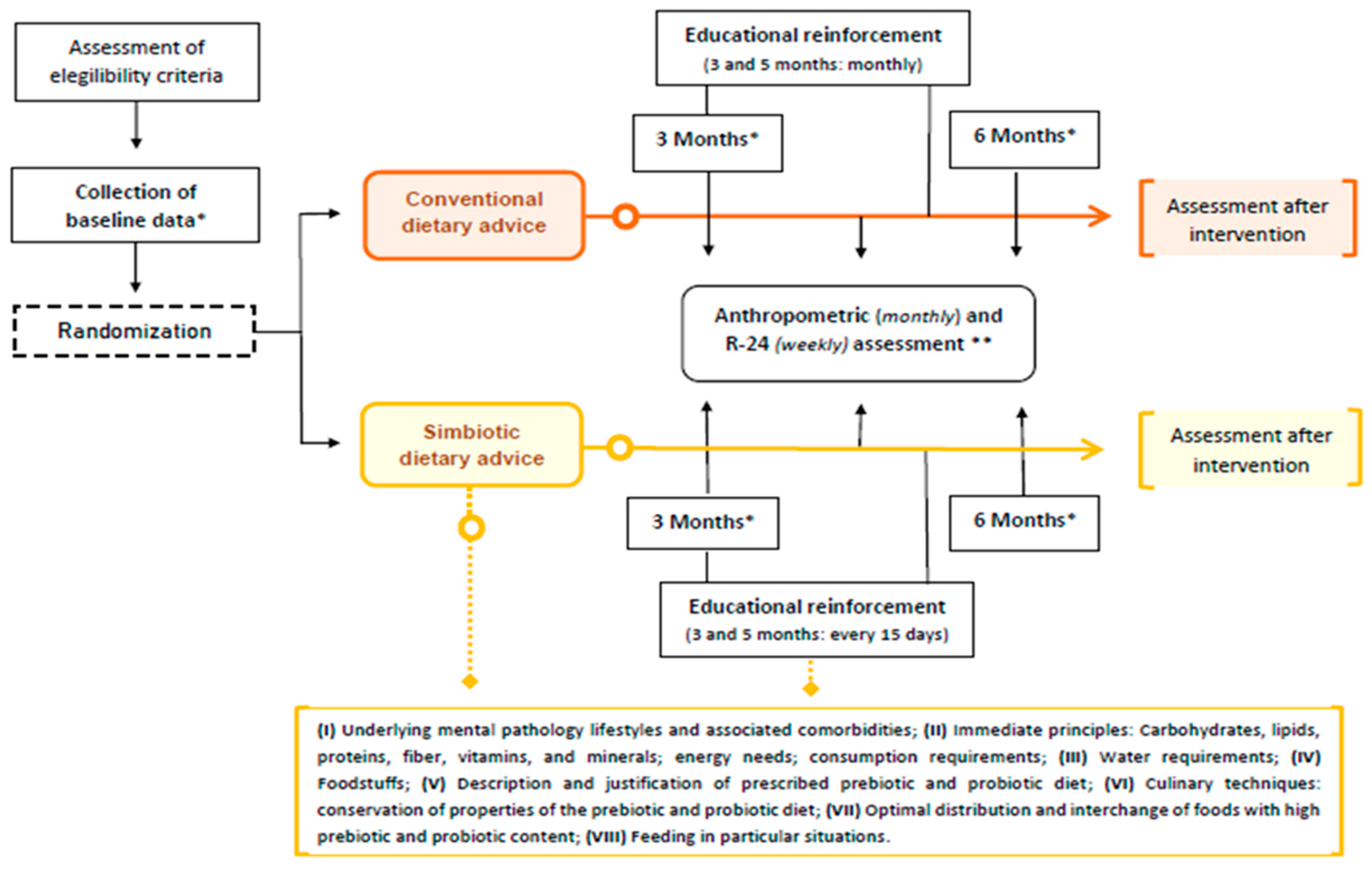
2.1. Study Design
2.2. Population Eligibility Criteria
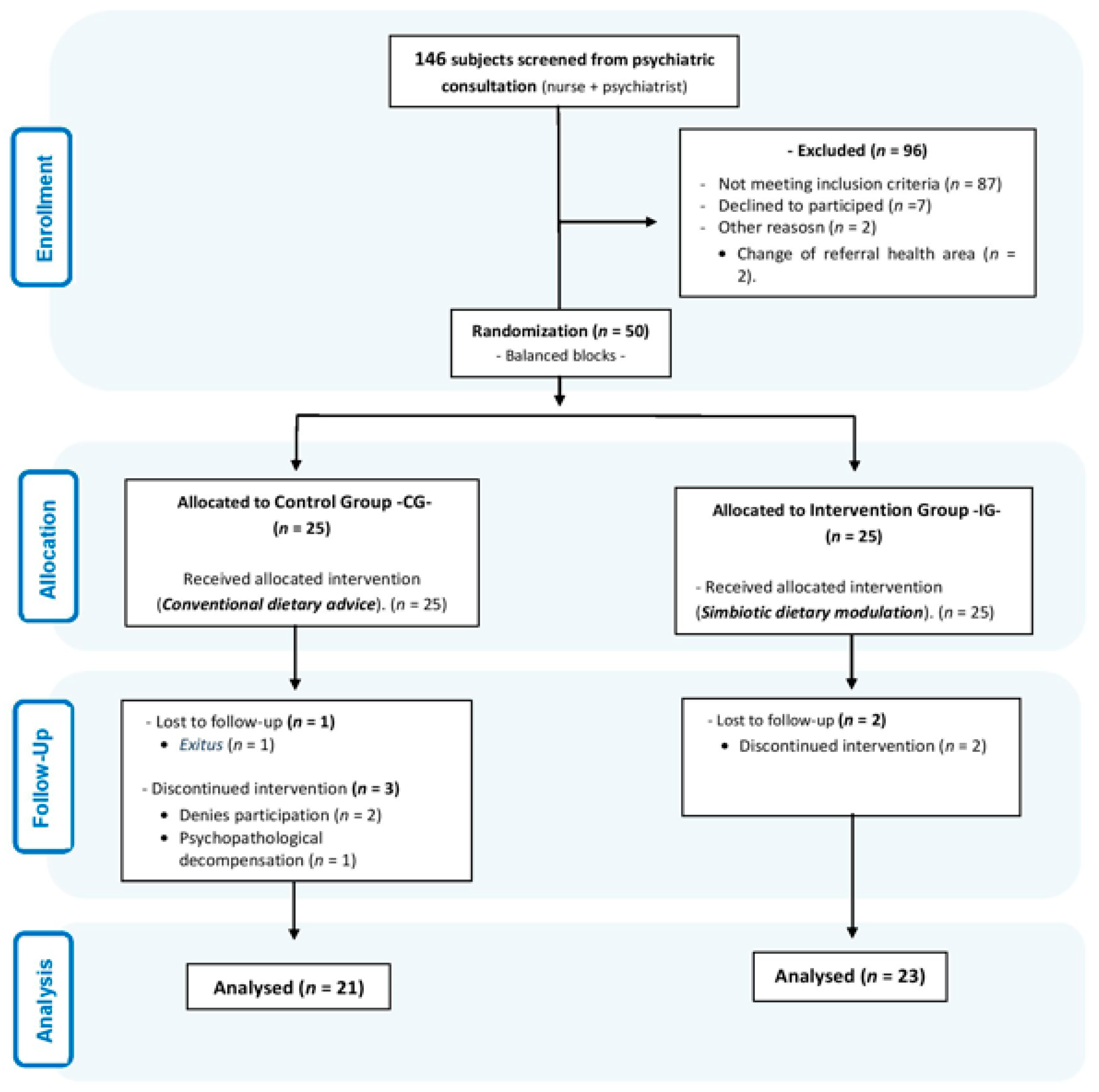
2.3. Sample Size
2.4. Intervention
2.5. Data Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration
References
- Aucoin, M.; LaChance, L.; Cooley, K.; Kidd, S. Diet and Psychosis: A Scoping Review. Neuropsychobiology 2020, 79, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Icaza, M.E. Gut microbiota in health and disease. Rev. Gastroenterol. Mex. 2013, 78, 240–248. [Google Scholar] [CrossRef]
- Castillo, F.; Marzo, M.E. Role of the gut microbiota in the development of various neurological diseases. Neurol. Sci. 2019, 37. [Google Scholar] [CrossRef]
- Henderson, D.C.; Borba, C.P.; Daley, T.B.; Boxill, R.; Nguyen, D.D.; Culhane, M.A.; Louie, P.; Cather, C.; Eden Evins, A.; Freudenreich, O.; et al. Dietary intake profile of patients with schizophrenia. Ann. Clin. Psychiatry 2006, 18, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Balanzá, V. Nutritional supplements in psychotic disorders. Actas Esp. Psiquiatr. 2017, 45, 16–25. [Google Scholar]
- Gómez, A.E. Nutrición y enfermedad mental. Esquizofrenia y ácidos grasos omega 3. Farm. Prof. 2007, 21, 60–63. [Google Scholar]
- Salagre, E.; Vieta, E.; Grande, I. The visceral brain: Bipolar disorder and microbiota. Rev. Psiquiatr. Salud Ment. 2017, 10, 67–69. [Google Scholar] [CrossRef]
- Soria, V.; Uribe, J.; Salvat, N.; Palao, D.; Menchón, J.M.; Labad, J. Psychoneuroimmunology of mental disorders. Rev. Psiquiatr. Salud Ment. 2018, 11, 115–124. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.P. Gut microbiota-brain axis. Chin. Med. J. 2016, 129, 2373–2380. [Google Scholar] [CrossRef]
- Cepeda, V.; Mondragón, A.; Lamas, A.; Miranda, J.M.; Cepeda, A. Use of prebiotics and probiotics in the management of anxiety. Farm. Comunit. 2019, 11, 30–40. [Google Scholar] [CrossRef]
- Patra, S. Psychobiotics: A paradigm shift in psychopharmacology. Indian J. Pharm. 2016, 48, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Andreo, P.; García, N.; Sánchez, E.P. La microbiota intestinal y su relación con las enfermedades mentales a través del eje microbiota-intestino-cerebro. Rev. Dis. Clin. Neurol. 2017, 4, 52–58. [Google Scholar]
- Forsythe, P.; Kunze, W.; Bienestock, J. Moody microbes or fecal phrenology: What do we know about the microbiota-gutbrain axis? BMC Med. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Solé, B.; Verdolini, N.; Amoretti, S.; Montejo, L.; Rosa, A.R.; Hogg, B.; Garcia-Rizo, C.; Mezquida, G.; Bernardo, M.; Martinez-Aran, A.; et al. Effects of the COVID-19 pandemic and lockdown in Spain: Comparison between community controls and patients with a psychiatric disorder. Preliminary results from the BRIS-MHC STUDY. J. Affect. Disord. 2021, 281, 13–23. [Google Scholar] [CrossRef]
- Baker, A.L.; Kay-Lambkin, F.J.; Richmond, R.; Filia, S.; Castle, D.; Williams, J.; Thornton, L. Healthy lifestyle intervention for people with severe mental disorders. Ment. Health Subst. Use 2011, 4, 144–157. [Google Scholar] [CrossRef][Green Version]
- Muhammad, D.G.; Abubakar, I.A. COVID-19 lockdown may increase cardiovascular disease risk factors. Egypt. Heart J. 2021, 73, 2. [Google Scholar] [CrossRef]
- Bennett, G.; Young, E.; Butler, I.; Coe, S. The Impact of Lockdown During the COVID-19 Outbreak on Dietary Habits in Various Population Groups: A Scoping Review. Front. Nutr. 2021, 8, 626432. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.L.; González, J.; Martínez, M.C. Metabolic control and prolactin in severe mental illness. Nursing interventions. Rev. Enferm Salud Ment. 2018, 9, 24–28. [Google Scholar] [CrossRef][Green Version]
- Franch, C.M.; Molina, V.; Franch, J.I. Determinants of metabolic risk in atypical antipsychotic treatment. Rev. Psiquiatr. Salud Ment. 2016, 23, 87–130. [Google Scholar] [CrossRef][Green Version]
- Franch, C.M.; Molina, V.; Franch, J.I. Metabolic syndrome and atypical antipsychotics: Possibility of prediction and control. Rev. Psiquiatr. Salud Ment. 2017, 10, 38–44. [Google Scholar] [CrossRef]
- Ocando, L.; Roa, A.; León, M.; González, R. Atypical antipsychotics and their role in the development of metabolic disease. Rev. Iberoam. Hipert. 2018, 13, 44–51. [Google Scholar]
- Gurusamy, J.; Gandhi, S.; Damodharan, D.; Ganesan, V.; Palaniappan, M. Exercise, diet and educational interventions for metabolic syndrome in persons with schizophrenia: A systematic review. Asian J. Psychiatr. 2018, 36, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Teasdale, S.; Abreu, S.; Bastos, T.; Probst, M.; Rosenbaum, S.; Ward, P.B.; Corredeira, R. Dietary Intake, Adherence to Mediterranean Diet and Lifestyle-Related Factors in People with Schizophrenia. Issues Ment. Health Nurs. 2019, 40, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Tyndall, S.F.; Vora, D.; Hasan, R.; Megna, J.L.; Leontieva, L. Diet Quality and Mental Health Amongst Acute Inpatient Psychiatric Patients. Cureus 2021, 13, e12434. [Google Scholar] [CrossRef]
- Kali, A. Psychobiotics: An emerging probiotic in psychiatric practice. Biomed. J. 2016, 3, 223–224. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Mörkl, S.; Wagner-Skacel, J.; Lahousen, T.; Lackner, S.; Holasek, S.J.; Bengesser, S.A.; Painold, A.; Holl, A.K.; Reininghaus, E. The Role of Nutrition and the Gut-Brain Axis in Psychiatry: A Review of the Literature. Neuropsychobiology 2018. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, M.; Ivarsson, A.; Carlsson, I.M.; Sandgren, A.; Jormfeldt, H. Health Effects of an Individualized Lifestyle Intervention for People with Psychotic Disorders in Psychiatric Outpatient Services: A TwoYear Follow-up. Issues Ment. Health Nurs. 2019, 40, 839–850. [Google Scholar] [CrossRef]
- Sugawara, N.; Sagae, T.; Yasui-Furukori, N.; Yamakazi, M.; Shimoda, K.; Mori, T.; Sugai, T.; Matsuda, H.; Suzuki, Y.; Ozeki, Y.; et al. Effects of nutritional education on weight change and metabolic abnormalities among patients with schizophrenia in Japan: A randomized controlled trial. J. Psychiatr. Res. 2018, 97, 77–83. [Google Scholar] [CrossRef]
- Andalusian Regional Ministry of Health. Dietary Advice in Primary Care. Plan for the Promotion of Physical Health and Balanced Diet 2004–2008. 2010. Available online: https://www.juntadeandalucia.es/export/drupaljda/Guia_Consejo_Dietetico_AP_2005_imp2010.pdf (accessed on 18 February 2020).
- Andalusian Regional Ministry of Health. Guide to Intensive Dietetic Counselling in Primary Health Care. Plan for the Promotion of Physical Health and Balanced Diet 2004–2008. 2007. Available online: https://www.repositoriosalud.es/handle/10668/1220 (accessed on 20 February 2020).
- Sevillano, A.; Molina, G.; García, J.A.; García, M.; Molina, R.; Romero, M. Efficacy of nutrition education for the increase of symbiotic intake on nutritional and metabolic status in schizophrenic spectrum disorders: A two-arm protocol. Front. Nutr. 2022, 9, 912783. [Google Scholar] [CrossRef]
- Martín, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernández, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rojas, R.; Pérez-Rodríguez, F.; Cámara Martos, F. Nutriplato 2.0 web para valoración de recetas y platos, de libre uso. XVI J. Nac. Nutr. Práctica. 2012, 32, 29–58. [Google Scholar]
- Stefańska, E.; Wendołowicz, A.; Lech, M.; Konarzewska, B.; Zapolska, J.; Waszkiewicz, N.; Ostrowska, L. Does the usual dietary intake of schizophrenia patients require supplementation with vitamins and minerals? Psychiatr. Pol. 2019, 53, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Stefańska, E.; Wendołowicz, A.; Konarzewska, B.; Waszkiewicz, N.; Ostrowska, L. The assessment of satisfaction of energy demand and of chosen macro- and micro-element content in the daily food rations of women diagnosed with schizophrenia with varied nutritional states. Psychiatr. Pol. 2019, 53, 613–628. [Google Scholar] [CrossRef]
- Adamowicz, K.; Kucharska-Mazur, J. Dietary Behaviors and Metabolic Syndrome in Schizophrenia Patients. J. Clin. Med. 2020, 9, 537. [Google Scholar] [CrossRef]
- Stefańska, E.; Lech, M.; Wendołowicz, A.; Konarzewska, B.; Waszkiewicz, N.; Ostrowska, L. Eating habits and nutritional status of patients with affective disorders and schizophrenia. Psychiatr. Pol. 2017, 51, 1107–1120. [Google Scholar] [CrossRef]
- Zurrón, P.; Casaprima, S.; García, L.; García-Portilla, M.P.; Junquera, R.; Canut, M.T.L. Eating and nutritional habits in patients with schizophrenia. Rev. Psiquiatr. Salud Ment. 2019, S1888–9891, 30098–30099. [Google Scholar] [CrossRef]
- Cheikh, L.; Hashim, M.; Mohamad, M.N.; Hassan, H.; Ajab, A.; Stojanovska, L.; Jarrar, A.H.; Hasan, H.; Abu Jamous, D.O.; Saleh, S.T.; et al. Dietary Habits and Lifestyle During Coronavirus Pandemic Lockdown: Experience from Lebanon. Front. Nutr. 2021, 8, 730425. [Google Scholar] [CrossRef]
- Kowalski, K.; Bogudzińska, B.; Stańczykiewicz, B.; Piotrowski, P.; Bielawski, T.; Samochowiec, J.; Szczygieł, K.; Plichta, P.; Misiak, B. The Deficit Schizophrenia Subtype Is Associated with Low Adherence to the Mediterranean Diet: Findings from a Case-Control Study. J. Clin. Med. 2022, 11, 568. [Google Scholar] [CrossRef]
- Caemmerer, J.; Correll, C.U.; Maayan, L. Acute and maintenance effects of non-pharmacologic interventions for antipsychotic associated weight gain and metabolic abnormalities: A meta-analytic comparison of randomized controlled trials. Schizophr. Res. 2012, 140, 159–168. [Google Scholar] [CrossRef]
- Teasdale, S.B.; Ward, P.B.; Rosenbaum, S.; Samaras, K.; Stubbs, B. Solving a weighty problem: Systematic review and meta-analysis of nutrition interventions in severe mental illness. Br. J. Psychiatry 2017, 210, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, V. Ethnicity, mortality, and severe mental illness. Lancet Psychiatry 2017, 4, 517. [Google Scholar] [CrossRef]
- Giannouli, V. Violence in severe mental illness: Is cognition missing in the associations with ethnicity, cannabis and alcohol? Australas. Psychiatry 2017, 25, 633. [Google Scholar] [CrossRef] [PubMed]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Sevillano-Jiménez, A.; Romero-Saldaña, M.; García-Mellado, J.A.; Carrascal-Laso, L.; García-Rodríguez, M.; Molina-Luque, R.; Molina-Recio, G. Impact of high prebiotic and probiotic dietary education in the SARS-CoV-2 era: Improved cardio-metabolic profile in schizophrenia spectrum disorders. BMC Psychiatry 2022, 22, 781. [Google Scholar] [CrossRef]
- Saiz, J.; Vega, D.C.; Sánchez, P. Bases Neurobiológicas de la Esquizofrenia. Clín. Salud. 2010, 21, 235–254. [Google Scholar]
| Variables | Total (n = 44) | Control Group (n = 21) | Intervention Group (n = 23) | p | |
|---|---|---|---|---|---|
| Socio-demographic variables | |||||
| Sex | |||||
| Men | 32 (72.7%) | 14 (31.8%) | 18 (40.9%) | 0.388 | |
| Women | 12 (27.3%) | 7 (15.9%) | 5 (11.4%) | ||
| Age (years) | 49.2 (11.2) | 48.8 (13.8) | 49.5 (10.1) | 0.897 | |
| Household composition | |||||
| Individual | 12 (27.3%) | 5 (11.4%) | 7 (15.9%) | 0.893 | |
| Horizontal | 3 (6.8%) | 1 (2.3%) | 2 (4.5%) | ||
| Complete | 3 (6.8%) | 1 (2.3%) | 2 (4.5%) | ||
| Family home | 7 (15.9%) | 4 (9.1%) | 3 (6.8%) | ||
| Supervised flat | 19 (43.2%) | 10 (22.7%) | 9 (20.5%) | ||
| Economic level | |||||
| High | 6 (13.6%) | 3 (6.8%) | 3 (6.8%) | 0.651 | |
| Medium | 26 (59.1%) | 11 (25%) | 15 (34.1%) | ||
| Low | 12 (27.3%) | 7 (15.9%) | 5 (11.4%) | ||
| Education level | |||||
| Uneducated | 4 (9.1%) | 2 (4.5%) | 2 (4.5%) | 0.590 | |
| Primary | 19 (43.2%) | 11 (25%) | 8 (18.2%) | ||
| Secondary | 17 (38.6%) | 7 (15.9%) | 10 (22.7%) | ||
| University | 4 (9.1%) | 1 (2.3%) | 3 (6.8%) | ||
| Area of residence | |||||
| Urban | 38 (86.4%) | 18 (40.9%) | 20 (45.5%) | 1.00 | |
| Rural | 6 (13.6%) | 3 (6.8%) | 3 (6.8%) | ||
| Clinical Variables | |||||
| Psychiatric diagnosis | |||||
| Schizophrenia | 37 (84.1%) | 19 (43.2%) | 18 (40.9%) | 0.419 | |
| Schizoaffective Disorder | 5 (11.4%) | 1 (2.3%) | 4 (9.1%) | ||
| Delusional Disorder | 2 (4.5%) | 1 (2.3%) | 1 (2.3%) | ||
| Duration of illness (years) | 21.6 (12.4) | 22.5 (12.6) | 20.9 (12.5) | 0.715 | |
| Age at first hospitalisation (years) | 31.4 (11) | 31.4 (11.4) | 31.4 (10.7) | 0.572 | |
| Consumption of toxics | |||||
| No | 15 (34.1%) | 5 (11.4%) | 10 (22.7%) | 0.169 | |
| Yes | 29 (65.9%) | 16 (36.4%) | 13 (29.5%) | ||
| Type of toxics | |||||
| Alcohol | 6 (13.6%) | 3 (6.8%) | 3 (6.8%) | 0.775 | |
| Tobacco | 29 (65.9%) | 15 (34%) | 14 (31.8%) | ||
| Cocaine | 3 (6.8%) | 1 (2.3%) | 2 (4.5%) | ||
| Opioids | 2 (4.6%) | 1 (2.3%) | 1 (2.3%) | ||
| Amphetamines | 3 (6.8%) | 2 (4.5%) | 1 (2.3%) | ||
| Cannabis | 10 (22.7%) | 5 (11.6%) | 5 (11.3%) | ||
| Cardio-metabolic condition | |||||
| No | 24 (54.5%) | 11 (25%) | 13 (29.5%) | 0.783 | |
| Yes | 20 (45.5%) | 10 (22.7%) | 10 (22.7%) | ||
| Type Cardio-metabolic condition | |||||
| AHT | 10 (22.7%) | 6 (13.6%) | 4 (9.1%) | 0.407 | |
| DM | 7 (15.9%) | 5 (11.3%) | 2 (4.5%) | ||
| Hyperlipemia | 17 (38.6%) | 8 (18.1%) | 9 (20.4%) | ||
| Therapeutic Variables | |||||
| Reason for Change: Antipsychotic Treatment | |||||
| Unchanged | 31 (70.5%) | 16 (51.6%) | 15 (48.4%) | 0.660 | |
| Lack of efficiency | 5 (11.4%) | 1 (2.3%) | 4 (9.1%) | ||
| Tolerability/safety issues | 2 (4.5%) | 1 (2.3%) | 1 (2.3%) | ||
| Patient’s own choice | 3 (6.8%) | 1 (2.3%) | 2 (4.5%) | ||
| Other: Clinical improvement | 3 (6.8%) | 2 (4.5%) | 1 (2.3%) | ||
| Tolerability and Modulation of Dietary and Nutritional Patterns | |||||
| Culinary knowledge and food responsibility | |||||
| Can cook and he/she is in charge of it | 27 (61.4%) | 9 (20.5%) | 18 (40.9%) | 0.004 | |
| Can cook but he/she is not in charge of it | 6 (13.6%) | 2 (4.5%) | 4 (9.1%) | ||
| Cannot cook and he/she is not in charge of it | 11 (25%) | 10 (22.7%) | 1 (2.3%) | ||
| Variables | Total (n = 44) | Control Group (n = 21) | Intervention Group (n = 23) | p |
|---|---|---|---|---|
| Macronutrients (RDA) | ||||
| Energy (%) | 177.4 (48.4) | 182 (47.3) | 173.2 (50.1) | 0.329 |
| Proteins (g) | 432 (152.5) | 443.2 (159.6) | 421.9 (148.6) | 0.597 |
| Lipids (g) | 207.8 (63.1) | 212.4 (68.2) | 203.5 (59.4) | 0.716 |
| Saturated fatty acids (g) | 392.3 (222.2) | 414.9 (260.6) | 371.7 (183.9) | 0.698 |
| Monounsaturated fatty acids (g) | 147.7 (60.9) | 140.9 (63.6) | 154 (59) | 0.613 |
| Polyunsaturated fatty acids (g) | 138.9 (103.7) | 145.1 (131.3) | 133.2 (72.8) | 0.716 |
| Cholesterol (mg) | 247.2 (135.8) | 224.6 (70.3) | 267.9 (175) | 0.787 |
| Carbohydrates (g) | 159.6 (53.4) | 161.8 (48.2) | 157.5 (58.7) | 0.518 |
| Oligosaccharides (g) Polysaccharides (g) | 303.4 (205.7) | 342.7 (203.8) | 267.5 (205.2) | 0.088 |
| 123.2 (74.6) | 139.8 (87.2) | 108 (58.8) | 0.378 | |
| Fibre (g) | 215.1 (124.8) | 196 (130.5) | 232.5 (119.5) | 0.245 |
| Micronutrients (RDA) | ||||
| Ca (mg) | 181.1 (68.8) | 194.1 (64.7) | 169.2 (71.2) | 0.124 |
| Mg (mg) | 292.8 (95.4) | 285.3 (88.8) | 299.7 (102.6) | 0.823 |
| P (mg) | 352.9 (107.7) | 372.7 (123.5) | 334.8 (89.9) | 0.209 |
| Na (mg) | 310.8 (93.6) | 320.9 (112.4) | 301.5 (73.7) | 0.630 |
| K (mg) | 236 (83.8) | 232.4 (90.2) | 239.2 (79.5) | 0.664 |
| Fe (mg) | 216 (70.5) | 211.1 (64.1) | 220.5 (77.1) | 0.953 |
| Cu (mg) | 173.7 (91.2) | 170 (118.9) | 177 (58.1) | 0.184 |
| Zn (mg) | 260.4 (101.3) | 251.2 (83.4) | 268.7 (116.6) | 0.916 |
| Mn (mg) | 714.7 (595.6) | 575.9 (360.2) | 841.3 (735) | 0.264 |
| I (ug) | 252.7 (102.1) | 278.4 (120.9) | 229.2 (76.7) | 0.177 |
| Se (mg) | 501.4 (229) | 471 (203) | 529.2 (251.7) | 0.518 |
| Thiamine (mg) | 261.4 (81.4) | 261 (74.1) | 261.7 (89.2) | 0.769 |
| Riboflavin (mg) | 289.2 (92.7) | 299.5 (92.1) | 279.8 (94.4) | 0.226 |
| Niacin (mg) | 388.5 (116.3) | 392.7 (113) | 384.7 (121.6) | 0.503 |
| Pantothenic acid (mg) | 97.3 (44.6) | 103.6 (46.9) | 91.6 (42.7) | 0.329 |
| Vit B6 (mg) | 314.1 (105.7) | 305.5 (94.4) | 321.9 (116.7) | 0.842 |
| Biotin (ug) | 117.7 (76.2) | 135.7 (87.3) | 101.2 (61.7) | 0.162 |
| Folic Acid (ug) | 205.4 (81.4) | 199.1 (86.1) | 211.2 (78.4) | 0.565 |
| Vit B12 (ug) | 636.2 (273.9) | 657.5 (321.6) | 616.6 (227.5) | 0.842 |
| Ascorbic Acid (mg) | 451.6 (244.3) | 431.2 (248.7) | 470.2 (244.2) | 0.647 |
| Vit A (ug) | 239.5 (93.4) | 250.7 (86.9) | 229.3 (99.8) | 0.549 |
| Vit D (ug) | 64.9 (65.9) | 69.5 (80.5) | 60.6 (50.6) | 0.897 |
| Vit E (mg) | 228.5 (143.7) | 191.8 (100.7) | 262.1 (169.4) | 0.065 |
| Food Group: Weekly Consumption | ||||
| Dairy Products (n°. consumed/week) | 21.2 (13) | 22.7 (14.9) | 19.9 (11.1) | 0.487 |
| Eggs, Meats and Fish (n°. consumed/week) | 23.3 (9.3) | 21.8 (10.9) | 24.6 (7.5) | 0.188 |
| Vegetables (n°. consumed/week) | 25.3 (12.9) | 23.7 (14.2) | 26.8 (11.8) | 0.188 |
| Fruits (n°. consumed/week) | 22.4 (17.7) | 19 (18.4) | 25.4 (16.8) | 0.086 |
| Legumes and Cereals (n°. consumed/week) | 6.6 (4.9) | 5.9 (3.9) | 7.2 (5.7) | 0.687 |
| Sugars and ultra-processed products (n°. consumed/week) | 53.9 (22.4) | 45.5 (14.9) | 61.6 (25.4) | 0.03 |
| Weekly food record | ||||
| R24-weekly (n°. of symbiotic foods consumed/week) | 24.4 (7.8) | 20.6 (7.8) | 27.8 (6.2) | 0.001 |
| R24-monthly (n°. of symbiotic foods consumed/week) | 97.7 (31.4) | 82.6 (31.3) | 111.4 (25) | 0.001 |
| R24-trimestral (n°. of symbiotic foods consumed/week) | 293.1 (94.3) | 247.9 (94.1) | 334.3 (75) | 0.001 |
| Anthropometric Profile | ||||
| Weight (kg) | 81.4 (17.6) | 76.6 (18) | 85.7 (16.3) | 0.086 |
| Waist circumference (cm) | 101.9 (17) | 97.6 (21) | 105.7 (11.5) | 0.312 |
| BMI (kg/m2) | 28.5 (5) | 27.5 (5.2) | 29.5 (4.8) | 0.307 |
| WHtR | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.0) | 0.518 |
| Height (cm) | 168.5 (9.2) | 166.4 (10.7) | 170.3 (7.4) | 0.245 |
| Therapeutic Variables | ||||
| N of associated antipsychotic | 1.3 (0.5) | 1.3 (0.5) | 1.3 (0.4) | 0.597 |
| DDD antipsychotics (mg) | 271.4 (242.5) | 286.7 (222.3) | 257.4 (242.5) | 0.458 |
| Total (n = 44) | Control Group (n = 21) | Intervention Group (n = 23) | p * | p ** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Basal | 6 Months | p | Basal | 6 Months | p | Basal | 6 Months | p | ||
| Macronutrients (RDA) | |||||||||||
| Energy (%) | 177.4 (48.4) | 128.2 (31.7) | <0.001 | 182 (47.3) | 130.9 (37.8) | 0.001 | 173.2 (50.1) | 125.8 (25.4) | <0.001 | 0.329 | 0.647 |
| Proteins (g) | 432 (152.5) | 328.4 (116.7) | <0.001 | 443.2 (159.6) | 311.1 (134.1) | 0.003 | 421.9 (148.6) | 344.2 (98.6) | 0.011 | 0.597 | 0.209 |
| Lipids (g) | 207.8 (63.1) | 143.2 (39.2) | <0.001 | 212.4 (68.2) | 149.6 (47) | 0.002 | 203.5 (59.4) | 137.3 (30.2) | <0.001 | 0.716 | 0.245 |
| Saturated fatty acids (g) | 392.3 (222.2) | 251.6 (93.8) | <0.001 | 414.9 (260.6) | 252.4 (99.4) | 0.002 | 371.7 (183.9) | 250.9 (90.7) | 0.008 | 0.698 | 0.897 |
| Monounsaturated fatty acids (g) | 147.7 (60.9) | 108.7 (40.2) | <0.001 | 140.9 (63.6) | 103.1 (35.9) | 0.006 | 154 (59) | 113.9 (43.9) | 0.018 | 0.613 | 0.418 |
| Polyunsaturated fatty acids (g) | 138.9 (103.7) | 120.6 (115.8) | 0.264 | 145.1 (131.3) | 144.6 (160) | 0.985 | 133.2 (72.8) | 98.7 (43.3) | 0.095 | 0.716 | 0.733 |
| Cholesterol (mg) | 247.2 (135.8) | 173.1 (80.7) | 0.001 | 224.6 (70.3) | 160.1 (76.7) | 0.002 | 267.9 (175) | 185 (84.2) | 0.027 | 0.787 | 0.285 |
| Carbohydrates (g) | 159.6 (53.4) | 118.7 (35.2) | <0.001 | 161.8 (48.2) | 120.3 (38.5) | 0.003 | 157.5 (58.7) | 117.3 (32.6) | 0.004 | 0.518 | 0.897 |
| Oligosaccharides (g) | 303.4 (205.7) | 204.8 (98.6) | 0.005 | 342.7 (203.8) | 214 (113.4) | 0.015 | 267.5 (205.2) | 196.5 (84.6) | 0.134 | 0.088 | 0.805 |
| Polysaccharides (g) | 123.2 (74.6) | 96.6 (40.7) | 0.019 | 139.8 (87.2) | 89.1 (44.4) | 0.003 | 108 (58.8) | 103.5 (36.6) | 0.762 | 0.378 | 0.177 |
| Fibre (g) | 215.1 (124.8) | 185.8 (111) | 0.229 | 196 (130.5) | 175.8 (116.8) | 0.560 | 232.5 (119.5) | 194.8 (107.3) | 0.288 | 0.245 | 0.431 |
| Micronutrients (RDA) | |||||||||||
| Ca (mg) | 181 (68.8) | 142 (56) | 0.004 | 194.1 (64.7) | 142.8 (64.6) | 0.02 | 169.2 (71.2) | 141.3 (48.2) | 0.099 | 0.124 | 0.751 |
| Mg (mg) | 292.8 (95.4) | 238.2 (102.7) | 0.002 | 285.3 (88.8) | 220.2 (116.8) | 0.013 | 299.7 (102.6) | 254.7 (87.3) | 0.07 | 0.823 | 0.118 |
| P (mg) | 352.9 (107.7) | 270.9 (77.8) | <0.001 | 372.7 (123.5) | 265.6 (88.2) | 0.003 | 334.8 (89.9) | 275.7 (68.6) | 0.009 | 0.209 | 0.318 |
| Na (mg) | 310.8 (93.6) | 201 (60.7) | <0.001 | 320.9 (112.4) | 200.5 (67.6) | 0.001 | 301.5 (73.7) | 201.5 (55.2) | <0.001 | 0.630 | 0.860 |
| K (mg) | 236 (83.8) | 195.5 (84.4) | 0.007 | 232.4 (90.2) | 178.4 (87.7) | 0.03 | 239.2 (79.5) | 211 (80.1) | 0.120 | 0.664 | 0.136 |
| Fe (mg) | 216 (70.5) | 169.5 (57.6) | <0.001 | 211.1 (64.1) | 166.9 (66.3) | 0.008 | 220.5 (77.1) | 171.8 (49.8) | 0.003 | 0.953 | 0.953 |
| Cu (mg) | 173.7 (91.2) | 154.8 (73.8) | 0.170 | 170 (118.9) | 133.9 (74.5) | 0.132 | 177 (58.1) | 173.8 (69.3) | 0.829 | 0.184 | 0.08 |
| Zn (mg) | 260.4 (101.3) | 202.3 (76.4) | 0.003 | 251.2 (83.4) | 206.3 (96.5) | 0.118 | 268.7 (116.6) | 198.6 (53.9) | 0.013 | 0.916 | 0.565 |
| Mn (mg) | 714.7 (595.6) | 632.8 (437.2) | 0.413 | 575.9 (360.2) | 495.5 (376.9) | 0.290 | 841.3 (735) | 758.3 (458.3) | 0.648 | 0.264 | 0.028 |
| I (ug) | 252.7 (102.1) | 194.9 (91.9) | 0.009 | 278.4 (120.9) | 204.2 (114.7) | 0.061 | 229.2 (76.7) | 186.4 (66.3) | 0.063 | 0.177 | 0.953 |
| Se (mg) | 501.4 (229) | 392.4 (147.6) | 0.004 | 471 (203) | 360.4 (136.2) | 0.034 | 529.2 (251.7) | 421.6 (154.4) | 0.055 | 0.518 | 0.162 |
| Thiamine (mg) | 261.4 (81.4) | 209.6 (65.2) | <0.001 | 261 (74.1) | 203.5 (76.8) | 0.006 | 261.7 (89.2) | 215.2 (53.7) | 0.024 | 0.769 | 0.503 |
| Riboflavin (mg) | 289.2 (92.7) | 237.2 (72) | 0.005 | 299.5 (92.1) | 230.3 (79.9) | 0.019 | 279.8 (94.4) | 243.5 (65) | 0.135 | 0.226 | 0.534 |
| Niacin (mg) | 388.5 (116.3) | 318 (98.4) | 0.001 | 392.7 (113) | 295.9 (114.4) | 0.002 | 384.7 (121.6) | 338.1 (78.3) | 0.104 | 0.503 | 0.107 |
| Pantothenic acid (mg) | 97.3 (44.6) | 82.3 (32.2) | 0.051 | 103.6 (46.9) | 73 (29.4) | 0.019 | 91.6 (42.7) | 90.8 (32.8) | 0.929 | 0.329 | 0.08 |
| Vit B6 (mg) | 314.1 (102.7) | 262.9 (97.8) | 0.007 | 305.5 (94.4) | 244.2 (104.5) | 0.025 | 321.9 (116.7) | 279.9 (90.2) | 0.128 | 0.842 | 0.155 |
| Biotin (ug) | 117.7 (76.2) | 104.6 (73.6) | 0.363 | 135.7 (87.3) | 93.5 (62.3) | 0.07 | 101.2 (61.7) | 114.7 (82.7) | 0.429 | 0.162 | 0.296 |
| Folic Acid (ug) | 205.4 (81.4) | 174.8 (69.9) | 0.02 | 199.1 (86.1) | 158.3 (64.7) | 0.057 | 211.2 (78.4) | 189.9 (72.4) | 0.195 | 0.565 | 0.142 |
| Vit. B12 (ug) | 636.2 (273.9) | 475.6 (198.3) | 0.002 | 657.5 (321.6) | 449.5 (183.2) | 0.021 | 616.6 (227.5) | 499.4 (212.3) | 0.04 | 0.842 | 0.549 |
| Ascorbic Acid (mg) | 451.6 (244.3) | 429 (206.7) | 0.529 | 431.2 (248.7) | 383.2 (226.1) | 0.292 | 470.2 (244.2) | 470.8 (287.3) | 0.992 | 0.647 | 0.254 |
| Vit. A (ug) | 239.5 (93.4) | 219 (80.1) | 0.212 | 250.7 (86.9) | 208.6 (75.7) | 0.079 | 229.3 (99.8) | 228.6 (84.4) | 0.974 | 0.549 | 0.366 |
| Vit. D (ug) | 64.9 (65.9) | 52.4 (56.1) | 0.13 | 69.5 (80.5) | 56.4 (78.4) | 0.201 | 60.6 (50.6) | 48.8 (23.1) | 0.361 | 0.897 | 0.445 |
| Vit. E (mg) | 228.5 (143.7) | 156.4 (52.8) | 0.002 | 191.8 (100.7) | 146.9 (64.3) | 0.059 | 262.1 (169.4) | 165.1 (39.1) | 0.014 | 0.065 | 0.062 |
| Food Group: Weekly Consumption | |||||||||||
| Dairy Products (n°. consumed/week) | 21.3 (13) | 22.4 (11.9) | 0.625 | 22.7 (14.9) | 24.4 (13.8) | 0.670 | 19.9 (11.1) | 20.5 (9.8) | 0.822 | 0.487 | 0.316 |
| Eggs, Meats and Fish (n°. consumed/week) | 23.3 (9.3) | 20 (7.6) | 0.097 | 21.8 (10.9) | 22.1 (8.7) | 0.927 | 24.6 (7.5) | 18.1 (5.9) | 0.009 | 0.188 | 0.09 |
| Vegetables (n°. consumed/week) | 25.3 (12.9) | 23.8 (11.1) | 0.517 | 23.7 (14.2) | 23 (12.9) | 0.848 | 26.8 (11.8) | 24.6 (9.4) | 0.407 | 0.188 | 0.284 |
| Fruits (n°. consumed/week) | 22.4 (17.7) | 18.2 (14.4) | 0.074 | 19 (18.4) | 15.3 (14) | 0.276 | 25.4 (16.8) | 20.8 (14.6) | 0.165 | 0.086 | 0.148 |
| Legumes and Cereals (n°. consumed/week) | 6.6 (4.9) | 7.2 (4.2) | 0.380 | 5.9 (3.9) | 6.4 (2.5) | 0.562 | 7.2 (5.7) | 7.9 (5.2) | 0.522 | 0.687 | 0.661 |
| Sugars and ultra-processed products (n°. consumed/week) | 53.9 (22.4) | 57.4 (28.4) | 0.505 | 45.5 (14.9) | 67.2 (34.1) | 0.006 | 61.6 (25.4) | 48.4 (18.5) | 0.03 | 0.03 | 0.037 |
| Weekly food record | |||||||||||
| R24-weekly (n°. of symbiotic foods consumed/week) | 24.4 (7.8) | - | - | 20.6 (7.8) | - | - | 27.8 (6.2) | - | - | 0.001 | - |
| R24-monthly (n°. of symbiotic foods consumed/week) | 97.7 (31.4) | - | - | 82.6 (31.3) | - | - | 111.4 (25) | - | - | 0.001 | - |
| R24- quarterly (n°. of symbiotic foods consumed/week) | 293.1 (94.3) | - | - | 247.9 (94.1) | - | - | 334.3 (75) | - | - | 0.001 | - |
| Anthropometric Profile | |||||||||||
| Weight (kg) | 81.4 (17.6) | 78.7 (16.2) | <0.001 | 76.6 (18) | 75.8 (17.7) | 0.382 | 85.7 (16.3) | 81.3 (14.6) | <0.001 | 0.086 | 0.275 |
| Waist circumference (cm) | 101.9 (17) | 101.6 (12.5) | 0.898 | 97.6 (21) | 101.2 (13.5) | 0.322 | 105.7 (11.5) | 102.1 (11.7) | <0.001 | 0.397 | 0.981 |
| BMI (kg/m2) | 28.5 (5) | 27.6 (4.7) | <0.001 | 27.5 (5.2) | 27.2 (5.3) | 0.323 | 29.5 (4.8) | 27.9 (4.3) | <0.001 | 0.307 | 0.869 |
| WHtR | 0.6 (0.09) | 0.6 (0.07) | 0.932 | 0.6 (0.12) | 0.6 (0.08) | 0.345 | 0.6 (0.06) | 0.6 (0.06) | <0.001 | 0.597 | 0.378 |
| Therapeutic Variables | |||||||||||
| N° of associated antipsychotic | 1.3 (0.5) | 1.2 (0.4) | 0.262 | 1.38 (0.49) | 1.28 (0.46) | 0.329 | 1.3 (0.47) | 1.26 (0.44) | 0.575 | 0.597 | 0.855 |
| DDD antipsychotics (mg) | 271.4 (242.5) | 241.2 (226.7) | 0.108 | 286.7 (222.3) | 260.5 (221.5) | 0.230 | 257.4 (263.7) | 247.4 (225.9) | 0.301 | 0.458 | 0.789 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevillano-Jiménez, A.; Romero-Saldaña, M.; García-Rodríguez, M.; Molina-Luque, R.; Molina-Recio, G. Nutritional Impact and Eating Pattern Changes in Schizophrenic Spectrum Disorders after Health Education Program on Symbiotic Dietary Modulation Offered by Specialised Psychiatric Nursing–Two-Arm Randomised Clinical Trial. Nutrients 2022, 14, 5388. https://doi.org/10.3390/nu14245388
Sevillano-Jiménez A, Romero-Saldaña M, García-Rodríguez M, Molina-Luque R, Molina-Recio G. Nutritional Impact and Eating Pattern Changes in Schizophrenic Spectrum Disorders after Health Education Program on Symbiotic Dietary Modulation Offered by Specialised Psychiatric Nursing–Two-Arm Randomised Clinical Trial. Nutrients. 2022; 14(24):5388. https://doi.org/10.3390/nu14245388
Chicago/Turabian StyleSevillano-Jiménez, Alfonso, Manuel Romero-Saldaña, María García-Rodríguez, Rafael Molina-Luque, and Guillermo Molina-Recio. 2022. "Nutritional Impact and Eating Pattern Changes in Schizophrenic Spectrum Disorders after Health Education Program on Symbiotic Dietary Modulation Offered by Specialised Psychiatric Nursing–Two-Arm Randomised Clinical Trial" Nutrients 14, no. 24: 5388. https://doi.org/10.3390/nu14245388
APA StyleSevillano-Jiménez, A., Romero-Saldaña, M., García-Rodríguez, M., Molina-Luque, R., & Molina-Recio, G. (2022). Nutritional Impact and Eating Pattern Changes in Schizophrenic Spectrum Disorders after Health Education Program on Symbiotic Dietary Modulation Offered by Specialised Psychiatric Nursing–Two-Arm Randomised Clinical Trial. Nutrients, 14(24), 5388. https://doi.org/10.3390/nu14245388









